Abstract
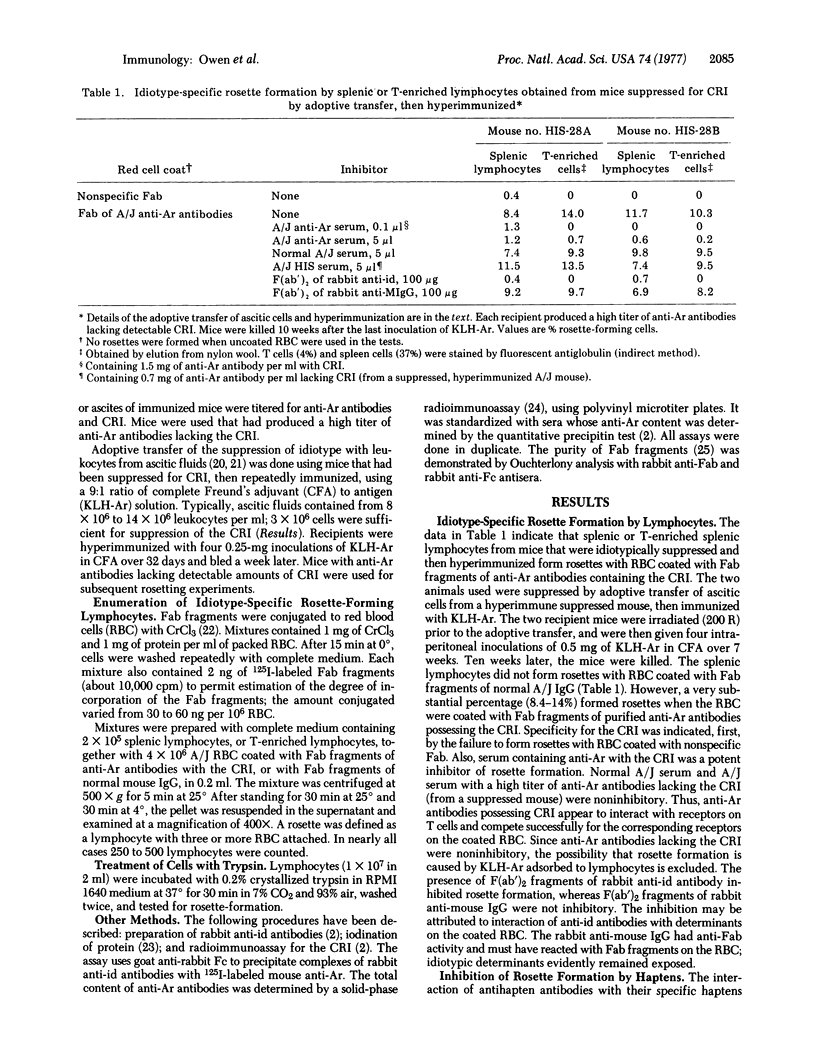
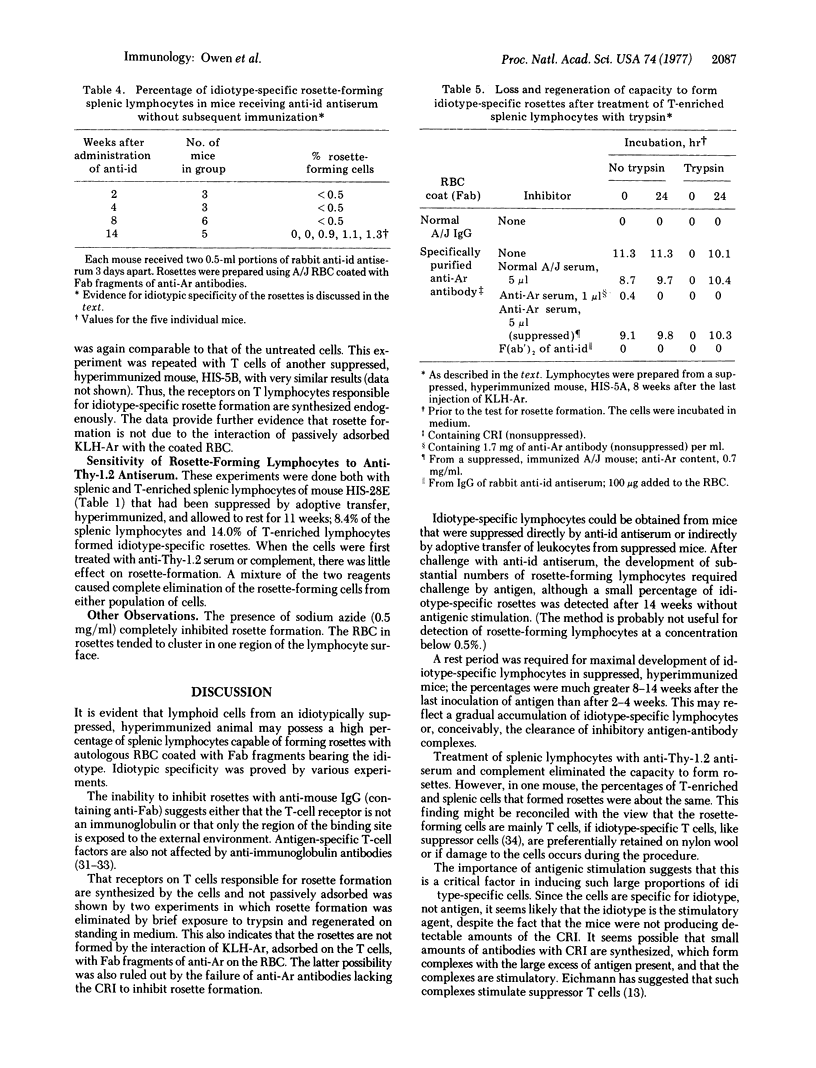
All A/J mice immunized with a conjugate of p-azophenylarsonate groups to keyhole limpet hemocyanin produce antibodies against azophenylarsonate, some of which share a crossreactive idiotype. The appearance of the idiotype can be suppressed, without reducing the response against azophenylarsonate, by injecting rabbit anti-idiotypic antibodies prior to immunization. We have now observed that mice suppressed in this way, or by adoptive transfer of leukocytes from other suppressed mice, and then immunized with the hemocyanin-azophenylarsonate conjugate, possess high proportions (up to 14%) of lymphocytes that form rosettes with A/J erythrocytes coated with Fab fragments possessing the idiotype. Idiotypic specificity was demonstrated by various experiments. Most of all of the rosette-forming lymphocytes appear to be thymus-derived lymphocytes (T cells). Treatment of T cells with trypsin eliminated the capacity to form rosettes, which was restored on standing overnight in medium. Thus, the receptors are synthesized by the cells and are not passively adsorbed. Treatment of mice with anti-idiotypic antiserum without antigenic stimulation did not elicit substantial numbers of rosette-forming cells. The requirement for antigen suggests that antigen-idiotype complexes may be a stimulatory agent. A prolonged rest period after immunization of suppressed mice was required for the induction of high percentages of rosette-forming cells. Rosette formation provides a convenient method for studying factors that induce the formation of idiotype-specific T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangasser S. A., Kapsalis A. A., Fraker P. J., Nisonoff A. Competition for antigen by cell populations having receptors with the same specificity but of different idiotype. J Immunol. 1975 Feb;114(2 Pt 1):610–614. [PubMed] [Google Scholar]
- Blomberg B., Geckeler W. R., Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972 Jul 14;177(4044):178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- Brient B. W., Haimovich J., Nisonoff A. Reaction of anti-idiotypic antibody with the hapten-binding site of a myeloma protein. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3136–3139. doi: 10.1073/pnas.68.12.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brient B. W., Nisonoff A. Quantitative investigations of idiotypic antibodies. IV. Inhibition by specific haptens of the reaction of anti-hapten antibody with its anti-idiotypic antibody. J Exp Med. 1970 Nov;132(5):951–962. doi: 10.1084/jem.132.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin J. L., Davie J. M. Specific isolation and characterization of antibody directed to binding site antigenic determinants. J Immunol. 1975 Jan;114(1 Pt 1):70–75. [PubMed] [Google Scholar]
- Cohn M., Notani G., Rice S. A. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969 Jan;6(1):111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2701–2705. doi: 10.1073/pnas.69.9.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Idiotypic identity of antibodies to streptococcal carbohydrate in inbred mice. Eur J Immunol. 1972 Aug;2(4):301–307. doi: 10.1002/eji.1830020402. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Pawlak L. L., Nisonoff A. Nature of anti-hapten antibodies arising after immune suppression of a set of cross-raactive idiotypic specificities. Eur J Immunol. 1973 Jan;3(1):44–48. doi: 10.1002/eji.1830030110. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Wang A. L., Pawlak L. L., Nisonoff A. Suppression of idiotypic specificities in adult mice by administration of antiidiotypic antibody. J Exp Med. 1972 Jun 1;135(6):1293–1300. doi: 10.1084/jem.135.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Okumura K., Cantor H., Sato V. L., Shen F. W., Boyse E. A., Herzenberg L. A. T-cell regulation of antibody responses: demonstration of allotype-specific helper T cells and their specific removal by suppressor T cells. J Exp Med. 1976 Aug 1;144(2):330–344. doi: 10.1084/jem.144.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L. The agglutination and sensitization of red cells by metallic cations: interactions between multivalent metals and the red-cell membrane. Br J Haematol. 1957 Jan;3(1):19–38. doi: 10.1111/j.1365-2141.1957.tb05768.x. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Sakato N., Eisen H. N. Recognition of immunoglobulin idiotypes by thymus-derived lymphocytes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2357–2360. doi: 10.1073/pnas.72.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., De la Croix F., Benacerraf B. Immunosuppressive factor(s) extracted from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). J Immunol. 1976 Feb;116(2):305–309. [PubMed] [Google Scholar]
- Klinman N. R., Pickard A. R., Sigal N. H., Gearhart P. J., Metcalf E. S., Pierce S. K. Assessing B cell diversification by antigen receptor and precursor cell analysis. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):489–502. [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonoff A., Ju S. Studies of a cross-reactive idiotype associated with anti-para-azophenylarsonate antibodies of A/J mice. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):347–356. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak L. L., Hart D. A., Nisonoff A. Requirements for prolonged suppression of an idiotypic specificity in adult mice. J Exp Med. 1973 Jun 1;137(6):1442–1458. doi: 10.1084/jem.137.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak L. L., Mushinski E. B., Nisonoff A., Potter M. Evidence for the linkage of the IGC H locus to a gene controlling the idiotypic specificity of anti-p-azophenylarsonate antibodies in strain A mice. J Exp Med. 1973 Jan 1;137(1):22–31. doi: 10.1084/jem.137.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Rubin B. Regulation of helper cell activity by specifically adsorbable T lymphocytes. J Immunol. 1976 Jan;116(1):80–85. [PubMed] [Google Scholar]
- Sher A., Cohn M. Effect of haptens on the reaction of anti-idiotype antibody with a mouse anti-phosphorylcholine plasmacytoma protein. J Immunol. 1972 Jul;109(1):176–178. [PubMed] [Google Scholar]
- Sirisinha S., Eisen H. N. Autoimmune-like antibodies to the ligand-binding sites of myeloma proteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3130–3135. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer D. S., Cosenza H., Lee W. M., Rowley D. A., Köhler H. Neonatal tolerance induced by antibody against antigen-specific receptor. Science. 1974 Nov 15;186(4164):640–643. doi: 10.1126/science.186.4164.640. [DOI] [PubMed] [Google Scholar]
- Takemori T., Tada T. Properties of antigen-specific suppressive T-cell factor in the regulation of antibody response of the mouse. I. In vivo activity and immunochemical characterization. J Exp Med. 1975 Nov 1;142(5):1241–1253. doi: 10.1084/jem.142.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J., Munro A. J. Removal of specific cooperative T cell factor by anti-H-2 but not by anti-Ig sera. Nature. 1974 Sep 6;251(5470):63–65. doi: 10.1038/251063a0. [DOI] [PubMed] [Google Scholar]
- Tung A. S., Ju S. T., Sato S., Nisonoff A. Production of large amounts of antibodies in individual mice. J Immunol. 1976 Mar;116(3):676–681. [PubMed] [Google Scholar]
- Wigzell H. Specific fractionation of immunocompetent cells. Transplant Rev. 1970;5:76–104. doi: 10.1111/j.1600-065x.1970.tb00357.x. [DOI] [PubMed] [Google Scholar]


