Abstract
The molecular mechanisms influencing healthspan are unclear but mitochondrial function, resistance to oxidative stress and proteostasis are recurring themes. Tumor necrosis factor Receptor Associated Protein 1 (TRAP1), the mitochondrial analogue of Hsp75, regulates levels of reactive oxygen species in vitro and is found expressed at higher levels in tumor cells where it is thought to play a pro-survival role. While TRAP1-directed compartmentalized protein folding is a promising target for cancer therapy, its role at the organismal level is unclear. Here we report that overexpression of TRAP1 in Drosophila extends healthspan by enhancing stress resistance, locomotor activity and fertility while depletion of TRAP1 has the opposite effect, with little effect on lifespan under both conditions. In addition, modulating TRAP1 expression promotes the nuclear translocation of homeobox protein Dve and increases expression of genes associated with the mitochondrial unfolded protein response (UPRmt), indicating an activation of this proteostasis pathway. Notably, independent genetic knockdown of components of the UPRmt pathway dampen the enhanced stress resistance observed in TRAP1 overexpression flies. Together these studies suggest that TRAP1 regulates healthspan, potentially through activation of the UPRmt.
Keywords: TRAP1, ageing, healthspan, mitochondria, UPR, Drosophila
Introduction
Age related impairment of locomotor, reproductive, and physiological functions is a universal phenomenon in animals. Intriguingly, several of the lifespan extension animal models are not healthy through their life (Bartke & Brown-Borg 2004). In worms, mitochondrial mutants such as isp-1 and nuo-2 live long, but are slow growing and have a reduced brood size (Rea et al. 2007). Similarly, in long lived dwarf mice with deficiencies in growth hormone, prolactin and thyroid stimulating hormone, reproductive fitness is compromised (Bartke & Brown-Borg 2004). Finally, Drosophila treated with the anti-convulsive agent Lamotrigine display significantly enhanced lifespan but poor locomotor performance at older ages (Avanesian et al. 2010). An emerging goal in aging research is developing interventions that not only increase longevity, but also increase the healthspan (Kirkland & Peterson 2009; Selman & Withers 2011).
The mechanisms that modulate healthy aging are unclear, but mitochondria are thought to play a central role (Wolff & Dillin 2006). Mitochondria generate high levels of reactive oxygen species (ROS) that damage cellular macromolecules and gradually impair cellular function. Some evidence suggests that accumulation of ROS contributes to the physiological decline associated with age, though this theory is not universally accepted (Wolff & Dillin 2006; Hekimi et al. 2011). Mice that overexpress catalase in their mitochondria have an enhanced lifespan, delayed age-associated pathologies, reduced mtDNA deletions with age and reduced levels of ROS (Schriner et al. 2005). Knockdown of components of the electron transport chain (ETC) not only decrease ATP production but surprisingly also increase lifespan (Wolff & Dillin 2006; Copeland et al. 2009). Furthermore, overexpressing mitochondrial LON protease in the fungal aging model P. anserina results in enhanced healthspan, suggesting that mitochondrial proteostasis is an important regulator of organismal health (Luce & Osiewacz 2009).
Mitochondrial function is tightly associated with energy sensing and stress response pathways that contribute to aging, in particular the mTOR and AMPK pathways (Haigis & Yankner 2010). These pathways are also associated with oxidative stress resistance and regulation of cellular proteostasis (Salminen et al. 2012). Compartments such as the endoplasmic reticulum and mitochondria are especially vulnerable to diminished proteostasis because of their high intrinsic protein folding requirements. To regulate protein quality control, these organelles have dedicated stress response mechanisms called the unfolded protein response (UPR) (Ron & Walter 2007; Haynes & Ron 2010). Accordingly, it has been hypothesized that enhanced proteome maintenance would push the limits of the minimal proteostasis boundary (folding energetics necessary for folding of a protein at a defined proteostasis network capacity) towards health (Powers et al. 2009).
Mitochondrial chaperone Tumor necrosis factor Receptor Associated Protein 1 (TRAP1) is a member of the Hsp90 family and is the mitochondrial analogue of Hsp75 (Felts et al. 2000). TRAP1, originally identified as a binding partner of the intracellular domain of TNFR1, also binds retinoblastoma protein during mitosis, and the tumor suppressors EXT1 and EXT2 (Altieri et al. 2012). Despite the high homology with Hsp90, TRAP1 does not interact with its traditional client proteins (Kang et al. 2007), indicating a distinct functional role for this mitochondrial chaperone. In vitro evidence suggests that TRAP1 regulates ROS levels and protects cells against oxidative damage (Hua et al. 2007). Furthermore, TRAP1 was found expressed at higher levels in tumor cells where it is thought to play a pro-survival role by inhibiting apoptosis (Kang et al. 2007; Costantino et al. 2009). Recent evidence also implicates TRAP1 in Parkinson’s disease. PINK1 mediated phosphorylation of TRAP1 is considered important in suppressing cytochrome c release from mitochondria to regulate apoptosis (Pridgeon et al. 2007). TRAP1 also ameliorates cellular toxicity induced by α-synuclein in dopaminergic neurons (Butler et al. 2012) and expression of TRAP1 was able to rescue mitochondrial dysfunction in Pink1 deficient flies (Zhang et al, 2013; Costa et al, 2013). In summary, TRAP1 lies at an exciting intersection between ROS, aging, mitochondrial proteostasis and human disease.
To determine whether modulating mitochondrial proteostasis would influence longevity and healthspan in vivo, we studied the effect of modulating TRAP1 expression levels in Drosophila. Here we demonstrate that TRAP1 regulates ROS levels, oxidative stress resistance and heat stress resistance in Drosophila, without significantly altering lifespan. Intriguingly, there is robust sex-specificity in TRAP1-mediated stress resistance. In addition, ubiquitous overexpression of TRAP1 enhances fertility and remarkably improves locomotor ability in aging flies. In contrast, loss of TRAP1 results in decreased fertility and locomotor ability, indicating strong modulation of healthspan by TRAP1. Finally, we show that dosage modulation of TRAP1 activates the mitochondrial UPR (UPRmt) in males but not the females, indicative of similar sex-specificity as observed in stress resistance. Finally, impairment of the UPRmt inhibits TRAP1 mediated resistance to stress. These findings suggest that TRAP1 mediated alterations in UPRmt can modulate stress resistance and healthspan.
Material and Methods
Drosophila Stocks and Culture
All fly stocks were maintained on standard cornmeal medium, unless stated otherwise, at 25°C and 60% humidity under 12 hr light: dark cycles. w; TRAP1Δ4/ Cyo; +/+ flies were obtained from Dr. Jessica Treisman, New York University. A full-length deletion of the TRAP1 locus resulting from an imprecise p-element excision was confirmed by qPCR in w; TRAP1Δ4/ TRAP1Δ4; +/+ flies. To generate transgenic overexpression strains, Drosophila TRAP1 cDNA was inserted in pUAST plasmid and microinjected into w1118; +/+; +/+ embryos (BestGene Inc). Insertions were verified by inverse PCR and two independent strains were used, w; +/+; UAS TRAP14M/+ and w; +/+; UAS TRAP17M/+. Overexpression in the w; +/+; UAS TRAP14M/ActinGal4 and w; +/+; UAS TRAP17M/ActinGal4 was confirmed by qPCR. All strains were backcrossed 5–6 generations in the w1118; +/+; +/+ background. w1118; +/+; +/+, ActinGal4 and elavGal4 drivers were obtained from the Bloomington Stock Center, Indiana. w; UAS CG5045 RNAi/+; +/+ and w; UAS DVE RNAi; +/+ strains were obtained from the Vienna Drosophila Resource Center. Geneswitch-tub5Gal4 flies were a gift from Dr. Scott Pletcher (University of Michigan).
Lifespan Analysis and Stress Resistance
w1118; +/+; +/+ strain was used as the background control for TRAP1 mutants. For the TRAP1 overexpressing flies, the transgene alone and the driver alone strains were used as controls. In both lifespan and stress assays (oxidative and heat stress resistance), significant alteration was deemed only when p<0.05 relative to all control strains. Statistical analysis was performed using JMP software and significance was determined using the standard chi-squared based log rank test. For detailed experimental design, see Supplementary Information Material and Methods.
Feeding behavior
CAFE (Capillary Feeder) assay was modified from Ja et al, 2007. Briefly, 5 male flies of each genotype were starved for 3 hr (same time duration as in oxidative stress resistance assay) before being transferred to a 2.5 × 9.5 cm vial with moistened filter paper at the base. Two 5µl calibrated glass micropipettes (VWR) filled with 5% sucrose were inserted midway into the vial. Blue dye was added to the sucrose solution to facilitate readings that were taken every 2 hrs, for a total of 8 hours. Amount of food consumed was corrected on the basis of evaporation in a blank vial and calculated per fly per hour. The experiment was repeated 3 times with independent cohort of flies and statistical significance determined using Student’s t-test.
Locomotor ability
Locomotor ability was assessed using the negative geotaxis assay in a longitudinal study. A total of 50 male and 50 female flies of each genotype were assayed every 10 days, starting with 10-day old flies. On the day of experiment, flies were transferred to empty 2.5 × 9.5 cm vials. After 120 sec of acclimatization, flies were gently tapped to the bottom three times and allowed to climb the walls. The number of flies that successfully crossed 7 cm in 10 sec was scored. Each experiment was conducted with 5 independent groups, and each group was tested three times after a 5 min interval between each test. Statistical significance was determined by one-way ANOVA with Dunnett’s post hoc comparison using SPSS software.
Fertility
To assess female fertility, 5 virgin females of each genotype were allowed to mate with 5 w1118 males, aged 3 days. Flies were transferred to fresh food every 3 days and total number of progeny eclosed from each vial was counted once every 3 days for 9 days. Fresh 3-day old w1118 males were replaced at every time-point. Brood size was defined as the total progeny eclosed over 60 days. All genotypes were tested in parallel and three independent repeats were performed. To determine the fertility of males, 1 male of each genotype was allowed to mate with 3 virgin w1118 females aged 3 days. Parent flies were removed after 3 days and total number of progeny eclosed from each vial was counted. Fresh 3-day old virgin w1118 females were replaced at every time-point. The experiment was conducted with 5 males of each genotype in parallel and was repeated twice. Statistical significance was determined when p<0.05, using Student’s t-test.
ROS measurement
ROS levels were measured using MitoSox (Invitrogen). Adult brains were dissected in cold HBSS, transferred to 5 µM MitoSox and incubated for 30 min at room temperature. Brains were washed 3 times with cold PBS and mounted in Vectashield (Invitrogen) between a slide and a coverslip placed on double-sided tape to prevent squashing. For quantification purposes, z-stacks were acquired through the entire thickness of the brain, and total fluorescent intensity was measured from 3D reconstruction images using ImageJ (NIH).
Immunohistochemistry
Drosophila larvae were dissected and stained as described previously (Baqri et al. 2009). Adult Drosophila brains were dissected in cold PBS, fixed overnight in 0.8% paraformaldehyde at 4°C, and washed thrice in PBS with 0.5% Triton and 0.5% BSA (PBST) for 15 min each. Samples were blocked in 10% BSA for 2 hrs, and incubated overnight in primary antibody at 4°C. Samples were washed four times with PBST for 30 min each, and incubated with secondary antibody for 12 hrs at 4°C. After three 30 min washes with PBST and a 15 min wash with PBS, brains were mounted in Vectashield with DAPI (Invitrogen). We used mouse anti-mitochondrial complex V monoclonal antibody at 1:500 (MitoSciences) and rabbit anti-Dve antibody at 1:1000 (Nakagoshi et al, 1998). All comparative images were either acquired on a Nikon TE swept field microscope or a Zeiss LSM 700 confocal microscope at identical laser settings, exposure and aperture. Fluorescently labeled secondary antibodies used were goat anti-mouse Alexa 568 (Invitrogen) and goat anti-rabbit Alexa 488 (Invitrogen).
Quantitative PCR
Total RNA was reverse transcribed using the Taqman kit by Applied Biosystems (Roche), according to manufacturer’s recommendations. The quantitative PCR was done using the Power SYBr Master Mix (Applied Biosystems). Gene expression was normalized based on 28S RNA expression from three replicate experiments. For list of primers used, see Supporting Information Materials and Methods.
Results
TRAP1 regulates ROS levels in vivo
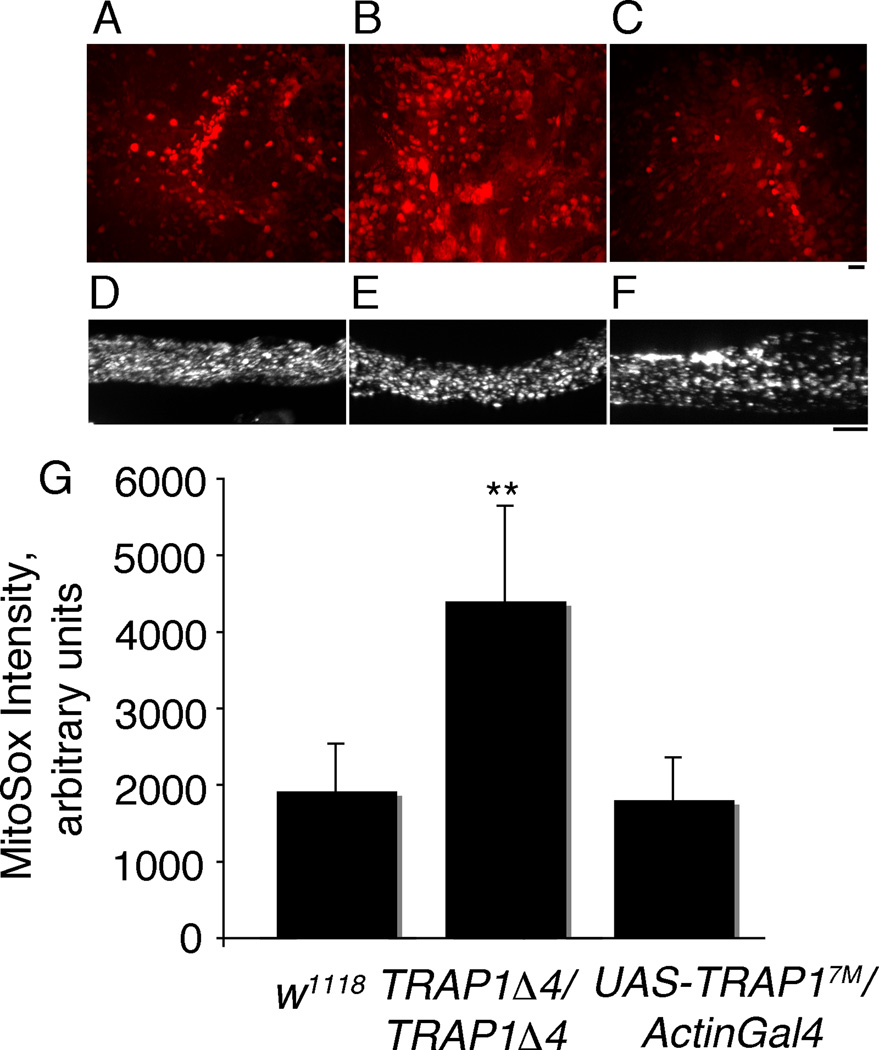
In vitro evidence indicates that loss of TRAP1 results in increased accumulation of cellular ROS (Hua et al. 2007; Im et al. 2007; Yoshida et al. 2013). To determine whether TRAP1 regulates ROS levels in vivo, we assayed ROS levels in the brains of adult Drosophila with MitoSox, a cell permeable dye that fluoresces when oxidized by ROS and is rapidly and selectively targeted to the mitochondria. There is a marked increase in MitoSox staining in the brains of 5 day old males in TRAP1 deletion mutant, w; TRAP1Δ4/ TRAP1Δ4; +/+, but not when TRAP1 was overexpressed using the constitutive and ubiquitous driver ActinGal4 (Figure 1 A, B, C and G). TRAP1 expression levels in mutant and overexpression strains were confirmed by qPCR (Supplementary Figure 1 A and B). Mitochondrial distribution was not significantly altered in the segmental nerves of these strains, indicating that increased MitoSox staining in the mutants was not due to changes in mitochondrial density (Figure 1 D , E and F). These data suggest that TRAP1 regulates generation of mitochondrial oxidants in vivo.
Figure 1. TRAP1 regulates ROS levels in vivo.
(A–C) Optic lobes of young adult Drosophila brains stained with Mitosox, and (D–F) larval segmental nerves of corresponding genotypes stained with antibody against mitochondrial Complex V. (A and D)w1118; +/+; +/+(B and E)w; TRAPΔ4/TRAPΔ4; +/+, (C and F) w; +/+; UAS-TRAP17M/ActinGal4. (G) MitoSox staining is significantly increased in w; TRAPΔ4/TRAPΔ4; +/+ mutants. While staining is marginally reduced in overexpression flies w; +/+; UAS-TRAP17M/ActinGal4, it is not significantly different from wildtype. Error bars denote standard deviation of means. N = 3–5. (**) indicate p < 0.001 from Student’s t-test. Mitochondrial distribution appears normal in mutant and overexpression animals in segmental nerves of Drosophila larvae. In all cases, scale bars equal 10 µm.
TRAP1 regulates resistance to oxidative stress in young and old Drosophila
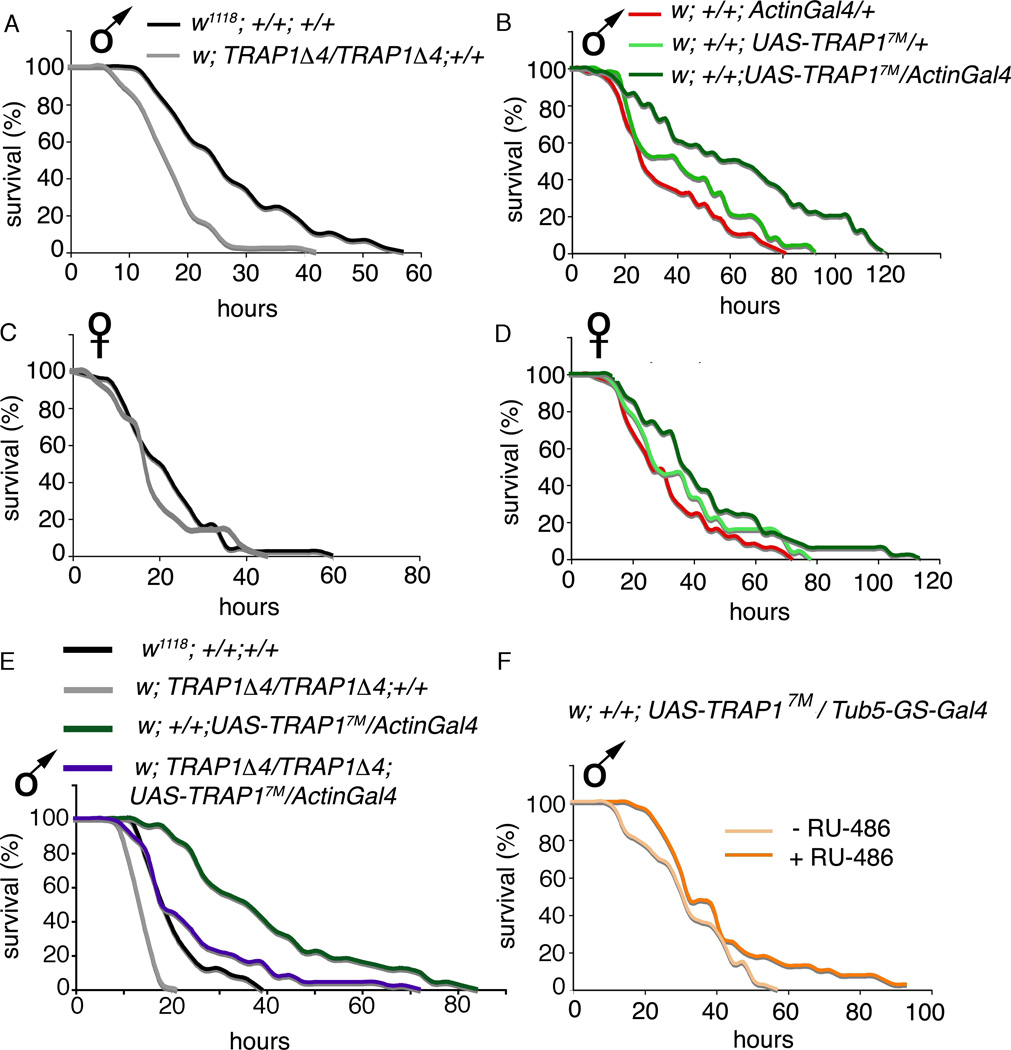
Increased ROS levels are associated with oxidative damage and diminished stress resistance in several animal models (Hekimi et al. 2011). To examine if TRAP1 regulates resistance to oxidative stress, we exposed 5-day old TRAP1Δ4 mutant flies to paraquat and assayed for survival. Paraquat is a methyl viologen that undergoes redox cycling to generate superoxide. In congruence with a recent study (Costa et al, 2013), we found that young TRAP1Δ4 mutant males were significantly more susceptible to oxidative stress as compared to males of the w1118 wildtype background strain (Figure 2 A). In contrast, resistance to oxidative stress was significantly increased when TRAP1 was ubiquitously overexpressed in two independent transgenic strains, w; +/+; UAS-TRAP17M/ActinGal4 and w: +/+; UAS-TRAP14M/ActinGal4 (Figure 2 B and Supplementary Figure 2 A). Interestingly, this response was sex specific: males were especially responsive to modulation of TRAP1 levels while female flies in both the mutant and overexpression strains did not show pronounced changes in oxidative stress resistance (Figure 2 C, D, and Supplementary Figure 2 B).
Figure 2. TRAP1 regulates oxidative stress resistance in young and old Drosophila males.
(A–E) Survival curves of young flies (5 days) of indicated genotypes exposed to 20 mM paraquat. (A) Median survival of male w; TRAP1Δ4/ TRAP1Δ4; +/+ (18.5 hrs ± 0.9) is significantly less than that of w1118; +/+; +/+ (28.4 hrs ± 1.6; p<0.0001). (B) Median survival of male w; +/+; UAS-TRAP17M/ActinGal4 (63.2 hrs ± 4.8) is significantly more than that of w; +/+; UAS-TRAP17M/ + (43.9 hrs ± 3.2; p=0.0002), and that of w; +/+; ActinGal4 (36.2 ± 2.8; p<0.0001). (C) Median survival of female w; TRAP 1A4/TRΔP 1Δ4; +/+ (20.3 hrs ± 1.4) is comparable to that of w1118; +/+; +/+ (22.4 hrs ± 1.4; p=0.4). (D) Median survival of female w; +/+; UAS-TRAP17M/ActinGal4 (43.9 hrs ± 3.3) is significantly higher than that of w; +/+; ActinGal4/+ (32.4 hrs ± 2.2; p=0.004) but comparable to that of w; +/+; UAS-TRAP17M/ + (36.9 hrs ± 2.7; p=0.1). (E) Median survival of young w; TRAP 1A4/TRAP 1A4; UAS-TRAP17M/ActinGal4 males (25.26 hrs ± 1.88) is significantly higher than w; TRAP 1Δ4/TRAP 1Δ4; +/+ (15 hrs ± 0.4; p<0.0001). (F) Median survival is increased in old (40 days) w; +/+; UAS-TRAP17M/ GS-tub5Gal4 males maintained on 5 mM RU-486 (41.47 hrs ± 2.99), relative to control flies of the same genotype, sex and age maintained on vehicle (32.88 ± 1.78; p = 0.03). In all cases, errors denote standard deviation. Statistical significance was determined using the standard chi-squared based log-rank test.
To confirm the role of TRAP1 in regulating oxidative stress resistance, we rescued loss of function by over-expressing TRAP1 in the TRAP1Δ4/ TRAP 1Δ4 mutant background. Median survival on paraquat was significantly enhanced in w;TRAP1Δ4/ TRAP1Δ4; UAS-TRAP17M/ActinGal4 flies as compared to w; TRAP 1Δ4/ TRAP1Δ4; +/+ mutants (Figure 2 E). The observed decrease of stress resistance in the overexpression strain itself, when endogenous TRAP1 is depleted in this background, also suggests that changes in survival are the result of TRAP1 dosage modulation as opposed to the effects of hybrid vigor.
It is known that oxidative stress resistance declines as a function of age, which we have verified in wild-type flies (Supplementary Figure 2 C and D). To determine whether TRAP1 over-expression in older flies could confer protection against oxidative stress, we transiently over-expressed TRAP1 in 40-day old flies using the inducible GeneSwitch-tub5Gal4 driver and assessed oxidative stress resistance against paraquat. GeneSwitch drivers allow temporal and spatial control because the expression of UAS effector lines is controlled by a chimeric Gal4 protein that is activated only in the presence of the steroid RU-486 which is not naturally expressed in Drosophila. Therefore, interpretation of these experiments relies on comparable ingestion of paraquat and RU-486 in wildtype, mutant and overexpression strains. To assess feeding behavior, we used a modified CAFE assay and found similar consumption of food across all groups (Supplementary Figure 3). Median survival was significantly increased in UAS-TRAP17M/ GS-tub5Gal4 males maintained on RU-486 as compared to control flies of the same genotype, sex and age maintained on the vehicle (Figure 2 F). These results suggest that a high transient dosage of TRAP1 is sufficient to improve resistance to oxidative stress in older flies. Together, these data implicate TRAP1 as an important modulator of oxidative stress resistance in young and aged Drosophila.
TRAP1 confers protection against heat stress
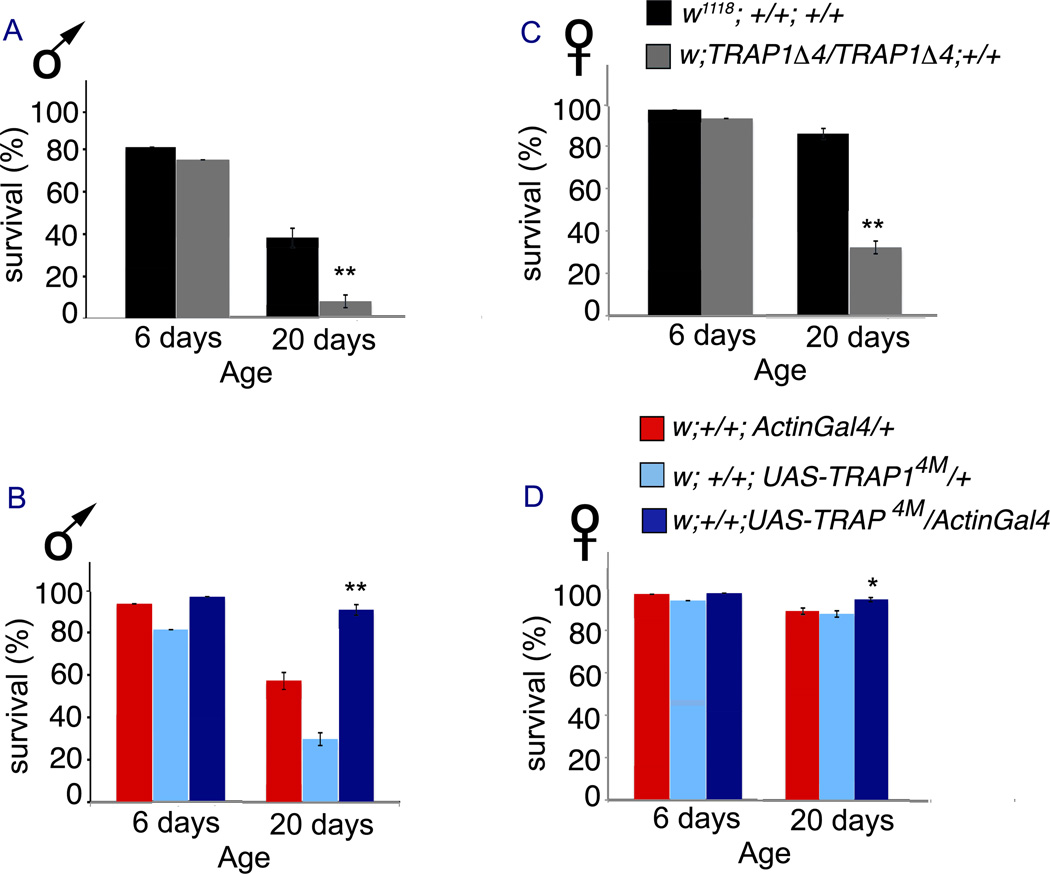
Because TRAP1 is an Hsp we subjected TRAP1 deletion and overexpression strains to acute heat stress as a second measure of stress resistance. Heat shock was applied twice, at 5 and 19 days of age, and survival was measured 24 hours later in both cases. Younger flies are largely resistant to heat shock; there is only a moderate decrease in survival of TRAP1Δ4 mutant males and females as compared to wildtype controls on the 6th day. Similarly, there is only a modest improvement in survival of UAS-TRAP14M/ActinGal4 males and females after the first heat shock (Figure 3). However, the differences are significant after the second heat shock on the 20th day. In both male and female TRAP1Δ4 mutants, survival was significantly decreased as compared to the wildtype control. In contrast, UAS-TRAP14M/ActinGal4 males and females overexpressing TRAP1 display significant improvement in survival compared to control animals (Figure 3). These results are consistent with TRAP1 being an important regulator of stress resistance.
Figure 3. TRAP1 confers resistance against heat stress.
Fly survival at 6 days and 19 days of age, 24 hrs after each heat stress is delivered. Data is presented as the percentage of flies subjected to the stress that survives. (A) On the 6th day, survival of male w; TRAP 1Δ4/TRAP 1Δ4; +/+ (75 ± 6%) trends towards being lower than w1118; +/+; +/+ (81 ± 8 %; p=0.05), but is significantly decreased (7.14 ± 0.66%) at 20 days of age relative to control (37.41 ± 0.77%; p<0.001). (B) Survival of female w; TRAP 1Δ4/TRAP 1Δ4; +/+ (93 ± 1%) is comparable to that of w1118; +/+; +/+ (97 ± 1%; p=0.1) on the 6th day, but significantly decreased at 20 days (32.05 ± 7%) compared to control (85.62 ± 3.5%; p<0.001). (C) On 6th day, survival of male w; +/+; UAS-TRAP14M/ActinGal4 (96.5 ± 1.5%) is significantly higher than w; +/+; ActinGal4/+ (81 ± 6%; p=0.01), but comparable to w; +/+; UAS-TRAP14M/ + (93.5 ± 0.5%; p=0.1). However, on the 20th day, survival of male w; +/+; UAS-TRAP14M/ActinGal4 (90.64 ± 1%) is remarkably higher than w; +/+; ActinGal4/+ (29.71 ± 2.16; p<0.0001), and w; +/+; UAS-TRAP14M/ + (57.14 ± 0.75%, p<0.001). (D) Survival of female w; +/+; UAS-TRAP14M/ActinGal4 (97.5 ± 1.5%) is not significantly different from w; +/+; ActinGal4/+ (94 ± 1%; p=0.1), and w; +/+; UAS-TRAP14M/ + (97 ± 1%; p=0.2) at 6 days. However at 20 days, survival of w; +/+; UAS-TRAP14M/ActinGal4 (94.62 ± 2%) is significantly more than w; +/+; ActinGal4/+ (87.18 ± 2.5%; p<0.001), and w; +/+; UAS-TRAP14M/ + (89.18 ± 2.5%; p=0.01). In all cases, errors denote standard errors of mean. Statistical significance was determined using one-way ANOVA.
Loss or overexpression of TRAP1 does not significantly alter lifespan
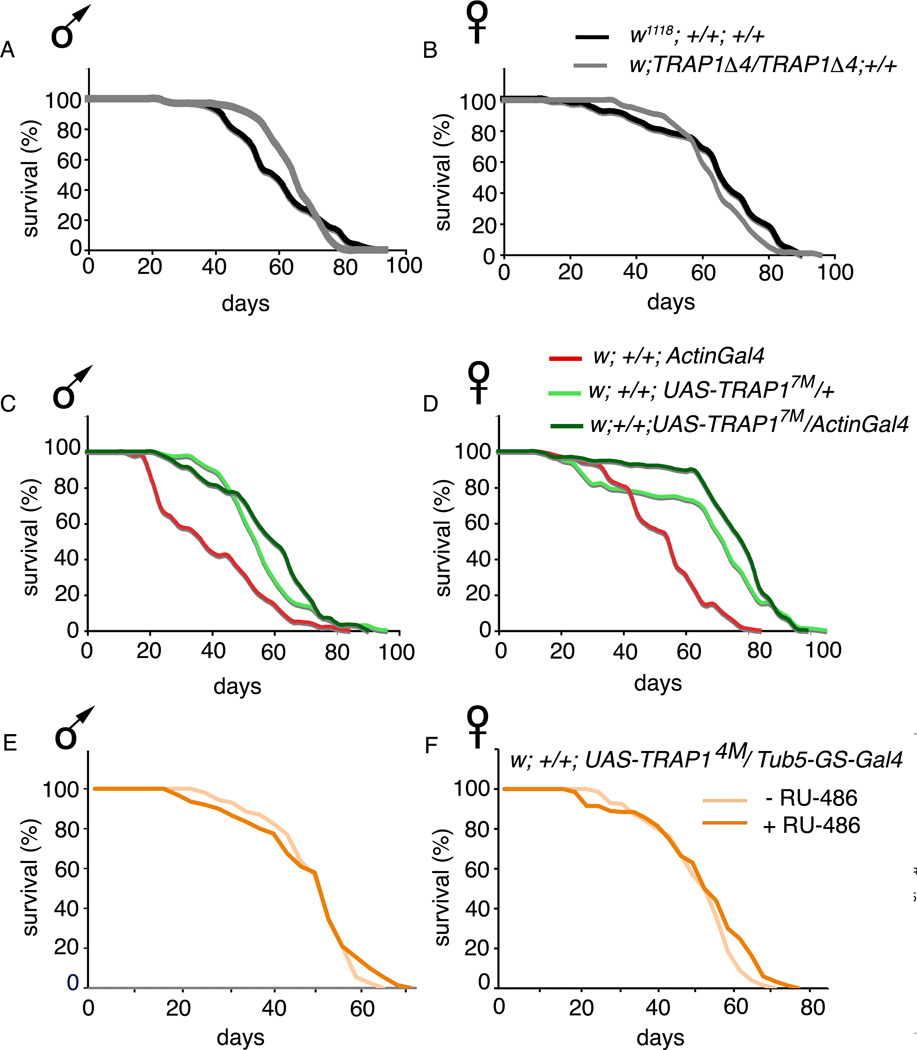
While recent reports indicate that ROS levels may not regulate longevity directly, overexpression of chaperones and enhanced resistance to oxidative stress are both associated with lifespan extension (Walker & Lithgow 2003; Chen et al. 2006; Sanz et al. 2010; Hekimi et al. 2011). It has been reported previously in multiple model systems that overexpressing small heat shock proteins (Hsps) such as Hsp16, Hsp 27 and Hsp22 leads to a sizeable extension in lifespan (Walker & Lithgow 2003; Morrow et al. 2004; Wang et al. 2004). However, overexpression of larger Hsps such as Hsp70 and Hsp60 do not affect lifespan dramatically (Tatar et al. 1997; Wadhwa et al. 2005), and in the case of induced Hsp70 expression can even be deleterious for growth in Drosophila cells (Feder et al. 1992). To examine whether TRAP1-mediated oxidative stress resistance is associated with alteration in longevity, we conducted lifespan analyses. A recent study suggested that TRAP1 mutant flies have reduced lifespan (Costa et al, 2013). However, when we separated the sexes in the lifespan analysis, we found that survival of TRAP1 mutant males and females is not significantly different from control groups (Figure 4 A and B). We did observe a marginal increase in median lifespan in females of one of the TRAP1 overexpressing strains, UAS-TRAP17M/ActinGal4 (Figure 4 D). However, this effect was relatively minor (~ 4–14% increase in median lifespan), unlike the substantial increase in oxidative stress resistance in TRAP1 overexpressing flies (~ 44% increase in median survival in males). We did not observe any increase in lifespan of males and females in a second overexpression strain, UAS-TRAP14M/ActinGal4 (data not shown). As described in the methods section, all transgenic strains and drivers used in these studies were backcrossed for at least 5 generations. However, lifespan can be significantly influenced by the slightest heterogeneity in genetic background. To control for this possibility, we overexpressed TRAP1 using the GeneSwitch-tub5Gal4 driver which increases TRAP1 mRNA levels by 5 and 10 fold in males and females respectively (Supplementary Figure 1 C and D). Average lifespan of UAS-TRAP14M/ GS-tub5Gal4 males and females maintained on RU-486 (Waskar et al. 2009) was comparable to controls maintained on vehicle only (Figure 4 E and F). Collectively, these observations suggest that knockout or overexpression of TRAP1 has little effect on lifespan.
Figure 4. TRAP1 has a marginal influence on lifespan.
Lifespan curves of indicated genotypes. (A) Median lifespan of male w1118; +/+; +/+ (60.4 ± 1.4 days) is comparable to that of w; TRAP 1Δ4/TRAP 1Δ4; +/+ (64.9 ± 1.1 days; p=0.48). (B) Median lifespan of female w1118; +/+; +/+ (64.6 ± 1.44 days) is comparable to w; TRAP 1Δ4/TRAP 1Δ4; +/+ (64.32 ± 0.98 days; p=0.08). (C) Median lifespan of male w; +/+; UAS-TRAP17M/ActinGal4 (58.6 ± 1.3 days) is significantly more than w; +/+; ActinGal4/+ (40.7 ± 1.4 days; p<0.001), and marginally more than that of w; +/+; UAS-TRAP17M/ + (56.5 ± 1.1 days; p=0.05). (D) Median lifespan of female w; +/+; UAS-TRAP17M/ActinGal4 (75.5 ± 1.4 days) is significantly more than that of w; +/+; ActinGal4/+ (53.5 ± 1.2 days; p<0.001), and marginally more than that of w; +/+; UAS-TRAP17M/ + (66.5 ± 1.9; p=0.044). (E) Median lifespan of w; +/+; UAS-TRAP14M/ GS-tub5Gal4 males maintained on RU-486 (43.66 ± 0.88 days) is similar to control flies of the same genotype, sex and age maintained on vehicle (44.86 ± 0.64 days; p = 0.15). (F) Median lifespan of w; +/+; UAS-TRAP14M/ GS-tub5Gal4 females on RU-486 (47.24 ± 0.97 days) is also comparable to controls (44.84 ± 0.81 days; p=0.04). In all cases, errors denote standard deviation. Statistical significance was determined using the standard chi-squared based log-rank test.
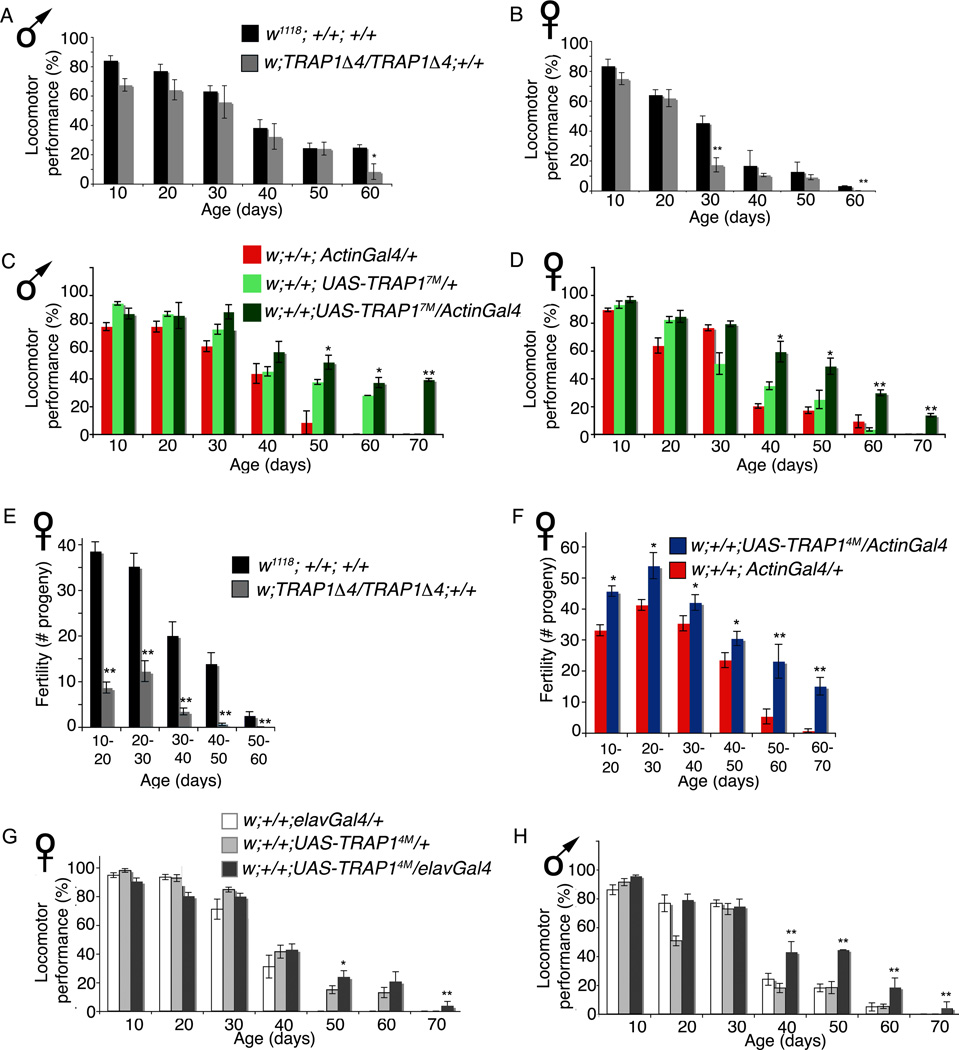
TRAP1 regulates locomotor performance and fertility in aging Drosophila
Recent studies on LON protease in the fungal aging model P. anserina have uncovered a role for mitochondrial proteostasis in healthspan regulation (Luce & Osiewacz 2009). Because TRAP1 is a mitochondrial chaperone, we sought to determine whether TRAP1 would regulate healthspan. In Drosophila, negative geotaxis (the ability of flies to climb vertically when startled) is an established paradigm to assess age-related locomotor impairment (Rhodenizer et al. 2008). In a longitudinal study, we found that locomotor performance in TRAP1 mutant flies was only moderately decreased at older age (Figure 5 A and B). In contrast, the effect on male and female TRAP1 overexpressing flies (UAS-TRAP17M/ActinGal4 and UAS-TRAP14M/ActinGal4) was much clearer and they climb significantly better than the driver alone and transgene alone controls at 60 and 70 days of age (Figure 5 C and D, Supplementary Figure 4 A and B). As an independent indicator of healthspan, we determined the fertility of TRAP1 mutant and overexpression flies. TRAP1 mutant females show severely compromised fecundity at all age-points (Figure 5 E). In contrast, overexpression of TRAP1 results in increased fecundity (Figure 5 F). In addition, TRAP1 mutant males appear to be less fertile than control males (Supplementary Figure 4 C), whereas males overexpressing TRAP1 produced significantly more progeny at older ages than control strains (Supplementary Figure 4 D). Together, these results indicate strongly that TRAP1 promotes healthspan in both male and female Drosophila.
Figure 5. TRAP1 modulates healthspan.
(A and B) TRAP1 mutant flies tend to climb more poorly than controls in the negative geotaxis assay and the difference becomes significant at older ages. There is a significant drop in performance of mutant females at 30 days. By 60 days, fewer male and female w; TRAP1A4/TRAP1A4; +/+ flies climbed successfully as compared to w1118; +/+; +/+(C and D) Locomotor performance after 40 days of age is well maintained in w; +/+; UAS-TRAP17M/ActinGal4 males and females on ubiquitous overexpression of TRAP1. (E)w; TRAP1Δ4/TRAP1Δ4; +/+ females produce fewer progeny than controls at all stages of their life. (F)w; +/+; UAS-TRAP17M/ActinGal4 females produce more progeny than controls at all stages of their life. (G and H) Male and female flies overexpressing TRAP1 exclusively in the nervous system displayed significant improvement in locomotor ability at older age-points in w; +/+; UAS-TRAP14M/ elavGal4. In all cases error bars denote standard error of means; (*) indicates p < 0.05, (**) indicates p < 0.001. Statistical significance was determined by one-way ANOVA with Dunnett’s post hoc comparison. (See also Figure S 5)
Pan-neuronal overexpression of TRAP1 attenuates age-related decline in locomotor performance but does not affect lifespan or oxidative stress resistance
Modifying mitochondrial physiology in one tissue may influence function in others through cell non-autonomous stress response pathways (Durieux et al. 2011). In addition, Butler and colleagues showed that overexpression of human TRAP1 in cultured rat cortical neurons increased resistance to rotenone induced oxidative stress (Butler et al. 2012). To test whether overexpressing TRAP1 exclusively in the nervous system would be beneficial, we used the pan-neuronal driver elavGal4 for tissue specific overexpression. In contrast to the in vitro result, pan-neuronal overexpression of TRAP1 in adult Drosophila does not significantly enhance oxidative stress resistance to paraquat (Supplementary Figure 5 A and B), nor does it appreciably change lifespan (Supplementary Figure 5 C and D). Nonetheless, overexpression of TRAP1 in the fly nervous system was sufficient to improve locomotor performance of both sexes at older age-points in w; +/+; UAS-TRAP14M/elavGal4 strain (Figure 5 G and H), although less significantly in the females. Given previous work implicating TRAP1 in Parkinson’s disease via its interaction with PINK1 (Pridgeon et al. 2007; Costa et al. 2013; Zhang et al. 2013) and α-synuclein (Butler et al. 2012), these data indicate that augmenting TRAP1 expression in the nervous tissue may be beneficial in human neurological diseases that impair motor behavior.
Dosage modulation of TRAP1 activates the mitochondrial unfolded protein response
The mitochondrial unfolded protein response (UPRmt) is a protective response pathway between mitochondria and the nucleus that is initiated in response to a mitochondrial stress signal (Haynes & Ron 2010). Induction of the UPRmt promotes expression of chaperones and proteases that enhance mitochondrial proteostasis in mammalian cell culture and C. elegans (Zhao et al. 2002; Yoneda et al. 2004). Because TRAP1 is a component of the mitochondrial proteostasis machinery by virtue of being a chaperone, we hypothesized that it is involved in the regulation of the UPRmt.
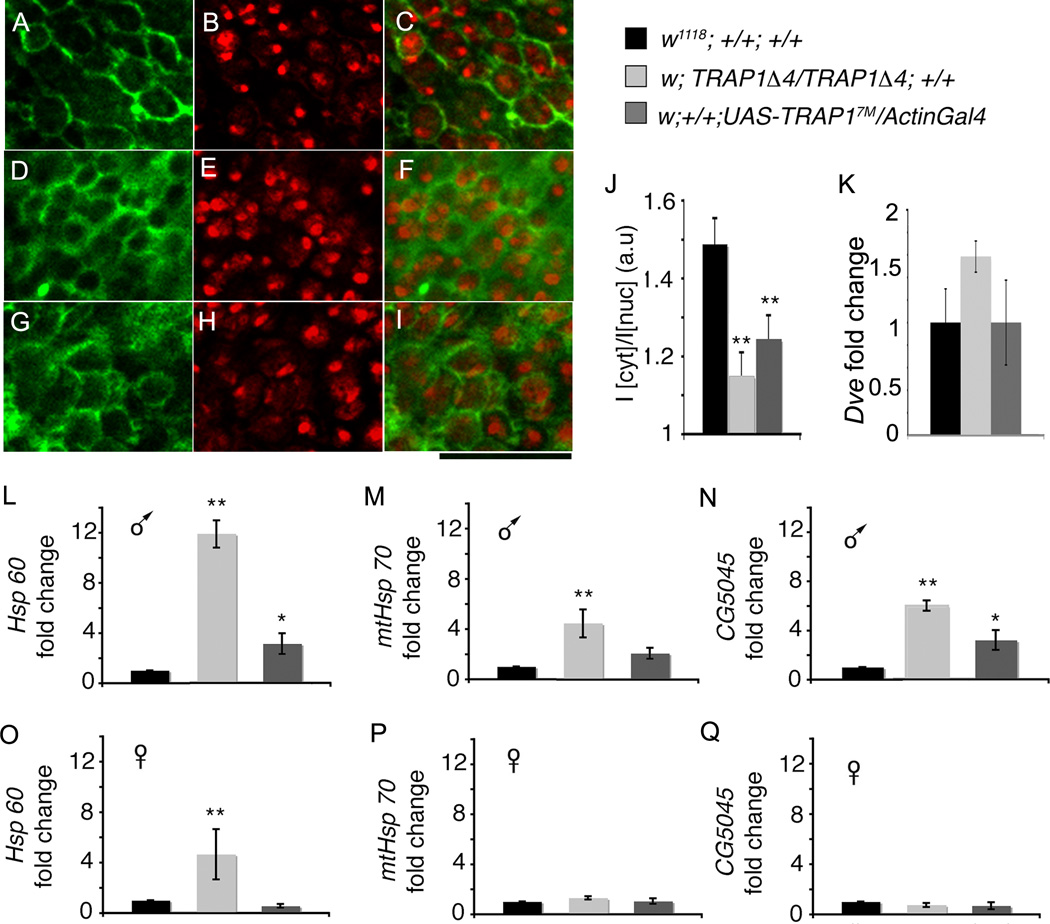
As part of the UPRmt in worms, transcription factors such as DVE-1 translocate from the cytoplasm to the nucleus to promote downstream transcription of chaperones and proteases (Haynes & Ron 2010). Immunohistochemical analysis of adult brains with antibody against Dve, the Drosophila homolog of DVE-1, reveals a ring like cytosolic localization pattern around the nucleus of neuronal cell bodies of wildtype flies (Figure 6 A , B and C). In contrast, Dve in TRAP1 mutants displayed a more diffuse staining pattern indicating nuclear translocation (Figure 6 D , E and F). We also note a similar change in sub-cellular localization of Dve in the TRAP1 overexpressing flies (Figure 6 G , H and I). These observations were verified by quantifying the ratio of cytosolic to nuclear intensity of DVE staining which corroborates a change in sub-cellular localization of DVE (Figure 6 J). Importantly, comparison of Dve mRNA levels between the strains by qPCR reveals that these changes in staining pattern are not due to an overall reduction in Dve transcription in TRAP1 mutants (Figure 6 K). These results demonstrate that dosage modulation of TRAP1 influences nuclear translocation of DVE in Drosophila, suggesting an induction of UPRmt.
Figure 6. Loss and overexpression of TRAP1 activates the UPRmt pathway.
(A – C) Anti-Dve antibody (green) reveals tight cytosolic localization of Dve in neuronal cell bodies in brains of adult w1118; +/+; +/+ males. Nuclear dye DAPI (red) stains the entire nucleus although it appears brighter in the nucleolus. The merged image of anti-Dve and DAPI stain reveals crisp cytosolic sub-cellular localization of Dve. (D – F) Dve staining appears diffuse in neuronal cell bodies of male w; TRAP1A4/TRAP1A4, +/+ flies and exhibits considerable overlap with the nucleus. (G – I) Dve staining appears similarly diffused and overlaps with nuclear stain in neuronal cell bodies of male w; +/+; UAS-TRAP14M/ActinGal4 flies. Scale bar equals 3.5 µm. (J) Ratio of staining intensity of Dve in the cytoplasm relative to the nucleus in indicated genotypes. (K) mRNA expression of Dve in w1118; +/+; +/+ females is not significantly different from mutant (p=0.2) and overexpression (p=0.9) strains. (L, M and N) Male w; TRAP1Δ4/TRAP1Δ4, +/+ and w; +/+; UAS-TRAP17M/ActinGal4 flies exhibit significantly increased mRNA expression of Hsp60, mtHsp70 and CG5045 as compared to w1118; +/+; +/+. (O, P and Q) Female w; TRAP1A4/TRAP1A4; +/+ exhibit significantly increased mRNA expression of Hsp60 as compared to w1118; +/+; +/+, but not that of mtHsp70 and CG5045. In all cases, data are presented as fold change from control. Error bars denote standard error of means; statistical significance was determined using one-way ANOVA. (*) indicates p < 0.05, (**) indicates p < 0.001.
To further test if TRAP1 regulates the UPRmt, we examined expression levels of genes known to be associated with the UPRmt, including Hsp60, mtHsp70 and a putative Drosophila protease CG5045 that is 77% identical to the worm ClpP (Flybase). In males, loss of TRAP1 led to strong upregulation of Hsp60, mtHsp70 and CG5045 (Figure 6 L, M, and N). As with resistance to oxidative stress, this response is sex-specific: in females only Hsp60 was increased significantly (Figure 6 O , P and Q). These results suggest that depletion of TRAP1 leads to induction of a robust UPRmt. In the TRAP1 overexpression strains we found expression of the UPRmt genes were modestly elevated in the overexpression strain in a similar sex dependent transcription profile as mutants but the magnitude increase was smaller in comparison (Figure 6 L – Q). These results suggest that overexpression of TRAP1 also induces an UPRmt response in males, albeit weaker than that in TRAP1 depletion.
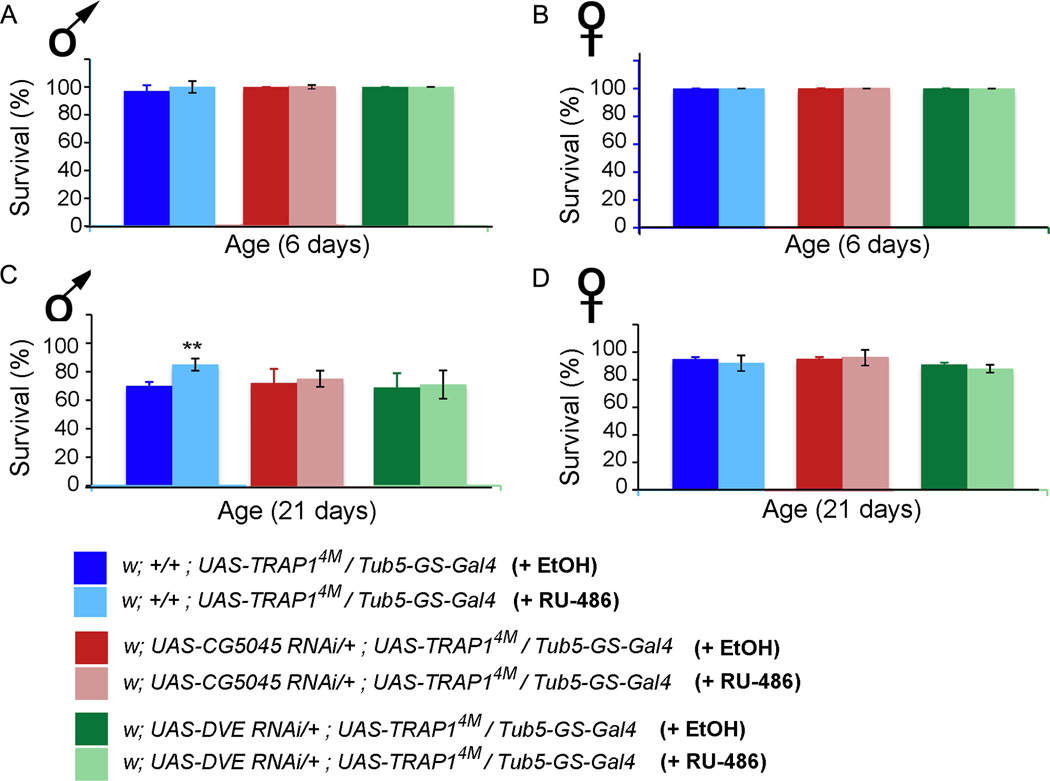
This result led us to ask if the increased resistance to stressors in TRAP1 overexpression animals is due to induction of the UPRmt. To address this, we sought to impair UPRmt induction in flies overexpressing TRAP1 and test their response to acute heat stress. We used the inducible GeneSwitch-tub5Gal4 driver to drive RNAi against transcription factor Dve and the protease CG5045 independently in UAS-TRAP14M background. As with constitutive overexpression of TRAP1 (Figure 3), conditional overexpression also significantly improves resistance to acute heat stress in 20-day old males but there is no significant difference in 5-day old flies as younger flies handle heat stress better (Figure 7). However, when Dve or CG5045 expressions were knocked down while simultaneously overexpressing TRAP1, the improvement in stress resistance was considerably dampened (Figure 7). Together, these data strongly suggest that the protective influence of TRAP1 overexpression is at least in part due to activation of the UPRmt pathway.
Figure 7. Activation of the UPRmt is required for TRAP1 mediated heat stress resistance in older males.
Fly survival at 6 days and 21 days of age, 24 hrs after each heat stress is delivered. (A) In 6-day old male flies with conditional Gal4, survival of w; +/+; UAS TRAP14M/ GS-Tub5Gal4 +/+ in control group (97 ± 4.2%) is comparable to identical flies maintained on RU-486 (99 +/− 1.4%; p=0.6), w; UAS CG5045 RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (100%) are the same as identical flies maintained on RU-486 (100%), and w; UAS DVE RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (100%) are the same as identical flies maintained on RU-486 (100%). (B) In 6-day old female flies with conditional Gal4, survival of w; +/+; UAS TRAP14M/ GS-Tub5Gal4 +/+ in control group (100%) is the same as identical flies maintained on RU-486 (100%), w; UAS CG5045 RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (100%) is the same as identical flies maintained on RU-486 (100%), and w; UAS DVE RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (100%) are the same as identical flies maintained on RU-486 (100%). (C) In 21-day old male flies with conditional Gal4, survival of w; +/+; UAS TRAP14M/ GS-Tub5Gal4 +/+ of flies maintained on RU-486 (85 +/− 4.2%) is significantly higher than controls (70 ± 2.8%; p = 0.05). However, w; UAS CG5045 RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (73 +/− 9.8%) is comparable as identical flies maintained on RU-486 (76 +/− 5.6%; p = 0.7), and w; UAS DVE RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (69 +/−1.4%) are similar to identical flies maintained on RU-486 (71 +/− 2.8%; p = 0.8). (D) In 21-day old female flies with conditional Gal4, survival of w; +/+; UAS TRAP14M/ GS-Tub5Gal4 +/+ in control group (95 +/− 1.4%) is comparable to identical flies maintained on RU-486 (92 +/− 5.6%; p = 0.5), w; UAS CG5045 RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (95 +/− 1.5%) is similar to identical flies maintained on RU-486 (96 +/− 5.6%; p = 0.8), and w; UAS DVE RNAi/+; UAS TRAP14M/ GS-Tub5Gal4 in control group (91 +/−1.4%) are the comparable to identical flies maintained on RU-486 (88 +/− 2.8%; p = 0.3). In all cases, errors denote standard deviation. Statistical significance was determined using one-way ANOVA.
Discussion
The molecular mechanisms influencing healthspan remain unclear (Tatar 2009; Yu & Driscoll 2011), but mitochondrial proteostasis is evolving as a central determinant. We find that dosage modulation of the mitochondrial chaperone TRAP1 in Drosophila regulates oxidative stress and heat stress resistance in males and modulates healthspan of both sexes, yet only has a negligible effect on lifespan. To gain a better understanding of the mechanism underlying these changes, we examined the effect of TRAP1 dosage on the UPRmt. We found that modulation of TRAP1 expression induces the nuclear translocation of transcription factor Dve and drives expression of Hsp60, mtHsp70 and a putative protease, CG5045. These results provide evidence for the presence of the UPRmt in Drosophila, confirming the conservation of this stress response pathway across phylogeny. Furthermore, dampening of TRAP1 mediated stress response upon impairment of the UPRmt indicates that alterations in mitochondrial proteostasis can influence stress resistance and healthspan,
Recently published studies have also indicated a similar role of TRAP1 in regulating stress resistance in flies (Costa et al, 2013, Zhang et al, 2013). However, we further demonstrate that dosage modulation of TRAP1 has remarkable sex-specific influences and is correlated with the sex-specific activation the UPRmt. Sex specificity has been reported previously in stress survival studies involving mitochondrial proteins (Magwere et al. 2006; Mourikis et al. 2006). One possible explanation is that mitochondria in female Drosophila have dissimilar bioenergetics from males, with higher oxygen consumption, higher hydrogen peroxide production and lower levels of catalase, along with higher mtDNA copy number (Yin et al. 2004; Ballard et al. 2007). In our oxidative stress and heat stress survival assays (Figure 2 and Figure 3), TRAP1 mutant and overexpression animals displayed considerable sex-specificity that correlated directly with the expression of mtHsp70 and CG5045, but not Hsp60 in males and females (Figure 6). These findings suggest that sex-specific differences in activation of proteostasis pathways underlie sex dependent differences in resistance to stress.
Our finding that both loss and overexpression of TRAP1 activate the UPRmt is indicative of the complex regulation of this response system. Because TRAP1 is a chaperone, induction of the UPRmt in TRAP1 mutants is likely a compensatory response to protect against excess unfolded proteins. While this response pathway may allow the TRAP1 mutants to have a near normal lifespan, the absence of TRAP1 function has obvious negative consequences on fitness. In contrast, the induction of the UPRmt in TRAP1 overexpressing flies could occur by two non-exclusive mechanisms. One possibility is that the mild stress of over-abundant TRAP1 in the mitochondrial matrix induces the UPRmt. Alternatively, given the potential role of TRAP1 in multiple signaling pathways involving Rb, myc, TNF, cyclophilin D (Kang et al. 2007; Altieri et al. 2012) overexpression of TRAP1 may lead to efficient protein folding, direct suppression of apoptosis, and the coordinated induction of other UPR genes through a TRAP1 mediated stress response. In the context of a minimal proteostasis boundary (Powers et al. 2009), either an increase in TRAP1 activity or the indirect induction of UPRmt would result in enhanced protein quality control in TRAP1 overexpression flies and would shift the proteostasis boundary towards health. Altogether, our results suggest that because of the critical roles played by TRAP1 in regulating several cellular processes, it is important that TRAP1 expression itself is finely regulated.
The role of TRAP1 in activation of the UPRmt has broad clinical relevance. TRAP1 is reportedly expressed at higher levels in cancer cells where it has been suggested to play a pro-survival role by inhibiting apoptosis (Kang et al. 2007; Costantino et al. 2009; Leav et al. 2010). Hsp90 molecules, including TRAP1, antagonize the cyclophilin D-dependent mitochondrial permeability transition, and this cytoprotective pathway is a potential target in cancer therapy (Kang et al. 2007). Our results demonstrate that complete loss of function of TRAP1 has little effect on organismal lifespan (Figure 4) and there is no discernible delay in development (data not shown). While caution must be used when speculating how results in model organisms translate to therapeutics in humans, these results suggest that aggressive disruption of TRAP1 in the context of cancer treatment may provide a means to sensitize tumors to chemotherapy without significantly reducing the lifespan of patients.
In addition, we show that overexpression of TRAP1, ubiquitously or in the nervous system, extends healthspan in both sexes. These results suggest that augmentation of TRAP1 expression may help offset normal age-related decline of physiological and motor capacity, as well as the pathological decline associated with neurological disorders.
Supplementary Material
(A and B) TRAP1 transcript level is almost completely eliminated in male and female w; TRAP1Δ4/TRAP1Δ4; +/+ mutants normalized to level in w1118; +/+; +/+ wildtype controls. In the w; +/+; UAS-TRAP14M/ActinGal4 overexpression strain, TRAP1 mRNA is increased almost 14-fold in males and over 17-fold in females. (C and D) Administration of the drug RU-486 results in conditional increase of TRAP1 mRNA in w; +/+; UAS-TRAP17M/ GS-tub5Gal4 to more than 10-fold in males and females, normalized to level in w; +/+; UAS-TRAP17M/ GS-tub5Gal4 flies that were exposed to the vehicle ethanol, but not the drug. Statistical significance was measured by Student’s t-test.
(A) Median survival on 20 mM paraquat of male 5-days old w; +/+; UAS-TRAP14M/ActinGal4 (61.62 ± 3.92 hrs) is significantly more than median survival of w; +/+; ActinGal4/+ males (36.18 ± 2.76 hrs; p<0.0001), and w; +/+; UAS-TRAP14M/+ (47.76 ± 3.1 hrs; p=0.016). (B) Median survival of female w; +/+; UAS-TRAP14M/ ActinGal4 (47.8 ± 2.7 hrs) is significantly more than w; +/+; ActinGal4/+ females (32.4 ± 2.2 hrs; p<0.0001), but comparable to median survival of w; +/+; UAS-TRAP14M/+ females (41.3 ± 3.0 hrs; p=0.24). (C and D) Oxidative stress resistance declines with age in both sexes. Young and old w1118; +/+; +/+ were subjected to identical treatment with 20 mM paraquat. (C) Median survival of young males (28.4 ± 1.6 hrs) is significantly higher than old males (13.8 ± 0.6 hrs; p<0.001). (D) Median survival of young females (22.4 hrs ± 1.4) is significantly higher than old females (18.1 ± 0.6; p=0.018). Statistical significance was determined using the standard chi-squared based log-rank test.
Feeding behavior was assessed in a CAFE assay in 5-day old Drosophila. (A) 5 male flies of a genotype are housed in each vial with two calibrated glass micropipettes filled with 5 µl of dyed 5% sucrose. (B) A blank vial without flies with similarly filled micropipettes is used to control for evaporation. (C) Representative image of a fly feeding from a micropipette. (D) There is no significant difference in consumption of liquid food in any of the strains tested. Statistical significance was determined by one-way ANOVA.
(A and B) Locomotor performance in the negative geotaxis assay of males and females of w; +/+; UAS-TRAP14M/ActinGal4 strains is improved in older flies as compared to transgene alone and driver alone controls of the same age. (C) Loss of TRAP1 has a marginal effect on male fertility. w; TRAP1Δ4/TRAP1Δ4; +/+ males exhibit a significant reduction in progeny at 40-days of age. (D) Overexpression of TRAP1 in w; +/+; UAS-TRAP17M/ActinGal4 results in males producing significantly more offspring as compared to controls at older age. Error bars indicate standard error of means; statistical significance was determined using one-way ANOVA followed by Dunnett’s post hoc comparison. (*) indicate p<0.05, (**) indicate p<0.001.
(A) Median survival of male w; +/+; UAS-TRAP17M/ elavGal4 (52.7 ± 3.0 hrs) is comparable to w; +/+; UAS-TRAP17M/+ (43.9 ± 3.2 hrs; p=0.2). (B) Median survival of female w; +/+; UAS-TRAP17M/ elavGal4 (49 ± 3.6 hrs) is higher than w; +/+; UAS-TRAP17M/+ (36.9 ± 2.7 hrs; p=0.01), but comparable to w; +/+; elavGal4/ + (47.6 ± 2.6 hrs; p=0.5). (C) Median lifespan of male w; +/+; UAS-TRAP17M/elavGal4 (50.8 ± 1.3 days) is significantly more than w; +/+; elavGal4/+ (56.9 ± 1.1; p=0.009), but significantly less than w; +/+; UAS-TRAP17M/+ (56.5 ± 1.1 days; p=0.02). (D) Median lifespan of female w; +/+; UAS-TRAP17M/elavGal4 (70.7 ± 2.1 days) is more than w; +/+; UAS-TRAP17M/+ (66.5 ± 1.9 days; p=0.0018), but comparable to w; +/+; elavGal4/+ (76.7 ± 1.4 days; p=0.82). Statistical significance was determined using the standard chi-squared based log-rank test.
Highlights.
We investigate the role of mitochondrial proteostasis in regulating healthspan
Mitochondrial chaperone TRAP1 promotes stress resistance, locomotion and fertility
TRAP1 activates the mitochondrial unfolded protein response (UPR)
TRAP1 mediated improvement in healthspan acts through the mitochondrial UPR
Acknowledgements
We thank Drs. Jessica Treisman and Scott Pletcher for sharing fly strains and Dr. Huiyuan Tang for help with RT-PCR. We thank Sam Lee and Andrew George for help with stock maintenance and behavioral assays. This work was supported by start-up funds from the Department of Zoology and MSU HBRI-II grant 91–4511 to K.E.M, a training grant to the MSU Neuroscience Program that supported R.M.B, NIH grant 45295 to L.S.K, and NSF grant IOS-0845847 to A.W.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
R.M.B and K.E.M designed the study; R.M.B, A.V.P, R.H.G collected data; R.M.B and A.W.S analyzed data; B.A.T and S.K contributed reagents; R.M.B, L.S.K, A.W.S, and K.E.M wrote the manuscript.
The authors declare no conflicts of interest.
References
- Altieri DC, Stein GS, Lian JB, Languino LR. TRAP-1, the mitochondrial Hsp90. Biochim Biophys Acta. 2012;1823:767–773. doi: 10.1016/j.bbamcr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanesian A, Khodayari B, Felgner JS, Jafari M. Lamotrigine extends lifespan but compromises health span in Drosophila melanogaster. Biogerontology. 2010;11:45–52. doi: 10.1007/s10522-009-9227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JW, Melvin RG, Miller JT, Katewa SD. Sex differences in survival and mitochondrial bioenergetics during aging in Drosophila. Aging Cell. 2007;6:699–708. doi: 10.1111/j.1474-9726.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- Baqri RM, Turner BA, Rheuben MB, Hammond BD, Kaguni LS, Miller KE. Disruption of mitochondrial DNA replication in Drosophila increases mitochondrial fast axonal transport in vivo. PLoS One. 2009;4:e7874. doi: 10.1371/journal.pone.0007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Butler EK, Voigt A, Lutz AK, Toegel JP, Gerhardt E, Karsten P, Falkenburger B, Reinartz A, Winklhofer KF, Schulz JB. The Mitochondrial Chaperone Protein TRAP1 Mitigates alpha-Synuclein Toxicity. PLoS Genet. 2012;8:e1002488. doi: 10.1371/journal.pgen.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- Copeland JM, Cho J, Lo T, Jr, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Costa AC, Loh SH, Martins LM. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson's disease. Cell Death Dis. 2013;4:e467. doi: 10.1038/cddis.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F, Landriscina M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J Cell Sci. 2010;123:3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Lapointe J, Wen Y. Taking a "good" look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- Im CN, Lee JS, Zheng Y, Seo JS. Iron chelation study in a normal human hepatocyte cell line suggests that tumor necrosis factors receptor associated protein 1 (TRAP1) regulates production of reactive oxygen species. J Cell Biochem. 2007;100:474–486. doi: 10.1002/jcb.21064. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFÉ assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leav I, Plescia J, Goel HL, Li J, Jiang Z, Cohen RJ, Languino LR, Altieri DC. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am J Pathol. 2010;176:393–401. doi: 10.2353/ajpath.2010.090521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce K, Osiewacz HD. Increasing organismal healthspan by enhancing mitochondrial protein quality control. Nat Cell Biol. 2009;11:852–858. doi: 10.1038/ncb1893. [DOI] [PubMed] [Google Scholar]
- Magwere T, West M, Riyahi K, Murphy MP, Smith RA, Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127:356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. Faseb J. 2004;18:598–599. doi: 10.1096/fj.03-0860fje. [DOI] [PubMed] [Google Scholar]
- Mourikis P, Hurlbut GD, Artavanis-Tsakonas S. Enigma, a mitochondrial protein affecting lifespan and oxidative stress response in Drosophila. Proc Natl Acad Sci U S A. 2006;103:1307–1312. doi: 10.1073/pnas.0510564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagoshi H, Hoshi M, Nabeshima Y, Matsuzaki F. A novel homeobox gene mediates the Dpp signal to establish functional specificity within target cells. Genes Dev. 1998;12:2724–2734. doi: 10.1101/gad.12.17.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol. 2008;43:739–748. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Kaarniranta K. Phytochemicals suppress nuclear factor-kappaB signaling: impact on health span and the aging process. Curr Opin Clin Nutr Metab Care. 2012;15:23–28. doi: 10.1097/MCO.0b013e32834d3ae7. [DOI] [PubMed] [Google Scholar]
- Sanz A, Fernandez-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging (Albany NY) 2010;2:200–223. doi: 10.18632/aging.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Selman C, Withers DJ. Mammalian models of extended healthy lifespan. Philos Trans R Soc Lond B Biol Sci. 2011;366:99–107. doi: 10.1098/rstb.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Can we develop genetically tractable models to assess healthspan (rather than life span) in animal models? J Gerontol A Biol Sci Med Sci. 2009;64:161–163. doi: 10.1093/gerona/gln067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390:30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, Kaur K, Aida S, Yaguchi T, Kaul Z, Hirano T, Taira K, Kaul SC. Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60. Biochem J. 2005;391:185–190. doi: 10.1042/BJ20050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskar M, Landis GN, Shen J, Curtis C, Tozer K, Abdueva D, Skvortsov D, Tavare S, Tower J. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany NY) 2009;1:903–936. doi: 10.18632/aging.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S, Dillin A. The trifecta of aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:894–903. doi: 10.1016/j.exger.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Yin PH, Lee HC, Chau GY, Wu YT, Li SH, Lui WY, Wei YH, Liu TY, Chi CW. Alteration of the copy number and deletion of mitochondrial DNA in human hepatocellular carcinoma. Br J Cancer. 2004;90:2390–2396. doi: 10.1038/sj.bjc.6601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117:4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Tsutsumi S, Muhlebach G, Sourbier C, Lee MJ, Lee S, Vartholomaiou E, Tatokoro M, Beebe K, Miyajima N, Mohney RP, Chen Y, Hasumi H, Xu W, Fukushima H, Nakamura K, Koga F, Kihara K, Trepel J, Picard D, Neckers L. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc Natl Acad Sci USA. 2013;110:1604–1612. doi: 10.1073/pnas.1220659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Driscoll M. EGF signaling comes of age: promotion of healthy aging in C. elegans. Exp Gerontol. 2011;46:129–134. doi: 10.1016/j.exger.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Karsten P, Hamm S, Pogson JH, Muller-Rischart AK, Exner N, Haass C, Whitworth AJ, Winklhofer KF, Schulz JB, Voigt A. TRAP1 rescues PINK1 loss-of-function phenotypes. Hum Mol Genet. 2013;22:2829–2841. doi: 10.1093/hmg/ddt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. Embo J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A and B) TRAP1 transcript level is almost completely eliminated in male and female w; TRAP1Δ4/TRAP1Δ4; +/+ mutants normalized to level in w1118; +/+; +/+ wildtype controls. In the w; +/+; UAS-TRAP14M/ActinGal4 overexpression strain, TRAP1 mRNA is increased almost 14-fold in males and over 17-fold in females. (C and D) Administration of the drug RU-486 results in conditional increase of TRAP1 mRNA in w; +/+; UAS-TRAP17M/ GS-tub5Gal4 to more than 10-fold in males and females, normalized to level in w; +/+; UAS-TRAP17M/ GS-tub5Gal4 flies that were exposed to the vehicle ethanol, but not the drug. Statistical significance was measured by Student’s t-test.
(A) Median survival on 20 mM paraquat of male 5-days old w; +/+; UAS-TRAP14M/ActinGal4 (61.62 ± 3.92 hrs) is significantly more than median survival of w; +/+; ActinGal4/+ males (36.18 ± 2.76 hrs; p<0.0001), and w; +/+; UAS-TRAP14M/+ (47.76 ± 3.1 hrs; p=0.016). (B) Median survival of female w; +/+; UAS-TRAP14M/ ActinGal4 (47.8 ± 2.7 hrs) is significantly more than w; +/+; ActinGal4/+ females (32.4 ± 2.2 hrs; p<0.0001), but comparable to median survival of w; +/+; UAS-TRAP14M/+ females (41.3 ± 3.0 hrs; p=0.24). (C and D) Oxidative stress resistance declines with age in both sexes. Young and old w1118; +/+; +/+ were subjected to identical treatment with 20 mM paraquat. (C) Median survival of young males (28.4 ± 1.6 hrs) is significantly higher than old males (13.8 ± 0.6 hrs; p<0.001). (D) Median survival of young females (22.4 hrs ± 1.4) is significantly higher than old females (18.1 ± 0.6; p=0.018). Statistical significance was determined using the standard chi-squared based log-rank test.
Feeding behavior was assessed in a CAFE assay in 5-day old Drosophila. (A) 5 male flies of a genotype are housed in each vial with two calibrated glass micropipettes filled with 5 µl of dyed 5% sucrose. (B) A blank vial without flies with similarly filled micropipettes is used to control for evaporation. (C) Representative image of a fly feeding from a micropipette. (D) There is no significant difference in consumption of liquid food in any of the strains tested. Statistical significance was determined by one-way ANOVA.
(A and B) Locomotor performance in the negative geotaxis assay of males and females of w; +/+; UAS-TRAP14M/ActinGal4 strains is improved in older flies as compared to transgene alone and driver alone controls of the same age. (C) Loss of TRAP1 has a marginal effect on male fertility. w; TRAP1Δ4/TRAP1Δ4; +/+ males exhibit a significant reduction in progeny at 40-days of age. (D) Overexpression of TRAP1 in w; +/+; UAS-TRAP17M/ActinGal4 results in males producing significantly more offspring as compared to controls at older age. Error bars indicate standard error of means; statistical significance was determined using one-way ANOVA followed by Dunnett’s post hoc comparison. (*) indicate p<0.05, (**) indicate p<0.001.
(A) Median survival of male w; +/+; UAS-TRAP17M/ elavGal4 (52.7 ± 3.0 hrs) is comparable to w; +/+; UAS-TRAP17M/+ (43.9 ± 3.2 hrs; p=0.2). (B) Median survival of female w; +/+; UAS-TRAP17M/ elavGal4 (49 ± 3.6 hrs) is higher than w; +/+; UAS-TRAP17M/+ (36.9 ± 2.7 hrs; p=0.01), but comparable to w; +/+; elavGal4/ + (47.6 ± 2.6 hrs; p=0.5). (C) Median lifespan of male w; +/+; UAS-TRAP17M/elavGal4 (50.8 ± 1.3 days) is significantly more than w; +/+; elavGal4/+ (56.9 ± 1.1; p=0.009), but significantly less than w; +/+; UAS-TRAP17M/+ (56.5 ± 1.1 days; p=0.02). (D) Median lifespan of female w; +/+; UAS-TRAP17M/elavGal4 (70.7 ± 2.1 days) is more than w; +/+; UAS-TRAP17M/+ (66.5 ± 1.9 days; p=0.0018), but comparable to w; +/+; elavGal4/+ (76.7 ± 1.4 days; p=0.82). Statistical significance was determined using the standard chi-squared based log-rank test.