Abstract
Rationale
Women are twice as likely as men to develop major depressive disorder. Exposure to chronic stress can induce depression in some vulnerable individuals, while others are resistant to depressive-like symptoms after equivalent levels of chronic stress.
Objectives
In female rats, individual differences in saccharin intake during chronic social defeat stress may predict subsequent cocaine self-administration, and may be attributed to alterations in mesolimbic dopamine activity.
Methods
Female rats were exposed to 21 days of chronic social defeat stress, during which they were evaluated for their anhedonia-like responses in the form of saccharin intake. After chronic social defeat stress, the rats were tested for behavioral cross-sensitization to cocaine and escalated cocaine self-administration in a 24-h “binge.” A separate group of animals underwent in vivo microdialysis of the nucleus accumbens (NAc) shell to assess dopamine (DA) in response to acute cocaine challenge.
Results
Cluster analysis revealed two phenotypes among the stressed female rats based on their saccharin intake while being exposed to stress, termed stress-resistant (SR, 28 %) and stress-sensitive (SS, 72 %). The amount of cocaine self-administered during the 24-h “binge” was positively correlated with preceding saccharin intake. The NAc DA response to a cocaine challenge was significantly lower in SR rats than in the SS and non-stressed control rats. No other significant differences were observed in behavioral cross-sensitization or cocaine self-administration prior to the “binge.”
Conclusion
Female rats showed individual differences in their anhedonic-like response to chronic social defeat stress, and these differences were reliably associated with subsequent cocaine-taking behavior.
Keywords: Chronic social defeat stress, Cocaine self-administration, Depression, Dopamine, Females, Individual differences
Introduction
Women are twice as likely as men to develop major depressive disorder (MDD, Abuse and Administration 2010). Exposure to chronic stress can induce depression in some vulnerable individuals, while others are resistant to such stress (McEwen and Stellar 1993). Substance use disorder (SUD) is frequently observed in patients with MDD (Kessler et al. 2005). The lack of treatment options available when both diseases occur simultaneously is a major concern, and may eventually give rise to increased comorbidity (Bracke 2000). Therefore, preclinical models using chronic social stress in females may be useful for characterizing individual differences associated with depressive- and addictive-like behaviors.
Stress plays a significant role in both drug abuse and depression (Anisman and Zacharko 1992; Blumner and Marcus 2009; Koob 2008). In comparison with males, females exhibit greater and longer lasting behavioral, physiological, and molecular responses to social and non-social stressors (Handa et al. 1994). This heightened stress response in females may at least partially mediate the increased vulnerability to escalated drug taking and depressive-like behaviors observed in both humans and rodents (Dalla et al. 2010; Wood 2010).
Stress can induce both increased drug self-administration as well as depressive-like responses, depending on schedule and severity. In males, brief episodes of social defeat stress promote drug self-administration, whereas chronic, inescapable social defeat stress potentiates depressive-like responses and suppresses drug-reinforced behaviors (Miczek et al. 2011). When subjected to equivalent levels of episodic social defeat stress, female rats self-administer more cocaine than males (Holly et al. 2012). However, detailed studies exploring the effects of chronic inescapable stress on cocaine self-administration in females have yet to be conducted.
Repeated exposure to stressful events can also precipitate depression in vulnerable individuals. Anhedonia, marked by a lack of interest in pursuing pleasurable activities, is one of the key symptoms of depression (American Psychiatric Association 2013). One of the assessments for anhedonia-like behavior in laboratory rodents is measurement of preference or intake of a sweet solution, such as saccharin or sucrose (Willner et al. 1998). Chronic social defeat stress reduces preference and intake for both saccharin and sucrose (Miczek et al. 2011; Rygula et al. 2005), which can be reversed with antidepressant treatment (Rygula et al. 2006; Rygula et al. 2008), providing some validation for chronic social defeat stress as inducing a cardinal symptom of depression.
However, there are individual differences in how rodents respond to chronic social stress. Some mice with distinctive adaptations in the mesolimbic dopamine system have been characterized as a stress-resistant phenotype and are resistant to chronic stress and do not show stress-induced alterations in social interactions, saccharin preference, social isolation-induced hyperthermia, and circadian rhythmicity (Krishnan et al. 2007). In contrast, the stress-vulnerable phenotype exhibits these anhedonia-like responses and is also susceptible to cardiovascular (Grippo et al. 2002), endocrine (Holsboer 1995), and metabolic dysfunction (Chrousos 2000). Investigating the underlying mechanisms of individual differences in anhedonic-like responses may lead to a better understanding of the relationship between stress, depression, and substance use. Furthermore, many preclinical studies exclusively use males to investigate individual differences in stress reactivity (Anisman et al. 1998; Bergstrom et al. 2008; Berton et al. 2007; Wilkinson et al. 2009; Zurawek et al. 2013), whereas females remain significantly understudied.
In this study, female rats were tentatively characterized as stress-resistant and stress-sensitive based on their saccharin intake during chronic social defeat stress exposure. These two phenotypes exhibited a distinct pattern with respect to subsequent cocaine self-administration as well as extracellular dopamine release in the nucleus accumbens in response to an acute cocaine challenge.
Materials and methods
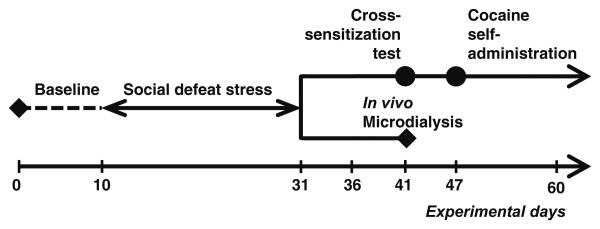
For experimental timeline, see Fig. 1.
Fig. 1. Timeline of the study.
Subjects
Female Long-Evans rats (Charles River Laboratories, Wilmington, MA) weighing 200–225 g (n=116) were used for in vivo microdialysis or behavioral sensitization followed by cocaine self-administration. Rats were individually housed in standard cages and adapted to the facility for at least 1 week after arrival. Male rats were housed in close proximity to the female rats to ensure regular estrous cycles. Separate “resident” multiparous female rats were housed in male–female pairs in large stainless steel cages (71×46×46 cm) as described previously (Shimamoto et al. 2011). The animals were housed in an environmentally controlled suite of a vivarium and procedure rooms that were kept at 21±1 °C, 35–40 % humidity on an inverted 12-h light-dark cycle (lights on at 20:00). Food and water were supplied ad libitum. During the experimental stress procedure, socially-stressed rats were housed in a protective wire mesh cage (20×30×20 cm) inside the large stainless steel cages where the resident male and female pair are co-housed in the intervals between the confrontations as described below. Following chronic social defeat stress, the rats were re-housed in custom-built polycarbonate cages (25×30×30 cm) with Cellu-Dri pellet bedding for subsequent cocaine self-administration or in vivo microdialysis experiments. All procedures were approved by the Institutional Animal Care and Use Committee of Tufts University, following NIH guidelines in the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Estrous cycle
Vaginal smears were analyzed daily to track estrous cycles. The Giemsa staining method was used for cytological examinations (Staples and Geils 1965). Vaginal smears were categorized as either pro/estrus or met/diestrus. Because the estrous phase on a first day of social defeat stress varied across rats, the regularity of estrous cycles was determined by calculating the ratio of pro/estrous to met/diestrus days during 10 days of baseline and 3 weeks of social defeat stress. Approximately 2 % of the female rats showed irregular estrous cycles during the baseline period and were excluded from further experiments.
Saccharin testing
To assess anhedonia-like responses, rats were tested for saccharin preference using a two bottle choice test during the 10-day baseline period and 3 weeks of social defeat stress. Three times per week, the rats were given access to two bottles in their home cages for 60 min, one filled with 0.02 % saccharin solution and the other with unsweetened drinking water. During the social defeat stress protocol, saccharin testing was performed prior to social defeat stress (11 a.m.–12 p.m.) to avoid acute stress effects. The position of the bottles was counterbalanced across rats. Intake of saccharin solution and water was measured and saccharin preference, defined as the volume of saccharin solution consumed divided by total intake volume, was calculated. Approximately 20 % of rats showed low (less than 90 %) saccharin preference during the baseline measurement period and were eliminated from further experiments.
Chronic social defeat stress
After collecting baseline measurements of both estrous cycles and saccharin preference, 91 rats were randomly divided into stressed (n=59) or non-stressed control (n=32) groups. Lactating dams with 3–12-day-old pups were used as aggressive stimulus rats in an adapted resident-intruder paradigm as described in detail previously (Holly et al. 2012; Shimamoto et al. 2011). Briefly, chronic social defeat stress consisted of two phases: (i) direct confrontation between the experimental rats and the “resident” female and (ii) threat period directly after the confrontation phase. For the 30 min direct confrontation, the resident male was removed and the experimental female was socially defeated by the resident female in the presence of pups, who were not removed in order to prevent reduction in maternal aggression (Lonstein and Gammie 2002). To minimize variation in aggression, experimental females were rotated between different lactating dams across social defeat sessions. After 30 min of direct confrontation, the resident male was returned and the experimental female rat was placed in a wire mesh protective cage inside the resident's large stainless steel cage with unrestricted access to food and water until the next direct confrontation occurred. This housing condition allowed for threat, while protecting the experimental female from any additional injury. This sequence was repeated twice daily for 21 consecutive days.
Behavioral cross-sensitization
Ten days after the last defeat, one cohort of rats (stressed, n= 50; control, n=26) was assessed for locomotor activity in their home cages after saline (i.p.) and cocaine (10 mg/kg, i.p.) (10 mg/kg, Covington and Miczek 2001). Prior to the test day, the rats were habituated with i.p. saline injection on three separate occasions to minimize injection stress. On the test day, the rats were first injected with saline and 5 min later their locomotor activity was recorded for 5 min, after which they were injected with cocaine. Additional 5 min observations were made beginning 5 and 25 min following the cocaine injection. Video recordings were measured by an observer (intra-observer reliability: r>0.95) for duration and frequency of walking, rearing, grooming, and immobility using a custom keyboard and computer software (The Observer Video-Pro version 9.0, Noldus Information Technology, Wageningen, The Netherlands). Vaginal smears were collected prior to testing to monitor estrous phases. Sixteen rats were not analyzed for cross-sensitization due to malfunction in video recording.
Cocaine self-administration
After behavioral sensitization testing, the rats (stressed, n=50; control, n=26) were implanted with a permanent indwelling catheter (SILASTIC silicon tubing; 0.63 mm i.d. × 1.17 mm o.d.) into the right jugular vein (Covington and Miczek 2001) under ketamine (100 mg/kg) and xylazine (3 mg/kg) anesthesia. The catheter was passed subcutaneously to the rat's back, where it exited through a small incision and was attached to a pedestal mounted to a harness system (SAI Infusion Technologies, Lake Villa, IL).
After 4 days of recovery, the rats began cocaine self-administration (0.75 mg/kg/infusion) as described previously (Covington and Miczek 2001). To ensure catheter patency, the catheters were flushed with heparinized saline (20 IU/mL) before each daily self-administration session, and 0.17-mL pulses of saline were delivered every 30 min, except during daily self-administration sessions. Cocaine self-administration was performed using an operant conditioning panel on one wall of the home cage that consisted of a stimulus light, two cue lights, and two retractable levers. Pressing the active lever resulted in an IV infusion of cocaine, followed by a 30 s time out period, during which the stimulus light was turned off. Pressing the inactive lever did not have any consequences but was recorded. Self-administration sessions were terminated after 5 h or 15 infusions.
The rats were initially trained on a fixed ratio 1 (FR1) schedule of reinforcement. Once the rats self-administered 15 infusions of cocaine (0.75 mg/kg/infusion) under an FR1 schedule in two consecutive sessions, the FR schedule was gradually increased to FR5, where every fifth lever press resulted in a cocaine infusion. After the rats exhibited reliable performance under the FR5 schedule for at least four consecutive sessions, they were tested on a progressive ratio (PR) schedule (0.3 mg/kg/infusion), where the number of lever presses for reinforcement increased in an exponential manner (Mutschler et al. 2001; Richardson and Roberts 1996). PR schedule performance was tested for at least three sessions. After completing the PR schedule, the rats were given continuous access to cocaine in a 24-h “binge” (0.3 mg/kg/infusion, FR5). Vaginal smears were collected before each session for FR, PR, and the 24-h “binge” to monitor estrous cycle. For the “binge” experiment, the smears were also collected after the session was terminated. Three rats lost catheter patency after FR and PR schedules were completed. Eighteen rats from the stressed group and 13 rats from the non-stressed control group completed the “binge,” with the remainder lost due to complications with anesthesia, loss of catheter patency, or illness/infection.
In vivo microdialysis and HPLC
After 21 days of social defeat stress, a separate group of animals (stressed, n=9; control, n=6) was used for in vivo microdialysis to assess the monoaminergic response to acute cocaine challenge (Holly et al. 2012; Miczek et al. 2011; Shimamoto et al. 2011). Approximately 1–2 days after the last social defeat, the rats underwent stereotaxic surgery with a unilateral guide cannula (Bioanalytical Systems Inc., West Lafayette, IN) aimed at the nucleus accumbens (NAc) shell with coordinates of AP, +2.1 mm from the bregma; ML, + 0.9 mm from the midline; and DV, −5.8 mm from the dura (Paxinos and Watson 1997) under ketamine (100 mg/kg) and xylazine (3 mg/kg) anesthesia. After at least 3 days of recovery period, the rats received daily injections of saline (i.p.) for 3 days prior to microdialysis to minimize injection and handling stress. The night before sample collection, the stylet in the cannula was replaced with a 2-mm active membrane probe (Bioanalytical Systems Inc., West Lafayette, IN) that was connected to a syringe filled with artificial cerebrospinal fluid (CMA Microdialysis Inc., North Chelmsford, MA). The infusion rate was set at 0.5 μL/min overnight. On the test day, the infusion rate was increased to 1.5 μL/min, and samples were collected every 10 min using a refrigerated (4 °C) fraction collector (CMA 142, CMA Microdialysis Inc., North Chelmsford, MA). Each sample vial contained 5 μL antioxidant (20 mM phosphate buffer including 25 mM EDTA-2Na, 0.5 mM ascorbic acid, pH=3.5). The first five samples served as baseline measurements of tonic dopamine (DA) and 5-HT levels. This was followed by saline (i.p.) and cocaine (10 mg/kg, i.p.), and 12 additional samples were collected to evaluate the time course of changes in DA and 5-HT concentrations. The average recovery rates of each probe for DA and 5-HT were 7.6 and 2.2 %, respectively. One rat was excluded from analysis due to irregular detection of monoamines.
DA and 5-HT samples were analyzed as described previously (Shimamoto et al. 2011; Miczek et al. 2011; Holly et al. 2012). Briefly, an HPLC system equipped with an LC10-AD pump (Shimadzu, Columbia, MD), a manual injector (model 7125; Rheodyne, Cotati, CA) with a 0.1-mL sample loop, and an electrochemical detection (ECD) system was used (DECADE II, Antec Leyden BV, Zoeterwoude, Netherlands). Monoamines were separated using a cation-exchange column (CAPCELL PAK, 1.5 mm×250 mm, 5 μm I.D., Shiseido, Tokyo, Japan) with column temperature set at 30 °C. Mobile phase consisted of 150 mM ammonium acetate, 50 mM citric acid, 27 μM EDTA, 10 % methanol, and 1 % acetonitrile with pH adjusted to 4.6. The flow rate was set at 0.2 mL/min. The concentrations of DA and 5-HT were calculated from standard curves with known concentrations of monoamines in the range (1.875–18.75 pg). Under these conditions, the limit of detection (LOD) for DA and 5-HT were 0.21 and 0.29 pg, respectively.
After sample collection, the experimental rats were perfused with 4 % paraformaldehyde solution prior to removing the brain for examination of probe placements (Fig. 4c).
Fig. 4.
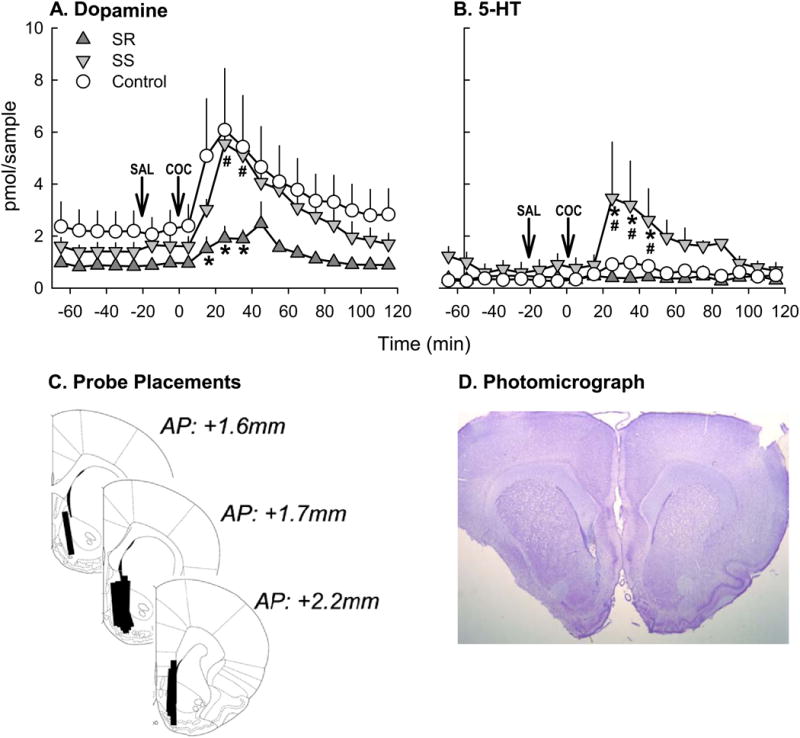
DA (a) and 5-HT (b) concentrations in the NAc shell before and after cocaine injection (10 mg/kg, i.p.) in SR (n=4), SS (n=5), and non-stressed control (n=5) female rats. *p<0.05 vs. control, #p<0.05 SS vs. SR. c Schematic of in vivo microdialysis probe placements within the NAc shell (n=14). d Photomicrograph with representative probe placement in the NAc shell
Statistical analysis
SAS for Windows V9.3 (SAS Institute, Cary, NC) was used for factor and cluster analyses, and Sigma Plot V11.0 (Systat Software, San Jose, CA) was used for all other statistical analyses.
A correlational analysis was performed to examine the relationship between the number of cocaine infusions during the 24-h “binge” and preceding saccharin intake (selected based upon factor analysis) within the stressed group (n= 18). Factor analysis and centroid hierarchical cluster analysis were used to separate the stressed rats into two phenotypes. Only factors with Eigenvalue >1 were considered.
Body weight, saccharin preference and intake, estrous cy-clicity, and locomotor activity during behavioral sensitization testing were all analyzed by two-way repeated measures analysis of variance (ANOVA). DA and 5-HT concentrations were assessed with one and two-way repeated measure ANOVA as appropriate, and cocaine self-administration data were analyzed by two-way ANOVA. When indicated by significant main effect, post hoc comparisons were performed using Holm-Sidak corrections for pairwise multiple corrections.
Results
Individual differences in saccharin intake during chronic social defeat stress
Cluster analysis revealed two subpopulations among the stressed females based on their saccharin intake during the 21-day period of social defeat stress. Based on the analysis, rats whose average saccharin consumption was less than 6 ml in 60 min were classified as stress-sensitive (SS, n=36, 72 %) while the rats showing saccharin intake greater than 6 ml were classified as stress-resistant (SR, n=14, 28 %, Fig. 2a). Saccharin intake and preference did not vary within the control group. Therefore, further comparisons were made between SS, SR, and non-stressed controls.
Fig. 2.
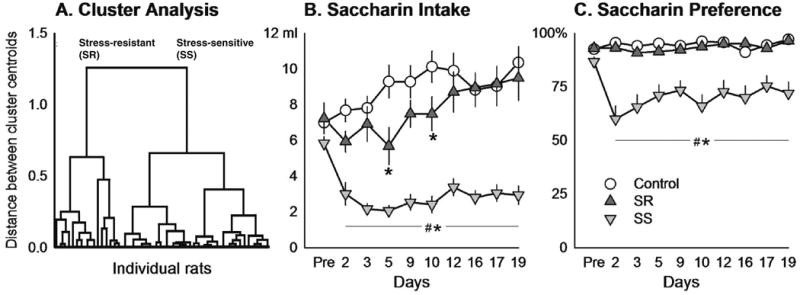
Physiological and endocrine changes during 21 days of chronic social defeat stress in females. a Characterization of females within the stressed group based on saccharin intake using cluster analysis (n=50). Females clustered separately into two distinct groups, subsequently termed stress-resistant (SR) and stress-sensitive (SS). b Average intake (ml/60 min) of 0.02 % saccharin solution before (Pre) and during social defeat stress in stress-resistant (SR, n=14), stress-sensitive (SS, n=36), and non-stressed control (n=26) female rats. *p<0.05 vs. control, #p<0.05 SS vs. SR. c Average saccharin preference (% of total 60 min fluid intake) before (Pre) and during social defeat stress in SR, SS, and control rats. Subjects and symbols are the same as in panel b
Effects of chronic social defeat stress on saccharin intake and physiological measures
Chronic social defeat stress significantly altered both saccharin intake and saccharin preference in female rats. For saccharin intake there were significant main effects of group (F(2, 657)=53.429, p<0.001), time (F(9, 657)=4.942, p<0.001), and a group×time interaction (F(18, 657)=4.416, p<0.001, Fig. 2b). In addition, for saccharin preference there was a significant group effect (F(2, 657)=18.164, p<0.001) and group×time interaction (F(18, 657)=2.750, p<0.001, Fig. 2c). There were no overall effects on water preference or intake. There were also no effects of stress on weight gain change (data not shown) or regularity of estrous cycle (F(2,207)=2.680, p= 0.076, data not shown).
Chronic social defeat stress differentially affects subsequent cocaine self-administration but not behavioral cross-sensitization in females
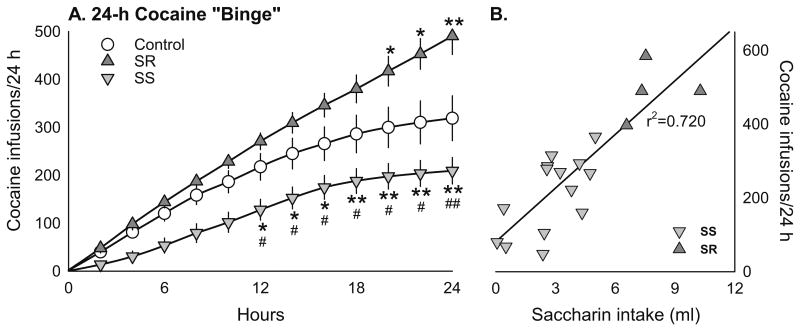
Chronic social defeat stress had a strong influence on cocaine intake during a 24-h “binge” regardless of estrous cycle phase before or after the session (Fig. 3a). There were significant effects of group (F(2, 30)=6.09, p=0.006), time (F(11, 330)= 94.21, p<0.001), and group×time interaction (F(22, 330)=3.51, p<0.001). Post hoc analysis revealed that the total number of cocaine infusions during the “binge” was significantly greater in the SR rats than in the non-stressed controls (t=2.85, p= 0.016) and SS rats (t=3.84, p=0.002). A correlational analysis revealed a significant positive correlation between the number of cocaine infusions taken by the stressed rats and their preceding saccharin intake during the course of stress exposure (r2=0.720, t=6.42, d.f. = 16, p<0.001, Fig. 3b). Chronic social defeat stress did not alter the cocaine self-administration FR response rate or responding under the PR schedule (data not shown).
Fig. 3.
a Cumulative cocaine intake (0.3 mg/kg/infusion) during a 24-h cocaine “binge” under the FR5 schedule in SS (n=14), SR (n=4), and non-stressed control (n=13) female rats. *p<0.05 vs. control, #p<0.05 SS vs. SR. b Correlation between the number of cocaine infusions during the “binge” and previous saccharin intake in SS (n= 14) and SR (n=4) rats
For behavioral cross-sensitization testing, there was no stress effect on frequency or duration of walking after cocaine challenge regardless of estrous cycle phase (data not shown). Factor analysis showed that saccharin intake, saccharin preference, and infusions during the 24-h “binge” loaded equally onto one factor (r=0.76, 0.91, and 0.79, respectively), while walking duration 5 and 25 min after cocaine injection during behavioral sensitization testing loaded equally onto a second factor (r=0.97, 0.96, respectively).
Chronic social defeat stress differentially affects extracellular DA and 5-HT levels in the NAc in response to cocaine challenge
Overall, cocaine increased DA levels in the NAc (Fig. 4a). There was a significant main effect of cocaine (F(18, 216)= 17.085, p<0.001), and group×cocaine interaction (F(36, 216)= 2.044, p<0.001), although no main effect of group was observed. DA levels after the cocaine challenge increased significantly in the non-stressed controls and the SS group 10–40 min after cocaine injection (p<0.05), whereas no such increase was observed in the SR group.
Chronic social defeat stress had a modest effect on 5-HT levels in the NAc after cocaine challenge (Fig. 4b). A marginal group effect (F(2, 162)=3.682, p=0.068) and a significant cocaine effect (F(18, 162)=2.291, p=0.003) were observed. Extracellular 5-HT was significantly greater in SS rats than in SR rats and non-stressed controls from 20–50 min after cocaine injection (p<0.05). Post-mortem histological examination confirmed that all probes were accurately positioned in the NAc shell (Fig. 4c), and a representative photomicrograph of an accurate probe placement in the NAc shell is shown in Fig. 4d. Neither DA nor 5-HT levels were influenced by estrous phase.
Discussion
The current experiments provide the first evidence for individual differences in anhedonic-like responses as a result of chronic social defeat stress in females. Moreover, we demonstrated in females that saccharin intake during chronic social defeat stress is positively correlated with cocaine self-administered during a 24-h “binge.” Using hierarchical cluster analysis, these stressed females were statistically separated into two groups, tentatively termed “stress-resistant” (SR) and “stress-sensitive” (SS). These two groups not only exhibited significant differences in the amount of cocaine self-administered during the 24-h “binge,” but also in their NAc DA response to experimenter-administered cocaine.
Characterization of chronic social defeat stress in females
The present results demonstrate distinct individual differences in intake and preference of a saccharin solution during chronic social defeat stress. These findings are concordant with previous results obtained in male mice and rats, emphasizing the importance of examining how individual subjects respond to equivalent levels of stress (Krishnan et al. 2007; Wood et al. 2010). Previous studies have focused on two distinct pheno-types, namely stress-resistant and stress-susceptible, as quantified by an array of measures such as sucrose/saccharin preference, social preference, social separation-induced hyperthermia, and circadian rhythmicity (Bergstrom et al. 2008; Krishnan et al. 2007). The proportion of females categorized as SS in the current study (72 %) is similar to the 40-70 % proportions reported in previous studies in males exposed to chronic social defeat stress (Berube et al. 2013; Der-Avakian etal. 2014; Wood etal. 2010). Thus, while the current study only focuses on saccharin intake and preference, it will be informative to examine chronically-stressed females across a range of other behavioral and physiological functions.
Stress-induced suppression in saccharin intake is associated with subsequent cocaine self-administration
A significant positive correlation was observed between saccharin intake during chronic social defeat stress and subsequent cocaine self-administration during a 24-h “binge,” indicating that differences in a putatively anhedonic response to chronic stress may predict later dysregulated cocaine taking. This finding is supported by similar studies in males showing individual differences in anhedonic-like responses during chronic stress are associated with differences in i.v cocaine self-administration and conditioned place preference (Kabbaj et al. 2001; Krishnan et al. 2007). While it is not entirely clear that saccharin intake and preference during stress is driving the correlation to subsequent cocaine self-administration during the 24-h “binge,” these variables also loaded equally onto a single factor in a factor analysis.
Female rats that exhibited reduced saccharin intake during chronic social stress (SS rats) self-administered significantly less cocaine during a 24-h “binge” than all other groups, corresponding to previous results with male rats (Lin et al. 2012; Miczek et al. 2011). One possible explanation for the decreased cocaine intake during the 24-h “binge” is that these SS females showed a reduced sensitivity to the reward effects of both saccharin and cocaine. However, as these SS females do not show altered DAergic response to cocaine nor differences in FR and PR schedule performance of cocaine self-administration compared to controls, reduced sensitivity to reward does not seem to entirely explain reduced “binge” self-administration. This finding of reduced “binge” intake appears to be at variance with clinical data demonstrating depression can aggravate subsequent drug use (Kessler et al. 2003). This apparent discrepancy may be due to the fact that many clinical patients are under the influence of antidepressants while abusing drugs (Pettinati et al. 2013). One preclinical study found that desipramine treatment reversed the suppressed cocaine self-administration in Flinders Sensitive Line, a putative genetic model of depression (Roth-Deri et al. 2009). Future work will address this issue by examining the effects of concurrent antidepressant treatment during chronic stress and/or cocaine self-administration.
In contrast, females whose saccharin intake and preference was unaffected by chronic social defeat stress (SR rats) exhibited increased cocaine self-administration during a 24-h “binge” compared to non-stressed controls. Thus, in a sense, SR females are not resistant to all effects of stress, but rather the anhedonic-like effects that can result from chronic social stress. Future work will investigate the mechanisms underlying the escalated cocaine self-administration observed in this group. However, it should be noted that both within males and females, greater preference and intake of saccharin in the absence of stress has also been positively correlated with subsequent psychostimulant self-administration (Carroll et al. 2002). As such, future studies also need to examine the association between additional measures of anhedonia-like responses and subsequent cocaine self-administration in chronically-stressed females.
Individual differences in NAc dopamine response to cocaine
Individual differences within the dopaminergic response to acute, experimenter-administered cocaine as a result of chronic social defeat stress may at least partially contribute to the observed differences in cocaine self-administration during the “binge”. Females showing reduced saccharin preference during chronic social stress, who later self-administered significantly less cocaine (SS rats), did not exhibit an altered DA response to cocaine compared to controls. Thus, stress-induced alterations within the mesolimbic dopamine system cannot fully explain the reduced cocaine self-administration observed in these animals. However, these females did show a significant effect of cocaine on NAc 5-HT, an effect not seen in controls or SR females. It is unclear from this study whether the increase in extracellular 5-HT after cocaine challenge is a result of altered expression of the serotonin transporter or increased phasic firing of serotonergic neurons. As such, future work should investigate the effect of susceptibility to social defeat stress on serotonergic signaling in both males and females.
Conversely, when those females who did not show a significant reduction in saccharin preference (SR rats) were examined separately, they showed a blunted dopamine response in the NAc. In comparison to both susceptible and control mice, male mice that are resilient to the anhedonic-like effects of chronic social defeat exhibited a significantly larger Ih current, which was paired with significantly greater K+channel-mediated peaks and sustained currents (Friedman et al. 2014). This led to an overall reduction in intrinsic excitability and current injection-evoked spike number compared to both controls and susceptible mice. A similar mechanism may be occurring in the SR females of the current study, such that the increased K+channel function may be blunting the firing of VTA DA neurons, resulting in decreased cocaine-induced efflux of DA in the NAc.
These data indicate that chronic social defeat stress may differentially alter the monoaminergic response to cocaine within the NAc in SS and SR rats. However, how these divergent responses to passive experimenter-administered cocaine relate to subsequent escalated active cocaine self-administration behavior is not immediately clear. Not only is the dopaminergic response to cocaine greater during active self-administration than passive cocaine administration (Lecca et al. 2007), the ventral striatal dopaminergic response adapts as animals gain cocaine self-administration experience (Willuhn et al. 2014). Thus, while the current in vivo microdialysis experiment may provide a clue to an altered dopaminergic or serotonergic response to initial cocaine exposure, it will be crucial to investigate the dopaminergic adaptations to repeated cocaine self-administration in these two phenotypes to elucidate a more causal role of dopaminergic function on dysregulated cocaine taking. The role of NAc dopamine in cocaine-related behaviors should also be more thoroughly investigated; as no differences between SS and SR females were observed in behavioral sensitization or FR/PR self-administration performance, there may be a differential effect on DA in each of these behaviors.
Conclusions and future directions
In conclusion, the present study demonstrated significant individual differences in response to chronic social defeat stress in female rats, as assessed by saccharin intake. Females that showed a reduction in saccharin intake during stress self-administered significantly less cocaine than controls during a 24-h “binge,” which is not associated with an altered dopaminergic response to acute experimenter-administered cocaine. On the other hand, females that did not show disruption of saccharin intake self-administered significantly more cocaine than controls during a 24-h “binge,” which may be a result of a blunted NAc dopaminergic response to cocaine.
As the tentatively termed “stress resilient” females in this series of experiments did show altered cocaine self-administration and NAc dopaminergic response to cocaine compared to controls, it is important to note that these females are vulnerable to some non-anhedonic effects of stress. Future studies should attempt to investigate innate characteristics of the mesolimbic dopamine system prior to stressful events to examine whether they may play a role in the development of subsequent resilience or susceptibility to the anhedonic-like effects of chronic social defeat stress. By identifying predetermined molecular traits, we could provide more insight into understanding vulnerability markers as predictors for maladaptive responses to chronic stress that could lead to substance use disorders. Other considerations should examine more closely the role of circulating ovarian hormones, as they may play a role in the increased vulnerability of women to develop MDD. Lastly, it is important to further validate this model of chronic social defeat stress in females by examining other anhedonic-like responses, such as social interaction or intracranial self-stimulation; examining physiological stress responses, such as corticosterone release; and incorporating antidepressant treatment strategies. Ultimately, the results reported here emphasize the importance of examining individual differences in the response to social defeat stress, particularly in females.
Acknowledgments
This research was supported by a National Institute on Drug Abuse Grant to KAM (DA031734) and a National Institute on Alcohol Abuse and Alcoholism Grant to KAM (AA013983). We appreciate the contribution of Mr. Durwood Marshall from Tufts University Technology Services for assisting with the cluster analysis. We also thank Mr. Tom Sopko for helping to prepare the manuscript.
Contributor Information
A. Shimamoto, Email: ashimamoto@mmc.edu, Department of Psychology, Tufts University, Medford, MA, USA; Department of Neuroscience and Pharmacology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd., Nashville, TN, USA.
Elizabeth N. Holly, Department of Psychology, Tufts University, Medford, MA, USA
Christopher O. Boyson, Department of Psychology, Tufts University, Medford, MA, USA
Joseph F. DeBold, Department of Psychology, Tufts University, Medford, MA, USA
Klaus A. Miczek, Department of Psychology, Tufts University, Medford, MA, USA; Department of Neuroscience, Tufts University, Boston, MA, USA
References
- American Psychiatric Association. DSM-5. 5th. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Anisman H, Lacosta S, Kent P, McIntyre DC, Merali Z. Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress. 1998;2:209–220. doi: 10.3109/10253899809167284. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J Psychiatry. 1992;(Suppl):36–43. [PubMed] [Google Scholar]
- Bergstrom A, Jayatissa MN, Mork A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE, III, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of [delta]fosb in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Berube P, Laforest S, Bhatnagar S, Drolet G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol Behav. 2013;122:237–245. doi: 10.1016/j.physbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Blumner KH, Marcus SC. Changing perceptions of depression: ten-year trends from the general social survey. Psychiatr Serv. 2009;60:306–312. doi: 10.1176/ps.2009.60.3.306. [DOI] [PubMed] [Google Scholar]
- Bracke P. The three-year persistence of depressive symptoms in men and women. Soc Sci Med. 2000;51:51–64. doi: 10.1016/s0277-9536(99)00432-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berlin) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. J Allergy Clin Immunol. 2000;106:S275–S291. doi: 10.1067/mai.2000.110163. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Psychiatry, Biol. 2014 doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, DeBold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology (Berl) 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. Neuroendocrinology of mood disorders. In: Bloom EH, Kupfer DJ, editors. Psychopharmacology-4th Generation of Progress. Raven; New York: 1995. [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di CG. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Epps SA, West CH, Boss-Williams KA, Weiss JM, Weinshenker D. Operant psychostimulant self-administration in a rat model of depression. Pharmacol Biochem Behav. 2012;103:380–385. doi: 10.1016/j.pbb.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., III Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Covington HE, 3rd, Miczek KA. Repeated self-administered cocaine binges in rats: effects on cocaine intake and withdrawal. Psychopharmacology (Berl) 2001;154:292–300. doi: 10.1007/s002130000646. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals: eighth edition. National Academy Press; Washington DC: 2011. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O'Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am J Psychiatry. 2013;170:23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Friedman A, Abraham L, Lax E, Flaumenhaft Y, Dikshtein Y, Yadid G. Antidepressant treatment facilitates dopamine release and drug seeking behavior in a genetic animal model of depression. Eur J Neurosci. 2009;30:485–492. doi: 10.1111/j.1460-9568.2009.06840.x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, Zernig G, Fuchs E, Flügge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav Pharmacol. 2008;19:183–196. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, DeBold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl) 2011;218:271–279. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples RE, Geils HD. An observation of the vaginal smear of the rat. J Endocrinol. 1965;32:263–264. doi: 10.1677/joe.0.0320263. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: volume I. Summary of National Findings. US Department of Health and Human Services; Rockville: 2010. [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, Laplant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl) 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI. The many facets of sex and drugs. Horm Behav. 2010;58:1. doi: 10.1016/j.yhbeh.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawek D, Faron-Gorecka A, Kusmider M, Kolasa M, Gruca P, Papp M, Dziedzicka-Wasylewska M. Mesolimbic dopamine D(2) receptor plasticity contributes to stress resilience in rats subjected to chronic mild stress. Psychopharmacology (Berl) 2013;227:583–593. doi: 10.1007/s00213-013-2990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]