Abstract
Peripheral blood telomere length has been associated with age-related conditions including Alzheimer’s disease (AD). This suggests that telomere length may identify subjects at increased risk of AD. Thus, we investigated the associations of peripheral blood telomere length with amnestic mild cognitive impairment (aMCI), a putative precursor of AD, among Mayo Clinic Study of Aging participants who were prospectively followed for incident aMCI. We matched 137 incident aMCI cases (mean age 81.1 years, [range 70.9–90.8]; 49.6% men) by age and sex to 137 cognitively normal controls. We measured telomere length (T/S ratio) at baseline and follow-up using quantitative PCR. Compared to the middle T/S quintile (Q3), the risk of aMCI was elevated for subjects with the shortest (Q1: HR, 2.85, 95% Confidence interval [CI] 0.98, 8.25; p = 0.05) and the longest telomere lengths (Q5: HR, 5.58, 95%CI, 2.21, 14.11; p = 0.0003). In this elderly cohort, short and long telomeres were associated with increased risk of aMCI. Our findings suggest that both long and short telomere lengths may play a role in the pathogenesis of aMCI, and may be markers of increased risk of aMCI.
Keywords: Alzheimer disease, aging cells, cohort study, leukocyte telomere length, mild cognitive impairment, population-based
1. Introduction
The persistent failure to develop effective disease-modifying therapies for Alzheimer’s disease (AD) has shifted the focus of research to identifying biomarkers for early detection in the preclinical or early clinical phase (Albert et al., 2011; Sperling et al., 2011). The rationale is that early detection using biomarkers, combined with effective intervention and treatment (when they become available) will reduce the risk of mild cognitive impairment (MCI) or progression from MCI to dementia. Peripheral blood leukocyte telomere length, a reliable surrogate for telomere length in other tissues, is a potential biomarker for early detection (Brouilette et al., 2007; Friedrich et al., 2000; Takubo et al., 2002). Telomere shortening occurs with increasing age due to repeated incomplete replications over time, and in some studies has been associated with cellular aging, mortality, and with cognitive impairment (von Zglinicki and Martin-Ruiz, 2005). The association of telomere length with cognitive impairment is not established; some studies have reported significant associations with cognition (Honig et al., 2006; Martin-Ruiz et al., 2006; Valdes et al., 2010; Yaffe et al., 2011), whereas others have not (Devore et al., 2011; Mather et al., 2010; Zekry et al., 2010b). While some of these studies have reported associations of telomere length with AD, few investigators have examined the association of telomere length with amnestic MCI, a putative precursor of AD, either prospectively or in a population-based setting. The objective of this study was to investigate the associations of peripheral blood telomere length measured at baseline with incident amnestic MCI (aMCI) in a subset of participants from the prospective, population-based, Mayo Clinic Study of Aging (MCSA).
2. Methods
2.1. Mayo Clinic Study of Aging
The details of the Mayo Clinic Study of Aging protocols have been previously published (Roberts et al., 2008). Briefly, we enumerated the Olmsted County, Minnesota population aged 70 – 89 years on October 1, 2004 and March 1, 2008. We randomly selected subjects using an age and sex stratified sampling scheme. We invited eligible subjects to an in-person evaluation that included a semi-structured interview by a nurse to assess memory and functional status and a clinical evaluation by a physician to assess cognition, cerebrovascular disease history, and a full neurological examination. Participants underwent neuropsychometric testing to assess performance in 4 cognitive domains: memory, executive function, language and visuospatial skills. Assessments for each individual were reviewed by the 3 evaluators and a diagnosis of normal cognition, MCI (amnestic MCI [aMCI] or non-amnestic [naMCI]) or dementia was assigned by consensus (Roberts et al., 2008).
2.2. Longitudinal follow-up
Follow-up of the study cohort was performed every 15–18 months using the same protocols as at baseline. To avoid bias, the evaluators are blinded to all previous clinical information when assigning a diagnosis at the follow-up visit.
2.3. Standard protocol approvals, registrations, and patient consents
All study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All subjects provided signed informed consent to participate.
2.4. Study sample
To avoid prevalence bias, we used a nested case-control design. From among 437 subjects who developed MCI during follow-up, we identified all incident aMCI cases who had DNA at baseline (n = 137). We individually matched each aMCI case by age at blood draw (± 2 years), sex, number of follow-up visits (± 2), and duration of follow-up (± 2 years) to cognitively normal controls (1:1 matching) selected from 691 eligible controls. A control had to be alive at the diagnosis of aMCI in the case, have DNA at baseline, at least one follow-up, and no diagnosis of aMCI.
2.5. Covariates
Demographic information was assessed by interview. Potential confounders (type 2 diabetes, cardiac disease, hypertension and stroke) were abstracted from the medical record. Body mass index was computed from measured weight and height, and the frequency of moderate physical exercise in the previous year or in midlife was assessed by questionnaire at baseline (Geda et al., 2010). Apolipoprotein E (APOE) genotyping was performed at baseline. The APOE ε4 allele is the most established genetic risk factor for late onset AD.
2.6. Measurement of telomere length
DNA was extracted from peripheral blood obtained at baseline and at each follow-up visit using Gentra AutoPure with Puregene chemistry. Relative telomere length (T/S ratio) was measured in peripheral blood DNA using the quantitative PCR method and primers to the telomeric hexamer repeats developed by Cawthon to amplify telomeric DNA (Cawthon, 2002). Two master mixes of PCR reagents were prepared using the T and the S primer pairs. 17 µL of T master mix was added to sample well, control well, and standard curve well of the first plate and 17 µL of S master mix was added to sample, control and standard curve well of the second plate. For each sample, triplicates of the DNA sample (5 ng/µL) were added to plate 1 and to the same well position in plate 2. For each standard curve, one reference DNA sample was serially diluted in TrisEDTAbuffer (TE) by 1:2-fold per dilution to produce 8 concentrations of DNA ranging from 0.78 to 50 ng/mL. Two microliters of each concentration was distributed to the standard curve wells on each plate. The plates were centrifuged for analysis using the ABI 7900HT instrument. The T and S PCRs were prepared identically with the exception of the oligonucleotide primers. The final concentrations of the reagents in the PCR were 15 mmol/L Tris–HCl, 0.2 mmol/L each dNTPs, 2.0 mmol/L MgCl2, 1% dimethyl sulfoxide, 150 nmol/L ROX dye, 0.2 × SYBR Green I (Molecular Probes), 5 mmol/L DL-Dithiothreitol (DTT), 1.25 U AmpliTaq Gold DNA polymerase (Applied Biosystems). The final telomere primer concentrations were tel 1b, 600 nmol/L; tel 2b 900 nmol/L. DNA was quantitated with PICO green. The S control gene (B2-globin on chromosome 11) concentrations were B-2 globin forward primer (hbg1) 300 nmol/L; B-2 globin reverse primer (hbg2) 700 nmol/L. The primer sequences (5’ – 3’ were: tel 1b CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT; tel 2b GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT; hbg1 GCTTCTGACACAACTGTGTTCACTAGC; hbg2 CACCAACTTCATCCACGTTCACC. All PCRs were run on the ABI Fast Real-Time 7900HT (Applied Biosystems). Other details have been previously described (Cawthon, 2002; Skinner et al., 2012).
The average telomere length for a sample was determined by comparing the intensity of the sample’s telomere signal (T) to that of a single-copy gene (S) of a reference DNA sample. The T/S ratio was computed using the median T and S of triplicates for each sample. Samples with a coefficient of variation greater than 10% were re-assayed in triplicate.
2.7. Inter- and intra-assay variability
We determined intra and inter-assay variability using stock DNA from one individual. For intra-assay variability, we compared results from the same specimen on which 25 telomere length measurements were performed on one PCR plate (one plate for telomere PCR, one plate for a standard hemoglobin (HBG) gene PCR). To determine inter-assay variability, 25 telomere length measurements from the same specimen were determined from one pair of telomere and HBG PCR plates and compared to a separate set of 25 telomere length measurements determined from a separate pair of telomere and HBG PCR plates performed on the same specimen. Our intra- and inter-assay variability was 3% and 3.5%, respectively, consistent with other studies.
2.8. Southern blots
We measured telomere lengths in 13 subjects (10 cases with MCI and 3 cognitively normal controls) using Southern blot TRF assays. MCI cases with short or long telomeres based on qPCR displayed heterogeneity in base pair length. There was consistency in telomere length by qPCR and TRF assays. Persons classified as having long telomeres by qPCR (samples 10 through 17) had longer average telomere lengths by Southern blot compared to those classified as having short telomeres (eFigure).
2.9. Statistical analyses
We categorized baseline T/S ratio into quintiles based on the distribution for the controls. We examined the associations of T/S quintiles with aMCI using conditional logistic regression models stratified on matched pairs, and adjusted for education (age and sex were matching variables). We also used unconditional logistic regression models that included age at blood draw, sex (both matching variables), and education (base model), to account for any residual confounding not captured by the conditional analyses. We examined potential confounding by APOE ε4 allele, obesity, diabetes, frequency of moderate exercise (≥ 1 vs. none per week) (Geda et al., 2010), cigarette smoking, cardiac disease, and stroke, with each variable added separately. Variables that were significantly associated with aMCI or T/S were simultaneously included in the full models. We also examined interaction of T/S ratio quintiles with age, sex, APOE ε4 allele, type 2 diabetes, and exercise.
Proportional hazards models: We also used proportional hazards models for nested case-control designs stratified on the matched pairs to take into account the prospective component of the study. To account for sampling of controls and to enhance representativeness of matched controls to the total eligible control cohort, we assigned a weight of 1 to cases and a weight equal to the inverse of the sampling fraction to controls (Ganna et al., 2012). We also performed analyses without stratifying on matched pairs (but included age at blood draw and sex as covariates) as was done for the logistic models to account for any residual confounding.
Finally, we used general additive models with a spline to test for a non-linear association of T/S ratio with aMCI case-control status (Nelder and Wedderburn, 1972; Stone, 1985). We used the log2 transformation of T/S ratio for all the analyses, given the skewed data and were back-transformed where necessary. Associations were considered significant at a p value < 0.05; and analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary NC).
3. Results
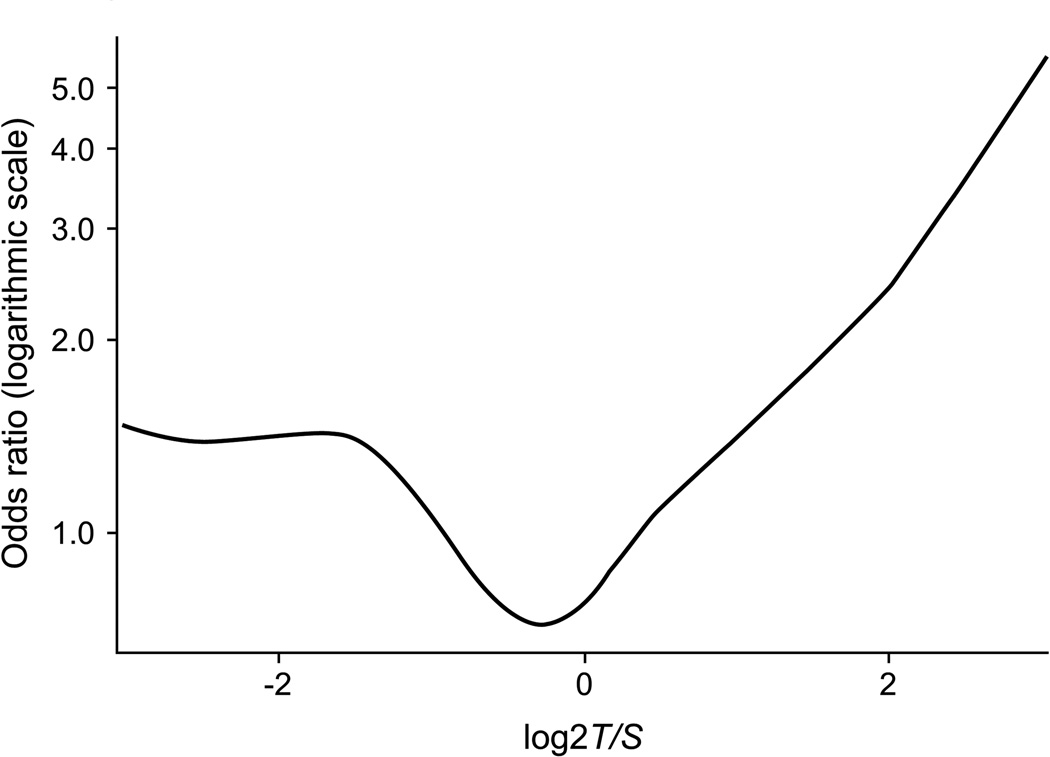
Table 1 shows the characteristics of aMCI cases and matched cognitively normal controls. In this elderly 71–90 year old cohort, there was no difference in log2 T/S in cases vs. controls (p = 0.44). There was no correlation of T/S ratio with age in aMCI cases and controls (combined; r = −0.089, p = 0.14; in controls: r = −0.112, p = 0.19; or in cases; r = −0.079, p = 0.36). There was also no sex difference in log2 T/S ratio (mean [range]) in men (−0.46 [−2.78–2.99]) vs. women (−0.46 [−3.03–2.51]; p = 0.84). Generalized additive models demonstrated a significant non-linear association between T/S and log odds of aMCI in a model adjusted for age, gender, education, APOE ε4 allele, and type 2 diabetes (p = 0.04; Fig. 1).
Table 1.
Baseline characteristics of amnestic MCI cases and matched controls.a
| Variable | aMCI cases (n=137) |
CN Controls (n=137) |
|---|---|---|
| Age, mean [SD] | 81.1 [5.1] | 81.0 [4.8] |
| (range) yrb | (70.9 – 90.8) | (71.0 – 90.1) |
| Men | 68 (49.6) | 68 (49.6) |
| Education, yr mean (SD) | 13.6 (3.0) | 14.3 (3.2) |
| APOE ε4 allele, any ε4 | 41 (29.9) | 28 (20.4) |
| Follow-up yrs, mean (SD) | 3.9 (1.3) | 3.9 (1.3) |
| Diabetes | 32 (23.4) | 17 (12.4)c |
| Hypertension | 112 (81.8) | 108 (78.8) |
| Stroke | 16 (11.7) | 13 (9.5) |
| Latelife BMI > 30 kg/m2,d | 36 (26.5) | 35 (25.7) |
| Midlife BMI > 30 kg/m2,e | 35 (29.4) | 23 (19.8) |
| Moderate exercisef | 99 (76.2) | 104 (78.8) |
| Cigarette smoking, ever | 73 (53.3) | 63 (46.0) |
| T/S ratio median (range) | 0.70 (0.12, 7.95) | 0.67 (0.15, 4.25) |
| Log2 T/S ratio mean [SD], range | −0.38 [1.1] | −0.54 [0.8] |
| (−3.0 – 3.0) | (−2.8 – 2.1) |
Estimates are n (%) or mean (range) unless otherwise stated; median (range) is provided when the variable is not normally distributed. Percents are based on non-missing data.
MCI, mild cognitive impairment; CN, cognitively normal.
Characteristics for amnestic MCI and their matched cognitively normal controls.
Age at sample draw; mean ([SD] (range).
p-value < 0.05.
Data were missing for 1 aMCI case and 1 CN control.
Data were missing for 18 aMCI cases and 21 CN controls.
Data were missing for 7 aMCI cases and 5 CN controls.
Fig. 1.
Odds ratio (logarithmic scale) for association of amnestic mild cognitive impairment with log2 T/S ratio using a generalized additive model adjusted for age, sex, education, APOE ε4 allele and type 2 diabetes. The spline demonstrates a significant non-linear association of log2 T/S with amnestic MCI (p = 0.04). X-axis tick marks represent T/S ratios of 0.25 (log2 = −2), 1 (log2 = 0), and 4 (log2 = 2).
3.1. Associations of T/S ratio with aMCI
Conditional logistic regression models: Compared to the middle quintile (Q3, reference) the OR for aMCI was non-significantly elevated nearly 2-fold for the shortest telomere length (Q1), and significantly elevated for the longest telomere length (Q5) in both the conditional (stratified on matched pairs) and unconditional models (Table 2). The results of unconditional logistic regression models were essentially the same and the data are not reported.
Table 2.
Cross-sectional associations of telomere length (T/S ratio) with amnestic MCI.
| Status |
Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| Variable | aMCI Cases N (%) |
Controls N (%) |
Odds Ratio (95% CI) |
p Value |
Odds ratio (95% CI) |
p Value |
| Conditional modela | ||||||
| T/S quintiles | ||||||
| Q1: <0.43 | 27 (19.9) | 22 (16.2) | 1.63 (0.70, 3.79) | 0.25 | 1.81 (0.76, 4.31) | 0.18 |
| Q2: 0.43 – 0.59 | 28 (20.6) | 31 (22.8) | 1.38 (0.66, 2.89) | 0.39 | 1.41 (0.66, 3.03) | 0.38 |
| Q3: 0.59 – 0.78 | 20 (14.7) | 32 (23.5) | 1.00 (reference) | -- | 1.00 (reference) | -- |
| Q4: 0.78 – 1.16 | 23 (16.9) | 31 (22.8) | 1.19 (0.53, 2.64) | 0.68 | 1.24 (0.54, 2.85) | 0.61 |
| Q5: > 1.16 | 38 (27.9) | 20 (14.7) | 2.76 (1.28, 5.98) | 0.01 | 3.05 (1.36, 6.85) | 0.007 |
MCI; mild cognitive impairment.
Conditional logistic regression models for matched cases and controls. Model 1 is adjusted for education (age and sex are included in the matching variables); model 2 additionally adjusts for APOE ε4 allele and type 2 diabetes.
Proportional hazards models: Compared to Q3, the hazard ratio (HR) of aMCI was non-significantly elevated for those with the shortest telomeres (Q1) and was significantly elevated for those with the longest telomeres (Q5) (Table 3). The unstratified analyses yielded essentially the same results; however, the association with short telomeres was statistically significant (HR, 1.98 (95% CI, 1.10, 3.54; p = 0.02) in both the base model and in the multivariable model additionally adjusted for APOE ε4 allele and type 2 diabetes (HR, 1.86, 95% CI, 1.03, 3.36; p = 0.04), and the association with long telomere also persisted in both the base (HR, 3.33, 1.90, 5.81; p < 0.0001) and in the multivariable (HR, 3.19, 95% CI, 1.81, 5.62; p < 0.0001) models.
Table 3.
Prospective association of telomere length (T/S ratio) with amnestic MCI
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Variable | Cases N (%) |
Controls N (%) |
Hazard Ratio (95% CI) |
p Value |
Hazard Ratio (95% CI) |
p Value |
| Matched analysisa | ||||||
| T/S quintiles | ||||||
| Q1: <0.43 | 27 (19.9) | 22 (16.2) | 2.85 (0.98, 8.25) | 0.05 | 2.54 (0.88, 7.32) | 0.09 |
| Q2: 0.43 – 0.59 | 28 (20.6) | 31 (22.8) | 2.02 (0.79, 5.18) | 0.14 | 1.95 (0.77, 4.92) | 0.16 |
| Q3: 0.59 – 0.78 | 20 (14.7) | 32 (23.5) | 1.00 (reference) | -- | 1.00 (reference) | -- |
| Q4: 0.78 – 1.16 | 23 (16.9) | 31 (22.8) | 1.45 (0.54, 3.92) | 0.47 | 1.42 (0.52, 3.83) | 0.49 |
| Q5: > 1.16 | 38 (27.9) | 20 (14.7) | 5.58 (2.21, 14.11) | 0.0003 | 5.04 (1.98, 12.84) | 0.0007 |
Proportional hazards models for nested case-control analyses stratified on matched pairs. Model 1 was adjusted for education; Model 2 also included APOE ε4 allele and type 2 diabetes.
Additional adjustment for exercise and obesity did not alter the findings for cross-sectional or prospective analyses and are not reported. There were no significant associations of Q2 or Q4 with aMCI, and no significant interactions of T/S with age, sex, type 2 diabetes, APOE ε4 allele or exercise.
Secondary analyses: Given the findings, we post hoc compared aMCI risk factors in cases and controls in Q1 and Q5. There were no differences in Q1 cases and controls in regard to APOE ε4 allele frequency (25.9% vs. 22.7%; p = 0.80), midlife obesity (23.1 vs. 21.1, p = 0.87), history of type 2 diabetes (22.2% vs. 13.6%, p = 0.44), and frequency of moderate exercise in midlife (65.4% vs. 77.3%, p = 0.37). By contrast, Q5 cases had non-significantly higher frequency of APOE ε4 allele (31.6% vs. 15.0% for controls; p = 0.17), midlife obesity [29.4% vs. 16.7%; p = 0.31), history of type 2 diabetes (21.1% vs. 15.0%, p = 0.58); and a lower frequency of exercise in midlife (63.9% vs. 75%; p = 0.39) than controls.
4. Discussion
In this elderly cohort with a restricted age range at enrollment, we observed several interesting and novel findings. The risk of aMCI was elevated in persons who had the shortest and longest telomere length at baseline when compared to the middle quintile, but there was no association with second and fourth quintiles. The association between telomere length and aMCI was non-linear.
The association of short telomere length with incident aMCI in our prospective analyses is in keeping with current hypothesis about telomere shortening as a marker for cognitive aging. The association with aMCI suggests that neurodegenerative mechanisms may be involved in the pathologic process. The association of long telomere length with aMCI is relatively novel. The underlying mechanisms for this association are not clear. The secondary analyses that showed a higher frequency of known risk factors for aMCI among Q5 cases compared to controls, but no differences among Q1 cases and controls, raises the question that telomere length may be surrogate marker for etiologic mechanisms or risk factors that influence cognition, rather than a direct predictor of cognitive outcomes. An alternative hypothesis is that long telomeres may be an indicator of risk of cognitive impairment at older ages. Possibly, a decline in telomere length beyond a critical threshold in some older persons may trigger telomere lengthening but may not necessarily result in improved cognition.
Potential mechanisms for telomere lengthening have been described and include telomerase activation and alternative lengthening of telomeres (ALT). With telomerase activation, telomerase elongates telomeres in certain cells, including brain cells, in response to cell damage (Greider and Blackburn, 1985; Mattson, 2000). Consistent with this, some studies have reported telomerase activation and longer hippocampal telomeres in AD brain tissue than in non-demented tissue (Thomas et al., 2008; Wikgren et al., 2012a). ALT is a homologous recombination-based mechanism that uses a DNA template to preserve telomere length, and results in very long telomeres (Bryan et al., 1995) and ALT-associated promyelocytic leukemia bodies (Muntoni and Reddel, 2005). We did not test for these mechanisms, but our findings suggest that these may be important pathways for future studies of both aMCI and AD.
Our findings are consistent with other studies. The increased risk of aMCI with longer telomere length is consistent with a study among persons from a memory clinic which reported that MCI cases who progressed to dementia had significantly longer telomere lengths compared to stable MCI (Moverare-Skrtic et al., 2012). Two cross-sectional studies reported associations of longer telomeres with worse performance on tests of episodic memory, but only in APOE ε4 carriers (Wikgren et al., 2012a; Wikgren et al., 2012b). Another study found longer telomeres in AD brain tissue than in control brains (Thomas et al., 2008).
The significant association of short telomeres with aMCI in our study is also consistent with other studies. Short telomeres were associated with MCI in the Nurses Health Study (Grodstein et al., 2008), with worse test scores in Mini Mental Status Exam, epidsodic memory and executive function in Chinese men (Ma et al., 2013), and verbal fluency in a Scottish cohort (age 79 years, n = 190) (Harris et al., 2006).
Similarly, shorter telomere length was associated with an increased risk of dementia in a large community-based nested case-control study (Honig et al., 2012; Honig et al., 2006). AD cases had have been reported to have shorter telomeres than controls in some cross-sectional studies (Hochstrasser et al., 2012); (Thomas et al., 2008); (mean age 70 years, range 64–89, n = 15) (Panossian et al., 2003).
Contrary to our findings, some longitudinal studies have observed beneficial effects of longer telomeres on cognitive performance and dementia (Devore et al., 2011; Martin-Ruiz et al., 2006; Yaffe et al., 2011). In a large multi-ethnic cohort of non-demented participants longer telomere length was associated with better scores in the Digit Symbol Substitution Test at baseline, and with less cognitive decline (Yaffe et al., 2011). In the Nurses Health Study, longer telomeres were modestly associated with slower cognitive decline (Devore et al., 2011). In a clinic-based non-representative study of stroke survivors, longer telomere length was associated with less cognitive decline and a reduced risk of dementia (Martin-Ruiz et al., 2006).
Other investigators have observed no association of telomere length with cognition in cross-sectional or longitudinal studies. In a non-representative cohort of old persons recruited from a geriatic hospital telomere length was not associated with incident MCI, MCI progression to dementia, or dementia (Zekry et al., 2010a; Zekry et al., 2010b). In two younger cohorts of narrow age range (mean age 47 years, and mean age 66.7 years), telomere length was not associated with changes in cognition (Mather et al., 2010). Other clinic-based cross-sctional studies have found no association of telomere length with MCI, with Mini-Mental Status Exam scores (Hochstrasser et al., 2012), or with dementia (Lof-Ohlin et al., 2008).
Several factors may contribute to inconsistencies across studies on telomere length and cognition (Mather et al., 2011). These include wide inter- and intra-person variability in telomere lengths, particularly in older persons (Martin-Ruiz et al., 2005); accelerated loss of shorter telomeres and greater mortality and cardiovascular disease in persons with shorter telomeres, resulting in a potential bias toward a greater number of subjects with longer telomeres in longitudinal studies (Mather et al., 2011; Mattson, 2000). Other factors are a narrow age range (Mather et al., 2010), unmeasured confounders (Mather et al., 2011), study design issues: cross-sectional vs. prospective design, cognitive tests used, covariates considered, short study duration, clinic- vs. population-based, small study sample, and method of measurement of telomere length (Mather et al., 2011).
We did not observe age and or sex differences in telomere length. The lack of an association with age may be due to the older and restricted age of the cohort (mean age 81.6; range 71–90 years). Consistent with our findings, other investigators have not observed sex differences in telomere length (Hochstrasser et al., 2012; Zekry et al., 2010b). Some studies among middle aged subjects, however, have demonstrated longer telomere lengths in adult women (Adams et al., 2007; Aviv et al., 2005; Bischoff et al., 2005; Bischoff et al., 2006; Der et al., 2012; Nordfjall et al., 2010; Wikgren et al., 2012b; Yaffe et al., 2011). One study found an inverse association of telomere length with age and longer telomere lengths in men than in women (Honig et al., 2012). We did not find interactions of telomere length with APOE ε4 carrier status in regard to risk of aMCI as some investigators have reported for other cognitive endpoints (Honig et al., 2006; Wikgren et al., 2012a; Wikgren et al., 2012b; Yaffe et al., 2011).
A potential limitation to our study is that the older age of the cohort may have contributed to greater intra- and inter-person variability in telomere length. Second, it is possible that peripheral blood telomere length may not be representative of brain cell telomere length. However, this remains to be determined in our future studies using autopsy cases. Finally, our study population was primarily of Northern European ancestry, thus extrapolations to other ethnicities should be performed with caution.
Our findings suggest that both short or long telomeres are associated with aMCI. They also suggest that telomere length alone may not be a reliable marker for risk stratification for aMCI; other factors will need to be taken into account. These data require replication in large prospective studies initiated in midlife. Studies examining telomere lengthening mechanisms in peripheral blood and brain tissue may further clarify the role of telomere length on risk of aMCI and AD, and help develop strategies for early detection of aMCI in late life.
Supplementary Material
Southern blot TRF assay measurement of base pair length (y-axis) in 10 MCI cases (sample ID 1 through 19): 5 cases classified in the shortest quintile based on qPCR, and 5 classified in the longest quintile by q PCR) and 3 controls with long telomeres based on qPCR.
Highlights.
The association of telomere length with cognitive impairment is non-linear.
Short telomere lengths are associated with risk of amnestic MCI in the elderly
Long telomere lengths are associated with amnestic MCI in the elderly
Telomere length alone may not be a reliable marker for early detection of aMCI.
Acknowledgments
The study was supported by the National Institute on Aging U01 AG006786, K01 AG028573, the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program, the Mayo Foundation for Medical Education and Research, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, von Zglinicki T. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6:125–128. doi: 10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Shay J, Christensen K, Wright W. The longevity gender gap: are telomeres the explanation? Sci Aging Knowledge Environ 2005. 2005:pe16. doi: 10.1126/sageke.2005.23.pe16. [DOI] [PubMed] [Google Scholar]
- Bischoff C, Graakjaer J, Petersen HC, Jeune B, Bohr VA, Koelvraa S, Christensen K. Telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8:425–432. doi: 10.1375/183242705774310079. [DOI] [PubMed] [Google Scholar]
- Bischoff C, Petersen HC, Graakjaer J, Andersen-Ranberg K, Vaupel JW, Bohr VA, Kolvraa S, Christensen K. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der G, Batty GD, Benzeval M, Deary IJ, Green MJ, McGlynn L, McIntyre A, Robertson T, Shiels PG. Is telomere length a biomarker for aging: cross-sectional evidence from the west of Scotland? PLoS One. 2012;7:e45166. doi: 10.1371/journal.pone.0045166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Prescott J, De Vivo I, Grodstein F. Relative telomere length and cognitive decline in the Nurses' Health Study. Neurosci. Lett. 2011;492:15–18. doi: 10.1016/j.neulet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Ganna A, Reilly M, de Faire U, Pedersen N, Magnusson P, Ingelsson E. Risk prediction measures for case-cohort and nested case-control designs: an application to cardiovascular disease. Am. J. Epidemiol. 2012;175:715–724. doi: 10.1093/aje/kwr374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch. Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses' health study. PLoS One. 2008;3:e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci. Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Hochstrasser T, Marksteiner J, Humpel C. Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp. Gerontol. 2012;47:160–163. doi: 10.1016/j.exger.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch. Neurol. 2012;69:1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann. Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Lof-Ohlin ZM, Hagnelius NO, Nilsson TK. Relative telomere length in patients with late-onset Alzheimer's dementia or vascular dementia. Neuroreport. 2008;19:1199–1202. doi: 10.1097/WNR.0b013e3283089220. [DOI] [PubMed] [Google Scholar]
- Ma SL, Lau ES, Suen EW, Lam LC, Leung PC, Woo J, Tang NL. Telomere length and cognitive function in southern Chinese community-dwelling male elders. Age Ageing. 2013;42:450–455. doi: 10.1093/ageing/aft036. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Anstey KJ, Milburn PJ, Easteal S, Christensen H. Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: a population study. BMC Geriatr. 2010;10:62. doi: 10.1186/1471-2318-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J. Gerontol. A. Biol. Sci. Med. Sci. 2011;66:202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Emerging neuroprotective strategies for Alzheimer's disease: dietary restriction, telomerase activation, and stem cell therapy. Exp. Gerontol. 2000;35:489–502. doi: 10.1016/s0531-5565(00)00115-7. [DOI] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Johansson P, Mattsson N, Hansson O, Wallin A, Johansson JO, Zetterberg H, Blennow K, Svensson J. Leukocyte telomere length (LTL) is reduced in stable mild cognitive impairment but low LTL is not associated with conversion to Alzheimer's disease: a pilot study. Exp. Gerontol. 2012;47:179–182. doi: 10.1016/j.exger.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Muntoni A, Reddel RR. The first molecular details of ALT in human tumor cells. Hum. Mol. Genet. 2005;14(Spec No. 2):R191–R196. doi: 10.1093/hmg/ddi266. [DOI] [PubMed] [Google Scholar]
- Nelder JA, Wedderburn RWM. Generalized linear models. J R Statist Soc A. 1972;135:370–384. [Google Scholar]
- Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Roos G. Large-scale parent-child comparison confirms a strong paternal influence on telomere length. Eur. J. Hum. Genet. 2010;18:385–389. doi: 10.1038/ejhg.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol. Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HG, Gangnon RE, Litzelman K, Johnson RA, Chari ST, Petersen GM, Boardman LA. Telomere length and pancreatic cancer: a case-control study. Cancer Epidemiol. Biomarkers Prev. 2012;21:2095–2100. doi: 10.1158/1055-9965.EPI-12-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CJ. Additive regression and other nonparametric models. Ann Stat. 1985;13:689–705. [Google Scholar]
- Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura K. Telomere lengths are characteristic in each human individual. Exp. Gerontol. 2002;37:523–531. doi: 10.1016/s0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Thomas P, NJ OC, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech. Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Deary IJ, Gardner J, Kimura M, Lu X, Spector TD, Aviv A, Cherkas LF. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol. Aging. 2010;31:986–992. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- Wikgren M, Karlsson T, Lind J, Nilbrink T, Hultdin J, Sleegers K, Van Broeckhoven C, Roos G, Nilsson LG, Nyberg L, Adolfsson R, Norrback KF. Longer leukocyte telomere length is associated with smaller hippocampal volume among non-demented APOE epsilon3/epsilon3 subjects. PLoS One. 2012a;7:e34292. doi: 10.1371/journal.pone.0034292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Karlsson T, Nilbrink T, Nordfjall K, Hultdin J, Sleegers K, Van Broeckhoven C, Nyberg L, Roos G, Nilsson LG, Adolfsson R, Norrback KF. APOE epsilon4 is associated with longer telomeres, and longer telomeres among epsilon4 carriers predicts worse episodic memory. Neurobiol. Aging. 2012b;33:335–344. doi: 10.1016/j.neurobiolaging.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, Simonsick EM, Kuller L, Li R, Ayonayon HN, Rubin SM, Cummings SR. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol. Aging. 2011;32:2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekry D, Herrmann FR, Irminger-Finger I, Graf C, Genet C, Vitale AM, Michel JP, Gold G, Krause KH. Telomere length and ApoE polymorphism in mild cognitive impairment, degenerative and vascular dementia. J. Neurol. Sci. 2010a;299:108–111. doi: 10.1016/j.jns.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Zekry D, Herrmann FR, Irminger-Finger I, Ortolan L, Genet C, Vitale AM, Michel JP, Gold G, Krause KH. Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiol. Aging. 2010b;31:719–720. doi: 10.1016/j.neurobiolaging.2008.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Southern blot TRF assay measurement of base pair length (y-axis) in 10 MCI cases (sample ID 1 through 19): 5 cases classified in the shortest quintile based on qPCR, and 5 classified in the longest quintile by q PCR) and 3 controls with long telomeres based on qPCR.