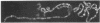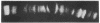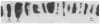Abstract
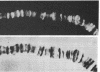
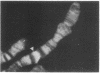
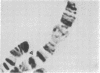
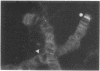
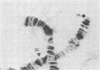
RNA polymerase (RNA nucleotidyltransferase) B (or II) and histone H1 of Drosophila melanogster were localized on salivary gland polytene chromosomes using the indirect immunofluorescence technique. RNA polymerase B is present almost exclusively in puffs and interband regions, whereas histone H1 is found primarily in bands. The puff at region 3C, known to be transcriptionally active in larval salivary glands, gives a bright fluorescence with antibodies against RNA polymerase B. This fluorescence disappears after exposure of the larvae to 37 degrees for 45 min. The heat shock treatment results in a general reduction of fluorescence intensity with the appearance of brightly staining heat shock puffs. Heat-induced removal of RNA polymerase molecules from a puff does not immediately alter its morphology. We propose than an interband represents that fraction of the total number of gene copies in a band that are active, the inactive copies being present in a condensed form in the adjacent band. Large puffs would originate through the decondensation and activation of most or all gene copies in a band.
Full text
PDF
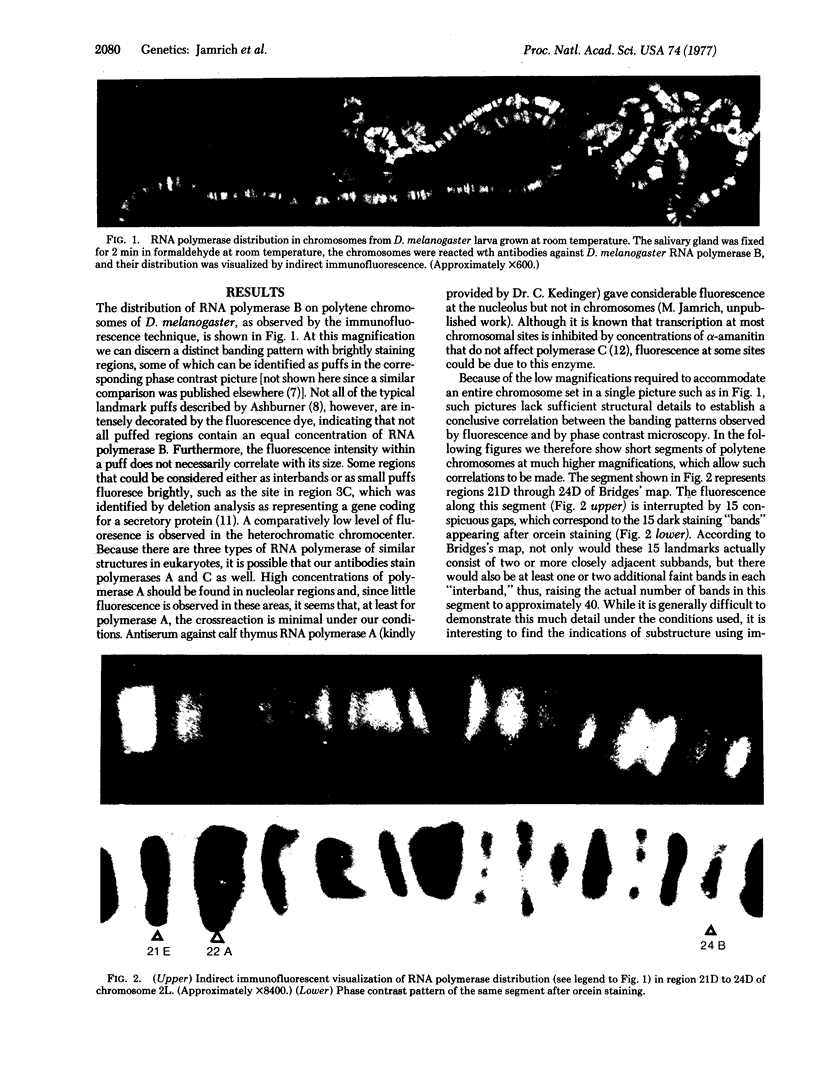

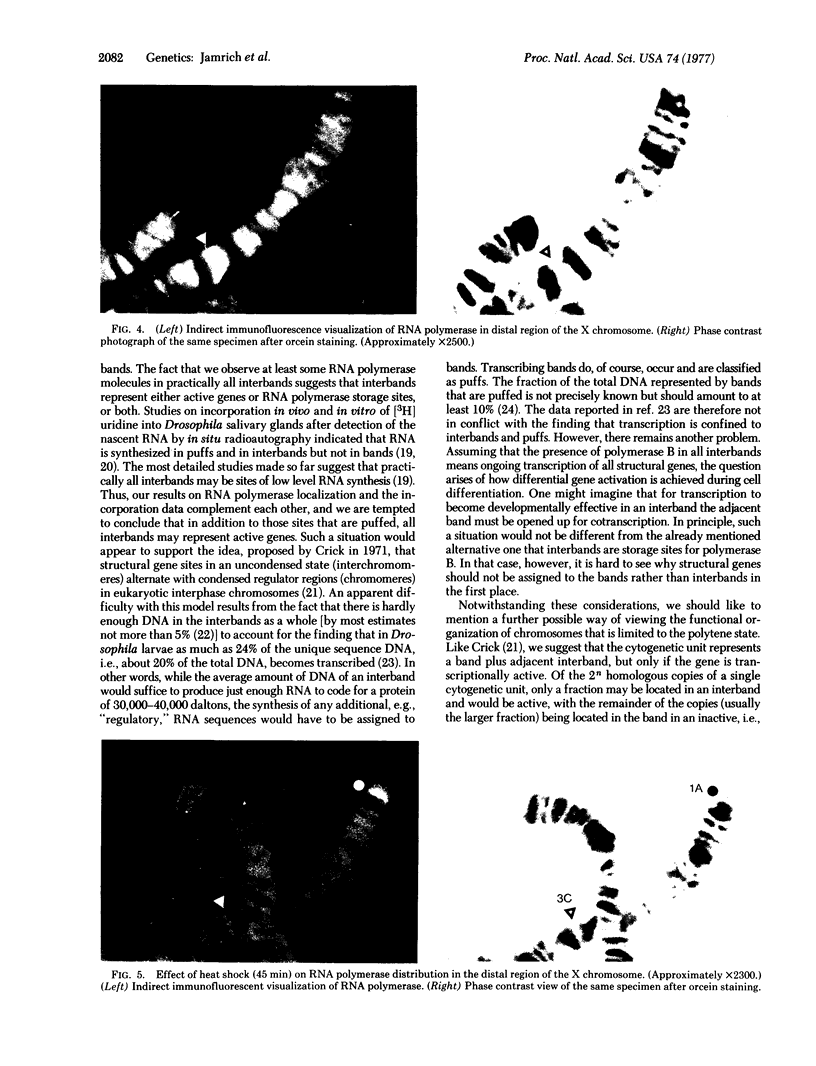
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Rudkin G. T., Cohen L. H. Locations of chromosomal proteins in polytene chromosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2038–2042. doi: 10.1073/pnas.73.6.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann W. Chromomeres and genes. Results Probl Cell Differ. 1972;4:1–33. doi: 10.1007/978-3-540-37164-9_1. [DOI] [PubMed] [Google Scholar]
- Beermann W. Effect of -amantine on puffing and intranuclear RNA synthesis in Chironomus salivary glands. Chromosoma. 1971;34(2):152–167. doi: 10.1007/BF00285183. [DOI] [PubMed] [Google Scholar]
- Crick F. General model for the chromosomes of higher organisms. Nature. 1971 Nov 5;234(5323):25–27. doi: 10.1038/234025a0. [DOI] [PubMed] [Google Scholar]
- Desai L. S., Pothier L., Foley G. E., Adams R. A. Immunofluorescent labeling of chromosomes with antisera to histones and histone fractions. Exp Cell Res. 1972 Feb;70(2):468–471. doi: 10.1016/0014-4827(72)90169-3. [DOI] [PubMed] [Google Scholar]
- Greenleaf A. L., Bautz E. K. RNA polymerase B from Drosophila melanogaster larvae. Purification and partial characterization. Eur J Biochem. 1975 Dec 1;60(1):169–179. doi: 10.1111/j.1432-1033.1975.tb20989.x. [DOI] [PubMed] [Google Scholar]
- Korge G. Chromosome puff activity and protein synthesis in larval salivary glands of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4550–4554. doi: 10.1073/pnas.72.11.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLING C. RIBONUKLEINSAEURE-SYNTHESE DER RIESENCHROMOSOMEN. AUTORADIOGRAPHISCHE UNTERSUCHUNGEN AN CHIRONOMUS TENTANS. Chromosoma. 1964 Apr 1;15:71–122. doi: 10.1007/BF00326915. [DOI] [PubMed] [Google Scholar]
- Pagés M., Alonso C. Activity of the endogenous RNA polymerase on fixed polytene chromosomes. Exp Cell Res. 1976 Mar 1;98(1):120–126. doi: 10.1016/0014-4827(76)90470-5. [DOI] [PubMed] [Google Scholar]
- Plagens U., Greenleaf A. L., Bautz E. K. Distribution of RNA polymerase on Drosophila polytene chromosomes as studied by indirect immunofluorescence. Chromosoma. 1976 Dec 16;59(2):157–165. doi: 10.1007/BF00328484. [DOI] [PubMed] [Google Scholar]
- Shields G., Dübendorfer A., Sang J. H. Differentiation in vitro of larval cell types from early embryonic cells of Drosophila melanogaster. J Embryol Exp Morphol. 1975 Feb;33(1):159–175. [PubMed] [Google Scholar]
- Silver L. M., Elgin S. C. A method for determination of the in situ distribution of chromosomal proteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):423–427. doi: 10.1073/pnas.73.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975 Apr;4(4):395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Turner S. H., Laird C. D. Diversity of RNA sequences in Drosophila melanogaster. Biochem Genet. 1973 Nov;10(3):263–274. doi: 10.1007/BF00485704. [DOI] [PubMed] [Google Scholar]