Abstract
Introduction
Ovarian and uterine carcinosarcoma (CS) are characterized by their aggressive clinical behavior and poor prognosis. We evaluated the efficacy of trastuzumab-emtansine (T-DM1), against primary HER2 positive and HER2 negative CS cell lines in vitro and in vivo.
Methods
Eight primary CS cell lines were evaluated for HER2 amplification and protein expression by FISH, immunohistochemistry, flow cytometry and qRT-PCR. Sensitivity to T-DM1-induced antibody-dependent-cell-mediated-cytotoxicity (ADCC) was evaluated in 4-hr-chromium-release-assays. T-DM1 cytostatic and apoptotic activities were evaluated using flow cytometry based proliferation assays. In vivo activity of T-DM1 was also evaluated.
Results
HER2 protein overexpression and gene amplification were detected in 25% (2/8) of the primary CS cell lines. T-DM1 and T were similarly effective in inducing strong ADCC against CS overexpressing HER2 at 3+ levels. In contrast, T-DM1 was dramatically more effective than T in inhibiting cell proliferation (P<0.0001) and in inducing G2/M phase cell cycle arrest in the HER2 expressing cell lines (shift of G2/M: mean ± SEM from 14.87 ± 1.23% to 66.57 ± 4.56%, P<0.0001). Importantly, T-DM1 was highly active at reducing tumor formation in vivo in CS xenografts overexpressing HER2 (P=0.0001 and P<0.0001 compared to T and vehicle respectively) with a significantly longer survival when compared to T and vehicle mice (P=0.008 and P=0.0001 respectively).
Conclusion
T-DM1 may represent a novel treatment option for the subset of HER2 positive CS patients with disease refractory to chemotherapy.
Keywords: T-DM1, Carcinosarcoma, Trastuzumab, HER2, trastuzumab emtansine
Introduction
Carcinosarcoma (CS), also known as Malignant Mixed Müllerian Tumors (MMMT) comprises one of the most rare and challenging tumors among all gynecological malignancies. CS are composed of a mixture of carcinomatous (malignant epithelial) and sarcomatous (mesenchymal) elements. The epithelial component is usually serous, but may also be endometrioid, clear cell, squamous cell or undifferentiated carcinoma. The sarcomatous component can be homologous (tissue native to the primary site) or heterologous (tissue not native to the primary site) [1]. These tumors can arise in any organ of the female genital tract such as endometrium, ovary, cervix, vulva, vagina or fallopian tube. The endometrium is the most common primary site.
Overall, uterine sarcoma accounts for 3–5% of all corpus uteri malignancies with an incidence in the United States of 1 to 4 per 100,000 women [2]. Surgical debulking is the mainstay of treatment, but the high rate of tumor recurrence (40–60%) and the poor median survival of 16–40 months after diagnosis suggest a need to identify more effective treatment modalities [3, 4]. CS arising from the ovary may account for only 1–4% of all ovarian cancers with most individuals diagnosed with advanced stage (i.e., III or IV) disease at the time of surgical staging [5, 6]. Ovarian CS has a worse prognosis than uterine CS, with a median survival rate of 8–32 months and recurrence rates of 50–100% [4, 7].
The pathogenesis and genetic alterations of CS remain unclear and different theories have been proposed to explain the biphasic nature of these tumors [8, 9]. Multiple studies have linked the clinically aggressive behavior of CS to the epithelial component of the tumor and consistent with this view, CS has been recently reclassified as metaplastic carcinomas [10, 11]. Because the carcinomatous component drives the biphasic tumor growth, it is speculated that therapeutic interventions targeting cellular pathways that drive epithelial cell proliferation may potentially be beneficial against these biologically aggressive tumors.
The Human Epidermal Growth Factor family of receptors (ErbB family) includes four structurally related members: HER1 (ErbB1, also known as EGFR), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). The ErbB family members are receptor tyrosine kinases (RTKs) with an analogous structure, consisting of an extracellular ligand-binding domain, a single hydrophobic transmembrane region and an intracellular segment that contains a conserved tyrosine kinase domain [12]. HER2 is known to play a pivotal role in cell growth, survival and differentiation in normal and tumor cells by enhancing kinase-mediated activation of downstream signaling pathways, such as those involving mitogen-activated protein kinase and phosphatidylinositide 3-kinase [13, 14]. The HER2/neu oncogene has been reported as overexpressed and/or amplified in various human tumor types including CS of the female genital tract [15–22]. HER2 overexpressing cancers have been associated with worse prognosis when compared with matched HER2 non-amplified cancers, including higher mortality in early-stage disease, reduced time to relapse and a higher incidence of metastases [23, 24].
Trastuzumab emtansine (T-DM1, Kadcyla™, Genentech, South San Francisco, CA) is a novel HER2-targeting immunotoxin developed by conjugation of Trastuzumab (T) (Herceptin, Genentech, South San Francisco, CA) with maytansinoid cytotoxin (DM1), a highly potent microtubule polymerization inhibitor [25]. T-DM1 is internalized upon binding to the extracellular sub-domain IV of the HER2 receptor. After lysosomal degradation, DM1 is released in the cytoplasm, leading to cell cycle arrest and apoptotic cell death [26]. Our group has recently identified HER2 as a potential target in CS raising the possibility that anti-HER2 therapies may be a viable treatment options in these settings [27]. This study represents the first evaluation of the activity of T-DM1 against HER2 positive and negative primary CS cell lines in vitro followed by developing a supportive CS in vivo model.
Methods
Establishment of Carcinosarcoma cell lines
Study approval was obtained from the Institutional Review Board, and all patients signed consent prior to tissue collection according to the institutional guidelines. A total of eight primary uterine and ovarian CS cell lines were established from fresh tumor biopsy samples, as described previously [27]. Tumors were staged according to the International Federation of Gynecology and Obstetrics staging system. Patient characteristics are noted in Table 1.
Table 1.
Patient Characteristics
| PATIENT | AGE (YEARS) | RACE | FIGO STAGE | HISTOLOGY | PRIMARY SITE | GENERATION TIME AND N OF PASSAGES | |
|---|---|---|---|---|---|---|---|
| EC* | SC§ | ||||||
| SARARK-1 | 70 | AA | IC | Homologous | uterus | 2005 25 |
|
| END¥+CCθ | ESSβ | ||||||
| SARARK-3 | 75 | C | IIIC | Heterologous | ovary | 2009 27 |
|
| SERΔ | CDRS⋄ | ||||||
| SARARK-4 | 77 | C | IIIC | Heterologous | ovary | 2010 15 |
|
| SER | CDRS# | ||||||
| SARARK-6 | 78 | C | IV/IIB | Homologous | ovary | 2009 18 |
|
| SER | CDR◆ | ||||||
| SARARK-7 | 55 | AA | IV | Heterologous | ovary | 2004 25 |
|
| CC+SER | CDRS | ||||||
| SARARK-8 | 46 | C | IIB | Homologous | uterus | 2010 6 |
|
| UND# | ESS | ||||||
| SARARK-9 | 66 | C | IIIC2 | Homologous | uterus | 2013 5 |
|
| SER | ESS | ||||||
| SARARK-10 | 62 | C | IVB | Homologous | uterus | 2013 8 |
|
| SER | ESS | ||||||
Abbreviations: AA, African-American; C, Caucasian; FIGO, International Federation of Gynecology and Obstetrics,
EC, epithelial component;
SC, stromal component;
END, endometrioid;
ESS, endometrial stromal sarcoma;
CC, clear cell;
CDR, chondroid;
CDRS, chondrosarcoma;
SER, serous;
UND, undifferentiated.
Immunostaining of tissue and cell blocks of primary carcinosarcoma
Tumor blocks and cell blocks were obtained from all CS cell line patients and reviewed by a gynecologic surgical pathologist. The level of HER2 expression was then evaluated as previously described [17]. HER2 staining intensity was graded on the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) 2007 breast scoring criteria: 0 (negative = no staining observed), 1+ (light staining = weak, incomplete membrane staining in any proportion of tumor cells or weak complete membrane staining in <10% of tumor cells), 2+ (moderate staining = weak complete membrane staining in at least 10% of tumor cells or intense complete membrane staining in 30% or less of tumor cells), or 3+ (strong staining = uniform, intense membrane staining in >30% of tumor cells) [28]. Appropriate positive and negative controls were used with each case.
Fluorescence in situ hybridization of cell blocks from primary CS
Fluorescence in situ hybridization (FISH) analysis was performed using the PathVysion HER2 DNA-FISH-Kit (Abbott Molecular Inc., Abbott Park, IL, USA) according to the manufacturer’s instructions and as previously described [27]. Fluorescent signals in at least 30 non-overlapping interphase nuclei with intact morphology were scored using an Olympus BX61 microscope (Olympus America Inc.) with a 60-x planar objective, using the filter that permits simultaneous green and red colors. Tumor cells were scored for the number of orange (HER2/neu) and green (CEP17) signals. HER2/neu amplification was defined by a HER2/neu/CEP17 copy ratio ≥2.0
Quantitative real-time polymerase chain reaction (qRT-PCR)
RNA extraction from all carcinosarcoma cell lines and from normal control tissue was performed using AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. Total RNA (5 μg) was reverse-transcribed using Superscript III (Invitrogen, Carlsbad, CA). Quantitative PCR was carried out to evaluate the expression level of HER2 (ERBB2, Assay ID: Hs00170433_m1, Applied Biosystems) in all samples with a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using the recommended protocol by the manufacturer. Each reaction was run in duplicate. The internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Assay ID: Hs99999905_ml, Applied Biosystems), was used to normalize variations in cDNA quantities from different samples. The comparative threshold cycle (Ct) method was used for the calculation of amplification fold as specified by the manufacturer and previously described [27]. Analyses were performed using SDS software 2.2.2 (Applied Biosystems/Life Technologies).
Flow cytometry assay for HER2 staining
Trastuzumab (Herceptin, Genentech, South San Francisco, CA), a humanized monoclonal antibody (mAb) of the IgG1 isotype that binds with high affinity to the extracellular domain of the HER2 receptor was used to assess HER2 expression on the surface of tumor cells. All the primary carcinosarcoma cell lines were incubated with 2.5 μg/ml of trastuzumab for 30 minutes on ice. Rituximab (Rituxan, Genentech, South San Francisco, CA), a chimeric anti-CD20 monoclonal-antibody (mAb) was used as a negative control (2.5 μg/ml). A fluorescein isothiocyanate-conjugated Goat F(ab′)2 Anti-Human immunoglobulin was used as a secondary reagent (Invitrogen, Life Technologies). Analysis was conducted with a FACScalibur, using Cell Quest software (BD Biosciences, San Diego, CA, USA).
Test for antibody-dependant cell-mediated cytotoxicity (ADCC)
Standard 4-hours chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque-separated peripheral blood lymphocytes (PBLs) from several healthy donors, in combination with T-DM1 or trastuzumab against the CS target cell lines at effector to target ratios (E:T) of 10:1 and 20:1. The release of 51Cr from target cells was measured as evidence of tumor cell lysis after exposure of the tumor cells to 2.5 μg/ml of T-DM1 or 2.5 μg/ml of T. Concentration-response experiments were performed in order to determine the optimal antibody dosing for ADCC experiments. The negative control condition was the incubation of target cells alone. As a positive control condition, 1% sodium dodecyl sulfate (SDS) was used to achieve complete lysis of target cells. Chimeric anti-CD20 mAb rituximab 2.5 μg/ml was used as the negative control for T-DM1 and T in all bioassays. Briefly, the cells were 51Cr–labelled for 1h, washed, incubated with the antibodies and the release of 51Cr in the supernatant was analyzed after 4h. 51Cr-released from the target cells by cytolysis was counted in gamma radiation counter (2470 WIZARD2 Automatic Gamma Counter, PerkinElmer). The percentage cytotoxicity of trastuzumab or T-DM1 was calculated by the following formula: % cytotoxicity = 100 × (E−S)/(T−S), where E is the experimental release, S is the spontaneous release by target cells, T is the maximum release by target cells lysed with 1% SDS. Results are mean ± SEM (standard error of mean).
Proliferation assay
CS cell lines were plated at log phase of growth in 6-well tissue culture plates at a density of 50,000–80,000 cells in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Mediatech, Manassas, VA), and 0.3% fungizone (Life Technologies, Carlsbad, CA). After 24 hours incubation, cells were treated with T-DM1, T and rituximab at the saturating concentration of 2 μg/ml, 20 μg/ml and 20 μg/ml respectively, determined by previously conducted concentration-response experiments. 72 hours after drugs treatment cells were harvested in their entirety, centrifuged and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS). Analysis was performed using flow cytometry based assay to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of 3 independent experiments per cell line were performed.
Cell cycle analysis of CS cell lines after exposure to T-DM1 by flow cytometry
CS cell lines were plated in six-well tissue culture plates and when in exponential growth treated with T-DM1 at concentration of 2 μg/ml or with control mAbs (T and rituximab, 20 μg/ml). After 24–48 hours of treatment cells were harvested, fixed with 70% ethanol for at least 30 minutes, then washed and stained with 50 μg/ml of propidium iodide (PI) in the presence of 100 μg/ml of ribonuclease A. Quantification of cell cycle distribution was determined by flow cytometric analysis of propidium iodide fluorescence using the FACScalibur and Cell Quest software with doublet discrimination. For each analysis 10,000 gated events were collected and data analysis was performed using Cell Quest and FlowJo v8.8.2. Results are mean ± SEM.
In vivo treatment
Six to eight week-old C.B-17/SCID mice were given a single subcutaneous injection of 7 × 106 CS SARARK-6 cells (HER2/neu amplified) in approximately 200 μL of a 1:1 solution of sterile PBS containing cells and Matrigel (BD Biosciences). Once the mean tumor volume was 0.15 cm3, the mice were randomized into 3 treatment groups. Each group consisted of 5 mice and injections with the treatment drugs were administered after an 8-day period to allow tumor establishment. The mice were randomized to receive T-DM1 (15 mg/kg), trastuzumab (15 mg/kg), or vehicle sterile water. Drug dosages were chosen accordingly to previous studies conducted on different xenograft models [29–31]. All treatment drugs were given as an intravenous (IV) injection once per week. The mice in each group were given series of 5 injections after which they were placed in follow-up and observed for overall survival as the primary outcome measure. Tumor measurements and mouse weights were recorded twice weekly. Mice were sacrificed if tumors reached 1cm3 using the formula (width)2 × height/2. Animal care and euthanasia was carried out according to the rules and regulations as set forth by the Institutional Animal Care and Use Committee (IACUC).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, Inc. San Diego, CA). For quantitative real-time PCR (qRT-PCR) data, right skewing was removed by taking copy number ratios relative to the lowest-expressing normal endometrium (NEC) or normal ovarian sample (NOVA) (relative copy number), and then log2 transforming them to ΔCt values. A Wilcoxon rank-sum test was used to compare CS cell lines to normal endometrium or ovary for differences in immunohistochemistry (IHC) staining intensity. A two-tailed unpaired t-test was used to evaluate the differences in HER2 expression by flow cytometry. The differences in ADCC levels by 4-hr chromium release assays as well as the inhibition of proliferation in the CS cell lines after exposure to T versus T-DM1 and cell cycle changes in the HER2 overexpressing and not overexpressing cell lines were evaluated by the two-tailed unpaired student t-test. Overall survival data were analyzed and plotted using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered statistically significant at P values < 0.05.
Results
HER2 expression by IHC in primary uterine and ovarian CS cell lines
Immunohistochemical analysis of HER2 expression was performed on tissue and cell blocks of all primary uterine and ovarian CS patients available to this study. High levels of HER2 protein expression were detected in 2 out of 8 of the CS cell lines tested. Specifically, SARARK-6 and SARARK-9 showed strong (3+) HER2 positivity. In contrast, negligible HER2 expression (i.e., 0/1+) was detected in SARARK-1, SARARK-3, SARARK-4, SARARK-7, SARARK-8 and SARARK-10 (Table 2). The cell lines SARARK-6 and SARARK-1 were therefore chosen as representative cell lines for the next set of experiments because of their differential HER2 expression and their similar growth rates (data not shown).
Table 2.
HER2 expression and amplification in carcinosarcoma cell lines.
| Patient | qRT-PCR | Flow Cytometry | IHC | FISH |
|---|---|---|---|---|
| mRNA copy number | MFI | Cell/tissue block | ||
| SARARK-1 | 27.9 | 17.2 | 0 | Not Amplified |
| SARARK-3 | 75.06 | 22.8 | 0 | NT* |
| SARARK-4 | 36.21 | 32.36 | 0 | Not Amplified |
| SARARK-6 | 5634.2 | 508.91 | 3+ | Amplified |
| SARARK-7 | 61.16 | 38.35 | 1+ | Not Amplified |
| SARARK-8 | 65.35 | 12.06 | 0 | Not Amplified |
| SARARK-9 | 897.64 | 300.74 | 3+ | Amplified |
| SARARK-10 | 10.9 | 23.05 | 1+ | NT |
Abbreviations: qRT-PCR, real time PCR; MFI, mean fluorescence intensity; IHC, immunohistochemistry; FISH, fluorescent in situ hybridization;
NT, not tested.
ErbB2 gene amplification by FISH
Fluorescence in situ hybridization (FISH) was performed on 6 out of the 8 cell blocks obtained from the primary cell lines tested in this study. ErbB2 gene amplification was detected in both primary CS cell lines found to overexpress HER2 at 3+ level by IHC (i.e., SARARK-6 and SARARK-9 cells, Table 2). In contrast, all CS cell lines tested by IHC and found negative for HER2 protein overexpression were also found not amplified in the FISH assays (Table 2).
HER2 expression by qRT-PCR in CS cell lines
Ovarian and uterine CS cell lines grown as primary cultures were tested by qRT-PCR to evaluate HER2 receptor expression at the mRNA level (i.e., mRNA copy number). Negative controls were normal endometrium and ovarian tissues. The HER2 mRNA levels in the high expressing cell lines were significantly higher compared to normal control tissue. The mRNA copy number for SARARK-6 and SARAK-9 (HER2 overexpressing cell lines) was 5634.2, and 897.64 respectively. This HER2 expression was significantly higher when compared with mRNA copy numbers from the low HER2 expressing cell lines SARARK-1, SARARK-3, SARARK-4, SARARK-7, SARARK-8 and SARARK-10 (range from 10.9 to 75.06) (P<0.01) (Table 2).
HER2 expression by flow cytometry
To assess the possibility to target HER2 also in CS cells, its expression on cell surface was evaluated on live cells by flow cytometry. HER2 expression was in good agreement with the expression results found by qRT-PCR and IHC in all cell lines. In this regard, very high expression levels of HER2 were found in both SARARK-6 and SARARK-9 cell lines that showed 3+ level by IHC and high expression at mRNA level. For these 2 tumors the mean fluorescence intensity (MFI) was 508.91 and 300.74 respectively. In contrast cell lines with low HER2 protein expression by qRT-PCR and IHC demonstrated negligible HER2 surface expression by flow cytometry [i.e., SARARK-1, SARARK-3, SARARK-4, SARARK-7, SARARK-8 and SARARK-10, (MFI range from 12.6 to 38.35), (Table 2). Figure 1 shows a representative histogram of the flow cytometry results for SARARK-6 (high HER2 expressing cell line, panel A) and SARARK-1 (low HER2 expressing cell line, panel B).
Figure 1. Representative flow cytometry analysis in HER2 positive and negative CS cell lines.
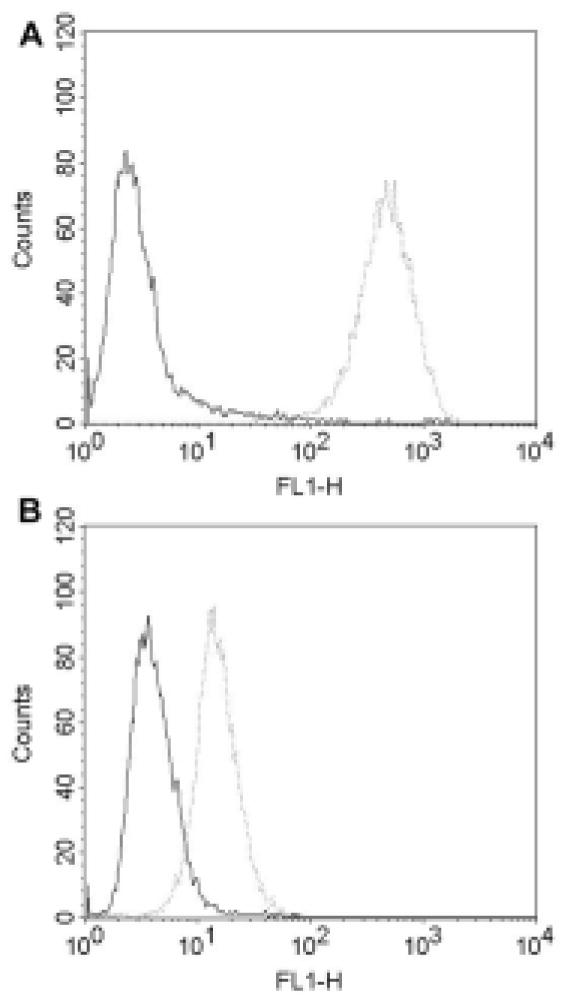
High HER2 expressing cell line (SARARK-6, panel A) versus the low HER2 expressing cell line (SARARK-1, panel B). Rituximab control mAb is the solid line and trastuzumab is the dotted line. High HER2 expressing cell line shows significantly higher mean fluorescence intensity with trastuzumab versus low HER2 expressing cell line.
ADCC mediated by T-DM1 and T in carcinosarcoma cell lines
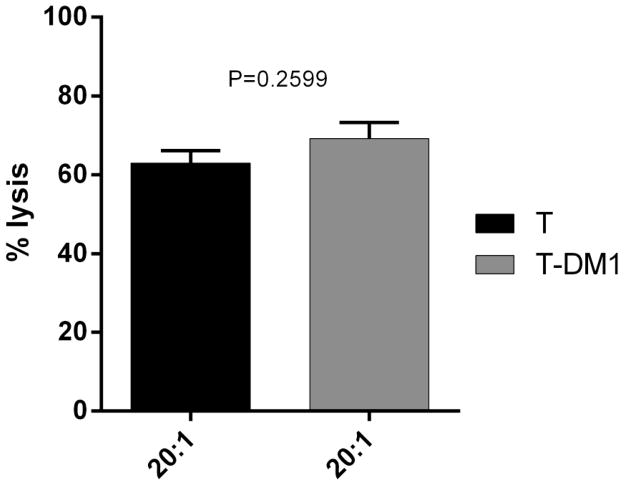
To address whether or not conjugation to DM1 effected the ability of trastuzumab to cause ADCC, CS cell lines were tested for their sensitivity to NK-mediated cytotoxicity after exposure to heterologous PBLs collected from several healthy donors in standard 4 h 51Cr release assays. CS cells were found to be resistant to PBL-mediated cytotoxicity when exposed to PBLs with or without rituximab control antibody as effectors (2.5 μg/ml) at E:T ratios of 10:1 and 20:1 (mean killing ± SEM of 2.1 ± 1.7% with PBLs alone and mean ± SEM of 3.5 ± 1.4% in the presence of PBL s+ rituximab at the highest concentration). Next we exposed CS cells to heterologous PBLs in the presence of T-DM1 (2.5 μg/ml) and T (2.5 μg/ml). We found both antibodies to be similarly effective in inducing strong ADCC against the high HER2 expressing cell line (SARARK-6) with mean cytotoxicity ± SEM of 68 ± 5% versus 63 ± 6% of T-DM1 vs T (P=0.259) (Figure 2). Negligible ADCC was detected against cell lines with low/negative HER2 expression when combined with PBLs and T-DM1 or T compared to control (data not shown).
Figure 2. Representative ADCC results of T-DM1 versus T.
HER2 overexpressing cell line (SARARK-6) was challenged with PBLs at a ratio of 20:1. No significant difference in ADCC is seen with T versus T-DM1 (P=0.259). Results represent average percent cytotoxicity ± SEM of two independent experiments.
T-DM1 is more effective than T at inhibiting CS cell proliferation
T-DM1 was dramatically more effective than T in inhibiting HER2 overexpressing cell proliferation and in causing cell death as determined by flow cytometry and propidium iodide uptake. Figure 3 shows representative experimental results for SARARK-6 (i.e., a CS with high HER2 expression) after T and T-DM1 exposure. We found a mean number of viable cells ± SEM of 17.98 ± 2.73% versus 81.75 ± 1.31% for T-DM1 versus T respectively (P<0.0001). These results are consistent with a significant higher activity of T-DM1 vs T against HER2 positive cells. No significant difference in cytostatic or apoptotic activity was detected using either T-DM1 or T against CS cell lines with low/negative HER2 expression [(i.e. SARARK-1 mean number of viable cells ± SEM of 88.07 ± 6.07% versus 90.0 ± 4.5% for T-DM1 versus T respectively (P=0.81)].
Figure 3. Representative proliferation assay result of T-DM1 versus T.
Cells were plated in 6 wells and treated with T-DM1 24 hours later, then counted after 72 hours by flow citometry. T-DM1 significantly inhibits SARARK-6 (high HER2 expressing cell line) proliferation compared to T (P<0.0001). Reported values are representative of 3 independent experiments; bars are mean ± SEM.
Effects of T-DM1 on Cell Cycle Progression
In order to determine T-DM1’s effect on the cell cycle, cells were exposed to T-DM1 for 24 or 48 hours and DNA was stained with propidium iodide for quantification of the different cell cycle phases. Exposure to T-DM1 resulted in a concentration-dependent increase in G2/M phase cells in all CS cell lines according with their HER2 expression levels (data not shown). We found a dramatic G2/M phase shift in the high HER2 expressing cell line (i.e., SARARK-6) from a mean ± SEM of 14.87 ± 1.23% in the untreated sample to a mean of 66.57 ± 4.56% after exposure to 2 μg/ml of T-DM1, as would be predicted by a drug that disrupts microtubule function (P<0.0001) (Figure 4). In contrast, no significant G2/M phase arrest was noted in the low HER2 expressing cell line (i.e., SARARK-1) after T-DM1 treatment with a shift from a mean ± SEM of 12.70 ± 3.37% to a mean of 15.81 ± 3.07% (P=0.8) (Figure 4). We also analyzed the effect of T in the same cell lines with a non-significant G2/M phase perturbation [mean ± SEM of 14.87 ± 1.23% to a mean of 17.3 ± 0.75% (P=0.2) for SARARK-6; mean ± SEM of 12.70 ± 3.37% to a mean of 8.87 ± 2.24% (P=0.4) for SARARK-1].
Figure 4. Analysis of cell cycle by flow cytometry.
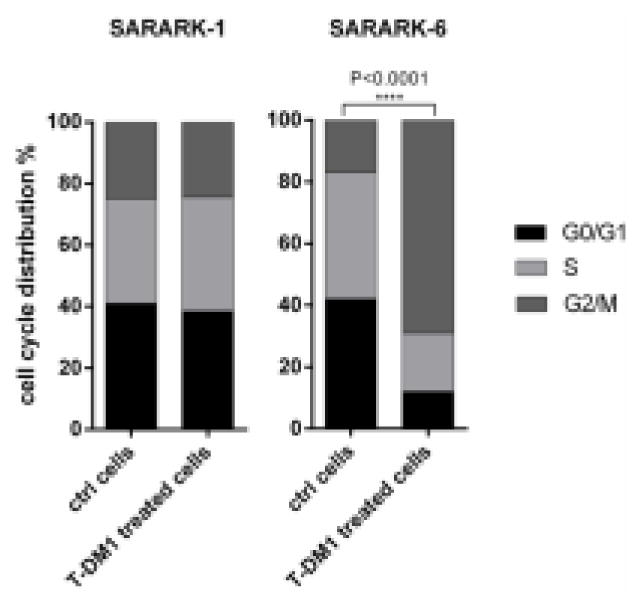
Representative experiment showing cell cycle arrest in G2/M phase after treatment with T-DM1 in the high HER2 expressing cell line SARARK-6 (P<0.0001). No change in cell cycle distribution is shown in the HER2 negative cell line SARARK-1. Data are representative of at least 3 independent experiments.
In-vivo therapeutic efficacy of T and T-DM1 in HER2 overexpressed CS xenografts
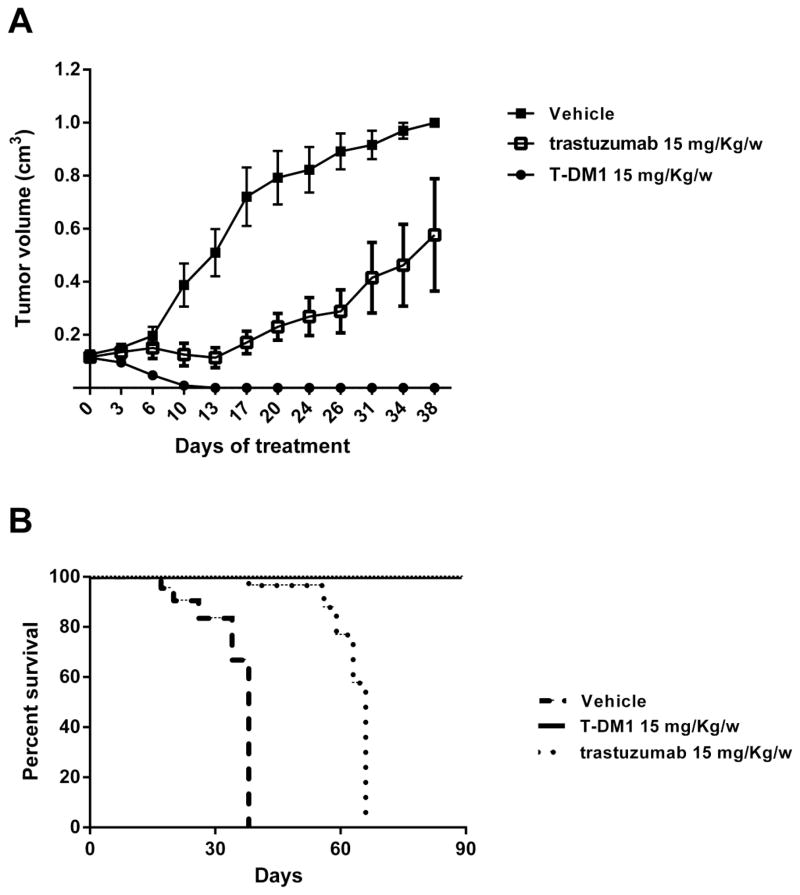
An in vivo experiment was used to determine the effect of T and T-DM1 on inhibition of tumor growth. Tumors were clearly visible in all mice inoculated with a mean volume of 0.15 cm3 before starting the drug treatment. Mice treated with T-DM1 achieved a grossly complete response when compared to T and vehicle group (P=0.0001 and P<0.0001 respectively) (figure 5A). Indeed, all animals treated with T-DM-1 (i.e., 5 out of 5) remained free of detectable tumor for the entire duration of the study period (i.e., over 90 days) with a P value of P=0.008 and P=0.0001 when compared to T and vehicle group respectively (Figure 5B). In contrast, all mice of the trastuzumab group required euthanasia or died from tumor burden between days 3 and 31 after suspension of trastuzumab injection. All mice in the vehicle group (n=5) had to be euthanized because of tumor burden between days 17 to 38 after tumor injection. Importantly, mice’s body weight was not affected (data not shown) and no signs of general toxicity were seen in any of the 3 treatment groups.
Figure 5. Tumor growth inhibition by T vs T-DM1.
Tumors were allowed to establish growth for a week after subcutaneous injection before initiation of treatment. Fifteen mice (5 mice per group) with CS xenografted tumor everexpressing HER2 (SARARK-6) were treated with a series of 5 IV injection of T-DM1 (15 mg/kg/w), T (15 mg/kg/w) and vehicle, respectively. T-DM1 treatment resulted in a dramatic reduction of tumors volumes and complete disappearance of established disease when compared to T and to vehicle group (P=0.0001 and P<0.0001 respectively) (panel A). Points are mean tumor volume (cm3); bars are SEM (standard error of the mean) (n=5 mice per group). T-DM1 treatment significantly prolonged overall survival in treated group when compared to trastuzumab group and to the vehicle group (P=0.008 and P=0.0001 respectively) (panel B).
Discussion
CS of the female genital tract are rare tumors characterized by aggressive clinical behavior. Although surgical debulking remains the mainstay of treatment, there continues to be the need for new effective adjuvant therapies given the high rate of tumor recurrence and poor survival in women diagnosed with CS [3, 4]. In the last few years, novel immunotherapeutic approaches using fully human anti-HER2 antibody-drug conjugates (ADCs) are increasingly being evaluated as targeted therapies for achieving clinically significant levels of cell killing against HER2 overexpressing tumors while minimizing damage to healthy tissues.
Consistent with this view, multiple groups including our own, have investigated the expression levels of HER2 in uterine and ovarian CS [19–22, 27]. For example, Raspollini et al. found HER2 overexpression in 7 of 24 CS cases (29.2%) tested by IHC [20]. Sawada et al. and Amant et al. found higher HER2 overexpression in CS [(i.e., 9 of 16 cases (56%), and 3 of 7 cases (43%), respectively] when considering both 2+ and 3+ positivity by IHC [21, 22]. Finally, Livasy et al. reported that up to 25% of CS (14/55) may overexpress HER2 at 3+ levels by IHC [19]. Our results evaluating HER2 expression in primary CS cell lines recently established in our laboratory are consistent with the previously reported results [19]. Indeed, we found ErbB2 to be amplified by FISH in 2 of 8 (25%) of uterine and ovarian CS cell lines available for study. We also evaluated and compared HER2 positivity in primary CS cell lines by real-time PCR as well as by flow cytometry. We found a significant correlation between the high mRNA copy numbers with the high surface protein expression by IHC (i.e., 3+) and flow cytometry. While the identification of CS overexpressing HER2 at 3+ level by IHC and/or harboring amplification of the ErbB2 gene by FISH may potentially represent the most effective way to guide selection of CS patients who may benefit more from T-DM1 therapy, demonstration of the contribution of HER2 protein expression and gene amplification to the sensitivity of a tumor to a specific targeted agent such as T-DM1 requires functional in vitro and in vivo validation. Previous studies have reported very little activity of a dose of unconjugated DM1 that was equivalent to the amount of DM1 conjugated to trastuzumab or to an aspecific antibody [29, 32].
Our experiments consistently demonstrate that T-DM1 has a strong growth inhibitory effect on HER2 positive CS cell lines in vitro as well as in vivo in SCID mice harboring CS xenografts. While both T-DM1 and T evoked similar ADCC against HER2 overexpressing CS cell lines in the presence of allogeneic PBLs, T-DM1 was found to be dramatically more effective than T in inhibiting cell proliferation and in inducing cell death after G2/M phase cell cycle arrest in the absence of mononuclear cells. Importantly, while both HER2 3+ CS cell lines were found to be resistant to the cytostatic effect of T they remained highly sensitive to the exposure to T-DM1.
Trastuzumab emtansine combines two mechanisms of action: a) Trastuzumab binds selectively to the HER2 positive tumor cells and blocks the uncontrolled signals that contribute to the growth and survival of the tumor and recruits natural killer (NK) cells for the initiation of ADCC; b) the DM1 (mertansine) is released within the cells and cause apoptosis by blocking cell duplication [32]. Because T-DM1 targets only cancer cells overexpressing HER2, exposure of healthy cells to DM1 is minimized. T-DM1 is the first ADC to receive full approval from the United States Food and Drug Administration (FDA) on the basis of the findings of EMILIA, a randomized phase III trial to assess the efficacy and safety of T-DM1 versus lapatinib plus capecitabine in patients with HER2 positive advanced breast cancer who received prior Herceptin and a taxane. In this study a total of 991 patients were treated with a median progression-free survival of 9.6 months in the T-DM1 arm compared with 6.4 months in the lapatinib/capecitabine arm and a Hazard Ratio for progression or death of 0.65 in favor of T-DM1 (P<0.001). Patients treated with T-DM1 showed a significantly higher objective response rate (ORR) of 43.6% compared with 30.8% in the lapatinib/capecitabine group (P<0.001), with significantly longer overall survival [33]. Overall T-DM1 appears to be safer than the lapatinib/capecitabine combination and well tolerated as a single agent given at 3.6 mg/kg every 3 weeks. This dosing differs from the 6 mg/kg every 3 weeks T dosing. Moreover T-DM1 has a shorter half-life than T (approximately 4 days for T-DM1 versus 3–4 weeks for T), which may also result in a decrease in adverse effects including cardiotoxicity associated with T [34].
In conclusion, this is the first report that explores the potential activity of T-DM1 therapy in HER2 overexpressing primary CS cell lines. Our in vitro and in vivo experiments suggest that T-DM1 and T are similarly effective in inducing strong ADCC against primary HER2 positive CS cell lines in the presence of PBLs. In contrast, our in vitro experimental results in the absence of PBLs clearly demonstrate that T-DM1 is considerably more effective than T in inhibiting cell proliferation and in inducing G2/M phase cell cycle arrest due to the action of the DM1 toxin. Importantly, our in vitro findings were confirmed in a relevant in vivo model of primary CS xenograft overexpressing HER2 at 3+ levels and demonstrating HER2 gene amplification, where complete tumor regression was achieved in all T-DM1 treated mice.
Due to the significant fraction of CS patients overexpressing HER2 [19–22, 24], T-DM1 may represent a novel treatment option for the subset of carcinosarcoma patients harboring disease refractory to traditional salvage chemotherapy and/or unresponsive to T. The investigation of T-DM1 in patients harboring HER2/Neu gene amplified chemotherapy-resistant CS is warranted.
Acknowledgments
We wish to thank Genentech for providing T-DM1. This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation and the Guido Berlucchi Foundation to ADS. RN was supported by the Italian Ministry of Health (RF-2010-2313497). This investigation was also supported by NIH Research Grant CA-16359 from the NCI.
List of abbreviations
- CS
carcinosarcoma
- HER2/ErbB2
epidermal growth factor type 2 receptor
- T-DM1
Trastuzumab-emtansine
- T
trastuzumab
- FISH
fluorescence in situ hybridization
- IHC
immunohistochemistry
- qRT-PCR
quantitative real time polymerase chain reaction
- ADCC
antibody-dependent-cell-mediated-cytotoxicity
- MMMT
Malignant Mixed Müllerian Tumors
- DM1
maytansinoid cytotoxin
- ASCO/CAP
American Society of Clinical Oncology and the College of American Pathologists
- PBL
peripheral blood lymphocytes
- IV
intravenous
- IACUC
Institutional Animal Care and Use Committee
- MFI
mean fluorescence intensity
- ADC
antibody-drug conjugate
- NK
natural killer
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.D’Angelo E, Prat J. Pathology of mixed Müllerian tumours. Hum Pathol Best Pract Res Clin Obstet Gynaecol. 2011;25:705–18. doi: 10.1016/j.bpobgyn.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol. 2004;93:204–8. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Galaal K, Godfrey K, Naik R, Kucukmetin A, Bryant A. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst Rev. 2011;1:CD006812. doi: 10.1002/14651858.CD006812.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg G, Shah JP, Kumar S, Bryant CS, Munkarah A, Morris RT. Ovarian and uterine carcinosarcomas: a comparative analysis of prognostic variables and survival outcomes. Int J Gynecol Cancer. 2010;20:888–894. doi: 10.1111/IGC.0b013e3181dc8292. [DOI] [PubMed] [Google Scholar]
- 5.Mano MS, Rosa DD, Azambuja E, Ismael G, Braga S, D’Hondt V, Piccart M, Awada A. Current management of ovarian carcinosarcoma. Int J Gynecol Cancer. 2007;17:316–24. doi: 10.1111/j.1525-1438.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 6.del Carmen MG1, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol. 2012;125:271–7. doi: 10.1016/j.ygyno.2011.12.418. [DOI] [PubMed] [Google Scholar]
- 7.Jonson AL, Bliss RL, Truskinovsky A, Judson P, Argenta P, Carson L, Dusenbery K, Downs LS., Jr Clinical features and outcomes of uterine an ovarian carcinosarcoma. Gynecol Oncol. 2006;100:561–564. doi: 10.1016/j.ygyno.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Cantrell LE, Van Le L. Carcinosarcoma of the ovary: a review. Obstet Gynecol Surv. 2009;64:673–80. doi: 10.1097/OGX.0b013e3181b8aff3. [DOI] [PubMed] [Google Scholar]
- 9.Inthasorn P, Beale P, Dairymple C, Carter J. Malignant mixed müllerian tumour of the ovary: prognostic factor and response to adjuvant platinum-based chemotherapy. Aust N Z J Obstet Gynaecol. 2003;43:61–4. doi: 10.1046/j.0004-8666.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 10.McCluggage WG. Uterine carcinosarcomas (malignant mixed Müllerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer. 2002;12:687–690. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 11.McCluggage WG. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321–325. doi: 10.1136/jcp.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tebbutt N1, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013;13:663–73. doi: 10.1038/nrc3559. [DOI] [PubMed] [Google Scholar]
- 13.Park JW, Neve RM, Szollosi J, Benz CC. Unraveling the biologic and clinical complexities of HER2. Clin Breast Cancer 2008. 2008;8:392–401. doi: 10.3816/CBC.2008.n.047. [DOI] [PubMed] [Google Scholar]
- 14.Tai Wanyi, Mahato Rubi, Cheng Kun. The role of HER2 in cancer therapy and targeted drug delivery. Journal of Controlled Release. 2010;146:264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: Implications for therapy. Cancer Treat Rev. 2014;40:770–80. doi: 10.1016/j.ctrv.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, De Las Casas LE, Roman JJ, Burnett A, Pecorelli S. Amplification of c-erbB-2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer 2005. 2005;104:1391–1397. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 17.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, Abu-Khalaf M, Buza N, Tavassoli FA, Hui P, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer. 2010;102:134–143. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimbaluk D, Rotmensch J, Scudiere J, Gown A, Bitterman P. Uterine carcinosarcoma: immunohistochemical studies on tissue microarrays with focus on potential therapeutic targets. Gynecol Oncol. 2007;105:138–144. doi: 10.1016/j.ygyno.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA. EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol. 2006;100:101–106. doi: 10.1016/j.ygyno.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 20.Raspollini MR, Susini T, Amunni G, Paglierani M, Taddei A, Marchionni M, Scarselli G, Taddei GL. COX-2, c-KIT and HER2/neu expression in uterine carcinosarcomas: prognostic factors or potential markers for targeted therapies? Gynecol Oncol. 2005;96:159–167. doi: 10.1016/j.ygyno.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 21.Sawada M, Tsuda H, Kimura M, Okamoto S, Kita T, Kasamatsu T, Yamada T, Kikuchi Y, Honjo H, Matsubara O. Different expression patterns of KIT, EGFR, and HER-2 (cerbB-2) oncoproteins between epithelial and mesenchymal components in uterine carcinosarcoma. Cancer Sci. 2003;94:986–991. doi: 10.1111/j.1349-7006.2003.tb01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amant F, Vloeberghs V, Woestenborghs H, Debiec-Rychter M, Verbist L, Moerman P, Vergote I. ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol. 2004;95:583–587. doi: 10.1016/j.ygyno.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 24.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 25.Boyraz B, Sendur MA, Aksoy S, Babacan T, Roach EC, Kizilarslanoglu MC, Petekkaya I, Altundag K. Trastuzumab emtansine (T-DM1) for HER2-positive breast cancer. Curr Med Res Opin. 2013;29:405–14. doi: 10.1185/03007995.2013.775113. [DOI] [PubMed] [Google Scholar]
- 26.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibodycytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 27.Guzzo F, Bellone S, Buza N, Hui P, Carrara L, Varughese J, Cocco E, Betti M, Todeschini P, Gasparrini S, Schwartz PE, Rutherford TJ, Angioli R, Pecorelli S, Santin AD. HER2/neu as a potential target for immunotherapy in gynecologic carcinosarcomas. Int J Gynecol Pathol. 2012;31:211–21. doi: 10.1097/PGP.0b013e31823bb24d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 29.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 30.Barok M, Tannerb M, Köninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–179. doi: 10.1016/j.canlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Cretella D, Saccani F, Quaini F, Frati C, Lagrasta C, Bonelli M, Caffarra C, Cavazzoni A, Fumarola C, Galetti M, La Monica S, Ampollini L, Tiseo M, Ardizzoni A, Petronini PR, Alfieri RR. Trastuzumab emtansine is active on HER-2 overexpressing NSCLC cell lines and overcomes gefitinib resistance. Molecular Cancer. 2014;13:143. doi: 10.1186/1476-4598-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128:347–56. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta M, Lorusso PM, Wang B, Yi JH, Burris HA, 3rd, Beeram M, Modi S, Chu YW, Agresta S, Klencke B, Joshi A, Girish S. Clinical implications of pathophysiological and demographic covariates on the population pharmacokinetics of trastuzumab emtansine, a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J of Clin Pharmacol. 2012;52:691–703. doi: 10.1177/0091270011403742. [DOI] [PubMed] [Google Scholar]