Abstract
Rationale
While alcohol intoxication is known to increase disinhibited behavior, the degree to which disinhibition occurs appears to depend on a number of factors including executive functioning ability. However, the neural mechanisms by which individual differences in executive functioning lead to variable degrees of disinhibition remain unclear.
Objectives
The aim of the current study was to examine the neural mechanisms by which individual differences in WM capacity moderate alcohol-induced disinhibition.
Methods
Seventeen heavy drinking males participated in a within-subjects design in which two sessions were completed: an alcohol session (.82g/kg) and a control session. Participants completed a Go/No-go task while undergoing functional magnetic resonance imaging (fMRI) after ingestion of the control or alcohol beverage. WM capacity was measured using an operation span task.
Results
Significant interactions of session and WM capacity emerged in contrasts examining successful response inhibition within superior temporal gyrus and unsuccessful inhibition in regions within the default mode network. In all cases, individuals with low WM capacity demonstrated a relative decrease in blood oxygen level dependent (BOLD) response during the alcohol compared to control session whereas the high WM capacity group demonstrated relative increases in BOLD response in the alcohol compared to control session.
Conclusions
Low WM capacity appears to be associated with decreased neural response to signals indicating a need for behavioral control, an effect that may lead to increased difficulty with inhibiting responses and increased negative consequences from alcohol intoxication.
Keywords: Disinhibition, alcohol intoxication, working memory, fMRI, dorsal ACC
The historical observation that alcohol intoxication promotes impulsive, disinhibited behavior has led to considerable research on alcohol’s acute effects on cognitive and behavioral control. Experimental studies generally support the notion that intoxication facilitates aggressive, risky or otherwise disinhibited behavior (Ito et al. 1996; Giancola and Corman 2007; George and Stoner 2000; Rehm et al. 2011). Identifying mechanisms of these effects could facilitate understanding of alcohol’s association with negative behavioral outcomes, and potentially inform knowledge of self-regulatory processes central to the etiology of alcohol use disorders, including impaired control over drinking (Field et al., 2010; Weafer & Fillmore, 2008).
Several theoretical models have been offered to explain alcohol-related increases in disinhibited behavior (e.g., Steele and Josephs 1990; Fillmore et al. 1999; Finn et al. 1999; Giancola 2000). Common to these theories is the notion that alcohol-induced deficits in behavioral control reflect acute impairment of neurocognitive mechanisms critical to ongoing behavioral monitoring, conflict detection, and response regulation (Steele and Josephs 1990; Easdon and Vogel Sprott 2000; Giancola 2000; Curtin et al. 2001; Easdon et al. 2005). Specifically, acute alcohol preferentially affects performance when there is a requirement to disengage from a prepotent response set in order to execute an alternate, adaptive response, such as in tasks that measure response inhibition (Curtin et al. 2001; Marczinski and Fillmore 2003; Marczinski and Fillmore 2005). In these tasks, initiation of a prepotent behavioral response set (e.g., responding rapidly to serial presentations of a “go” stimulus) is followed by the introduction of infrequent cues that signal the need for an alternative response (e.g., withholding behavior in response to a “stop” cue). A common finding is that alcohol impairs performance on trials requiring a shift in response set (e.g., inhibit prepotent responses), while often leaving intact the ability to execute the prepotent response (Mulvihill et al. 1997; Fillmore et al. 1999; Marczinski and Fillmore 2005).
At the neural level, alcohol appears to attenuate neural activity signaling evaluative (i.e., detecting response conflict) and regulative (i.e., adjusting behavior accordingly) components of cognitive control (Ridderinkhof 2002; Curtin and Fairchild 2003; Easdon et al. 2005; Schuckit et al. 2011; Bartholow et al. 2012). Alcohol intoxication is associated with reduced error related negativity to errors without (Ridderinkhof 2002) and with explicit feedback (Euser et al. 2011; Nelson et al. 2011). Studies using fMRI have largely corroborated evidence from the ERP literature, with alcohol exerting its effects selectively on the detection of response conflict (Marinkovic et al. 2012) and subsequent errors (Anderson et al. 2010). These effects have been localized to dorsal ACC, rostral ACC, and bilateral insula, and result from greater BOLD signal in placebo compared to intoxicated sessions. In addition, the experience of errors appears to be associated with decreased suppression of default mode activation during intoxication, possibly suggesting reduced ability to engage control networks and disengage from the prepotent response set (Anderson et al. 2010).
Notably, both animal (Poulos et al. 1998) and human (Weafer & Fillmore 2008) studies suggest individual variability in alcohol-induced impulsivity or response inhibition. Moreover, both theoretical considerations (Field et al. 2010) and empirical data (Weafer & Fillmore 2008), suggest that these differences have potential implications for consumption patterns. Identifying neurocognitive processes relevant for behavioral dysregulation during intoxication therefore has implications for alcohol use disorder etiology (Field et al. 2010), making it important to identify factors that predict liability for alcohol-related impairment. For example, one model of impulsivity suggests that lower executive functioning predicts a greater increase in impulsive behavior under intoxicated versus sober conditions (Finn et al. 1999). This prediction was supported in that individuals with lower working memory capacity demonstrated more impulsive responding (as measured by a Go/NoGo Task) under intoxicated versus sober conditions, a finding not observed among those with higher working memory capacity (Finn et al. 1999).
The current study used fMRI to examine acute alcohol effects on BOLD response during response inhibition. The study addressed two primary aims. First, given the recent emergence of neuroimaging work in this area (Anderson et al. 2010; Marinkovic et al. 2011; Marinkovic et al. 2013), we aimed to further characterize brain regions associated with alcohol-induced changes in neural activation during response inhibition. Based on evidence from EEG studies (Ridderinkhof 2002) and initial fMRI studies (Anderson et al. 2010; Marinkovic et al. 2011; Marinkovic et al. 2013), we predicted that participants would display reduced activation in regions important for cognitive control (Aron et al. 2004) and error monitoring (Gehring and Fencsik 2001; Taylor et al. 2006; Chevrier and Schachar 2010) during intoxicated versus sober conditions. A second aim was to evaluate individual differences in working memory capacity as a moderator of alcohol’s effects on neural correlates of response inhibition. We predicted that baseline working memory capacity would moderate alcohol’s effects on neural activation during response inhibition, such that alcohol-induced decreases in activation in areas critical for response inhibition (Aron et al. 2004; Aron 2007; Simmonds et al. 2008; Swick et al. 2011) would be more pronounced among individuals with low versus high working memory capacity.
Method
Twenty-two men participated in the study (mean age: 25.94 years, SD = 3.45; range: 21–35 years). Potential participants were identified based on participation in a prior fMRI study (Claus et al. 2011) and screened to ensure that they met the following inclusion criteria: age 21–35; regular heavy drinker (defined as at least 5 episodes of 5+ standard drinks in the last 30 days); no reported medical conditions or medications for which alcohol was contraindicated; not currently taking psychiatric medication; no recent use of drugs other than cannabis; no history of treatment for alcohol problems; not currently trying to reduce drinking or seek treatment for alcohol use. Additionally, participants were excluded if they endorsed any criteria that would prohibit completing MRI scans (e.g., metal implants; claustrophobia; head injury). Participants’ mean score on the Alcohol Use Disorders Identification Test (AUDIT) was 12.76 (SD = 6.89). The racial composition of the sample included Caucasian (12), Latino (3), Native American (3), Asian (1), and mixed race (3). Six participants endorsed Hispanic ethnicity. Participants who met inclusion criteria were scheduled for two fMRI sessions spaced 1–6 weeks apart depending on scanner availability (M = 16.8 days, SD = 13.5). Scan sessions included administration of alcohol or control beverages in a within-subjects, counterbalanced design. Participants were instructed to abstain from alcohol or recreational drugs for 24 hours, to refrain from eating for 3 hours, and not to drive to the session. On arrival participants provided a breathalyzer reading and a urine toxicology screen (for opiates, cocaine, and amphetamines).
Beverage manipulations
Alcohol condition
In the alcohol condition participants consumed a bolus dose (0.82 g/kg) of 100-proof vodka mixed with fruit juice at a 1:4 ratio. Participants received beverages in three equivalent portions and were allotted 3 minutes to consume each. The procedure utilized ideographic wait times following beverage consumption, an approach demonstrated to reduce variance in BAC (George et al., 2009; Schacht et al. 2010). Prior research on this procedure suggested that the current dosage would yield a peak breath alcohol concentration of approximately .09g% (Schacht et al., 2010). Upon finishing the beverages participants provided BrAC readings (Alco-Sensor IV, Intoximeters Inc., St. Louis, MO) every three minutes until a criterion BrAC (.06g%) was reached. Upon reaching the criterion BrAC, participants proceeded to the MRI room. Additional BrAC measures were obtained immediately prior to and following the fMRI session. Following the alcohol session participants remained in a private room until BrAC descended below .03g%, at which point they were compensated and discharged.
Control condition
During the control session participants consumed fruit juice in an amount equivalent to the body-weight adjusted volume the participant consumed (or would consume) in the alcohol session. Our procedure included a modified yoked control design (Giancola and Zeichner 1997) to reduce experimental variance in wait times between beverage administration and the onset of experimental procedures, given the use of ideographic wait times in the alcohol session. For participants randomized to complete the alcohol session first, the wait period (i.e., between beverage consumption and the onset of experimental procedures) in the control session matched the wait period from the prior alcohol session. For participants randomized to receive the control condition first, wait times were assigned by yoking the participant to another participant who had already completed an alcohol session. This approach serves to minimize overall variability in wait time.
Randomization occurred before the first session in accordance with a counterbalanced design. Because a placebo manipulation was not utilized, participants were aware of condition assignment. However, to minimize expectancies leading up to the session, they were informed that randomization to alcohol or control conditions would occur independently at each session and were not informed about assignment until arrival at each session. This study used a no-alcohol control based on the prediction that a credible placebo could not be implemented at this dosage, particularly in the context of a within-subjects design with lengthy assessment procedures. Imaging studies with similar designs suggested an inability of placebo to induce perceived intoxication (Anderson et al., 2011), which can pose concerns for interpretation (Martin & Sayette, 1993). Therefore, the effects measured in this study reflect the influence of pharmacology inclusive of any potential expectancy effects.
Self-report and behavioral assessments
Alcohol use and problem drinking
The Alcohol Use Disorders Identification Test (Babor et al. 2001) is a 10-item measure assessing hazardous drinking. Items cover quantity/frequency of drinking (3 items), dependence symptoms (3 items) and alcohol-related consequences (4 items). Previous research supports the validity and psychometric properties of the AUDIT (Kokotailo et al. 2006). The Timeline Followback (Sobell et al. 1992), a calendar-based approach for assessing substance use, provided estimates of participants’ recent (past 30 days) drinking (number of drinking days, drinks per drinking day, and number of heavy episodes (defined as 5 or more drinks on one occasion), as well as number of tobacco and marijuana use days. Participants also completed a generic measure of drinking history (e.g., age of onset).
Go/No-go (GNG)
The GNG task was based on the task presented by Kaufman and colleagues (2003). During the task, participants viewed an alternating sequence of X’s and Y’s, and responded with a button press on every trial. However, if the letter was repeated (e.g. X followed by X), participants were instructed to withhold their response. NoGo trials occurred approximately 10% of the time within the trial sequence. Each letter was presented for 700 milliseconds (msec) and was followed by a 300 msec fixation cross. Each 2-second functional scan included 2 trials, with the position order of inhibition trials counterbalanced within a functional run. Participants completed 2 runs of the task; each run was 5:32 minutes in length.
Working memory
Working memory capacity was assessed using the automated operation span task, which has been used extensively in studies of individual differences in executive function (e.g. Unsworth et al. 2009). In this task, participants are required to remember letters while performing basic math problems under time restrictions. On each trial, participants alternate between a single letter presentation and a true/false math problem (e.g. 2*4 + 3 = 12), and then must recall the letters in order at the end of each sequence, which includes 2–8 letters. From this measure, a total score is computed that represents each individual’s operation span, which is used to determine high or low working memory capacity.
fMRI methods
Image acquisition
All MRI data was collected on a 3T Siemens Trio (Erlangen, Germany) whole body scanner. Participants were placed in the scanner and a piece of tape was placed across the forehead to serve as feedback for movement reduction. Following localizer scans, an echo-planar gradient-echo pulse sequence (TR=2000ms, TE=29, flip angle=75°) was acquired with an 8-channel head coil, and images were acquired parallel to the ventral surface of a participant’s orbitofrontal cortex to reduce signal dropout and distortion in this region (Deichmann et al. 2003). Each volume acquired consisted of 33 axial slices (64×64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap). In addition, a high resolution T1-weighted MP-RAGE anatomical image was acquired (TR=2530ms, TE=1.64ms, flip angle=7°, 192 sagittal slices, 256×256 matrix, slice thickness = 1mm, no gap) for each participant.
Image analysis
All image analyses were completed using FMRIB’s Software Library (FSL) version 4.1.0 (Smith et al. 2004). The first 3 volumes of each functional run were discarded to allow the magnet to reach steady state. MCFLIRT (Motion Correction using FMRIBs Linear Image Registration Tool; Jenkinson et al. 2002) was used to realign functional images. Images were then deskulled using BET (Brain Extraction Tool; Smith 2002), spatially smoothed with a 5 mm full-width half-max Gaussian kernel, temporally filtered using a high-pass filter of 50 sec, prewhitened using FILM (FMRIBs Improved Linear Model) and grand mean intensity normalized; all of these steps were performed using FEAT (FMRIB Expert Analysis Tool; Smith et al. 2004). In addition, motion parameters for each participant were entered as covariates of no interest in the statistical model. The MP-RAGE anatomical image was deskulled using BET, and used for registration to the MNI 152 template brain.
Analyses focused on each given trial type within the Go-NoGo task as well as contrasts of interest. For the first analysis, customized regressors were created for each participant for 3 conditions of interest: Correct NoGo, Incorrect NoGo, and Incorrect Go. Each task regressor included the onset time of the event with a duration of 700ms; correct Go trials were the implicit baseline. Statistical analyses were performed using the general linear model as implemented in FEAT. Customized square waveforms representing the condition of interest were convolved with a double gamma hemodynamic response function. Time series analyses were conducted using FILM (Woolrich et al. 2004) with local autocorrelation estimation. In addition, temporal derivatives were added to account for variability in that deviated from the canonical hemodynamic response function. This first level analysis generated parameter estimates and contrast images of Correct NoGo>Correct Go (i.e. baseline) and Incorrect NoGo>Correct Go.
Contrast maps were then registered to the participant’s high-resolution anatomical image and the MNI 152 brain template using FLIRT (FMRIB’s Linear Image Registration Tool; Jenkinson et al. 2002) and individual runs within a session were combined using a fixed effects model in FEAT. Difference maps were computed for the control – alcohol sessions for each contrast of interest, and these contrasts were tested at the group level using FLAME (FMRIB’s Local Analysis of Mixed Effects) Stage 1. To protect against false positives, we used 3dClustSim to determine the minimum cluster size required to reduce alpha to .05 using a voxelwise threshold of p < .01.
Results
Preliminary analyses identified four participants who did not have reliable data on the Go/NoGo task, presumably due to not following or understanding instructions (error rates on Go trials > 50%). An additional participant had missing data for one session due to discontinuing the study, and a final participant was dropped because of a malfunction that resulted in loss of data for the alcohol session. These participants were removed from subsequent analyses, leaving a total of 17 participants for primary analyses (see Table 1 for descriptive information). All participants were verified as having a BrAC of 0.00g% upon arrival to the sessions. During alcohol sessions the criterion BrAC (.06g%) was attained, on average, 25.33 minutes (SD = 4.86) following the start of beverage consumption. Participants had a mean BrAC of .070g% (SD = .009) immediately before entering the scanner and .070g% (SD = .015) immediately upon completing the scan. Thus, the use of idiographic schedules appeared to yield minimal variability in BrAC values relative to the high variability often observed in oral alcohol administration paradigms (O'Connor et al. 1998; Ramchandani et al. 1999; O'Connor et al. 2000).
Table 1.
Drug use for all subjects, and high and low WM capacity groups.
| Variable | All | Low WM | High WM | t | p |
|---|---|---|---|---|---|
| N | 17 | 7 | 7 | - | - |
| Age | 25.94 (3.45) | 25.57 (3.31) | 27.28 (3.73) | 1.3 | .23 |
| Age of First Drink | 14.76 (4.19) | 11.86 (4.85) | 16.00 (1.41) | 2.2 | .05 |
| Drinking days | 15.17 (7.84) | 15.29 (10.24) | 15.14 (6.79) | .03 | .97 |
| Drinks per drinking day | 4.50 (2.83) | 5.59 (3.82) | 4.22 (1.75) | .86 | .41 |
| Heavy drinking episodes | 7.00 (9.06) | 8.14 (10.56) | 7.57 (9.81) | .10 | .92 |
| Peak standard drinks | 8.41 (3.99) | 10.14 (4.88) | 7.57 (3.05) | 1.18 | .26 |
| AUDIT score | 12.76 (6.89) | 14.43 (8.36) | 11.57 (5.86) | .74 | .47 |
| Cigarette smoking days | 7.88 (12.87) | 10.86 (14.15) | 8.29 (14.16) | .34 | .74 |
| Marijuana use days | 6.70 (12.20) | .29 (.49) | 12.1 (15.19) | 2.06 | .06 |
| Working memory span | 46.07 (19.13) | 31.29 (8.79) | 60.86 (14.33) | 4.65 | <.01 |
Notes. AUDIT – Alcohol Use Disorder Identification Test. *Working memory span was measured using an automated operation span task, provided to us by Dr. Randall Engle.
Behavioral Results
Analysis of behavioral data focused first on the effects of beverage condition on 5 task parameters: proportion Correct Go, proportion Incorrect NoGo, d’, response time (RT) to Correct Go trials, and RT to Incorrect NoGo trials. d’ is a measure of response sensitivity and is computed by subtracting the z-score of proportion Incorrect NoGo from the z-scored proportion Correct Go value. Paired sample t-tests indicated no effect of beverage condition on proportion Correct Go (Alcohol M [SD] = .939 [.057], Control M [SD] = .950 [.044], t(16) = 1.11, p = .28). However, alcohol significantly impaired response inhibition, increasing the proportion of Incorrect NoGo trials (Alcohol M [SD] = .674 [.161], Control M [SD] = .589 [.184], t(16) = 2.76, p = .01) and d’ (Alcohol M [SD] = 1.25 [.75], Control M [SD] = 1.62 [.85], t(16) = 2.62, p = .04). Response times did not differ across alcohol and control conditions in either the Go trials (Alcohol M [SD] = 307 milliseconds (msec) [64], Control M [SD] = 322 msec [56], t(16) = 1.55, p = .14) or Incorrect NoGo trials (Alcohol M [SD] = 282 msec [69], Control M [SD] = 298 msec [52], t(16) = 1.70, p = .11).
Associations of working memory with Go/NoGo task performance
Three participants did not have WM data, leaving 14 participants for analyses involving WM. A median split was computed for all WM analyses using the final score for the operation span task, which adjusts for the number of math errors committed. Because the median value was equivalent to the score for two participants, the total operation span score (total number of correctly identified letters, irrespective of order) was used to classify one participant as low working memory and one as high. Importantly, all working memory analyses were run using 7 participants in each group as well as with the two participants whose scores were equal to the median removed. In all analyses, behavioral and fMRI, significant relationships remained in both sets of analyses. The low WM group reported a younger age of first regular drinking t(12) = 2.17, p = .05); otherwise, the two groups did not differ significantly on alcohol or other substance use outcomes (Table 2).
Table 2.
Main effects for the Correct NoGo > Go and Incorrect NoGo > Go contrasts.
| Contrast | Region | Voxels | Max z | x | y | z |
|---|---|---|---|---|---|---|
| Correct NoGo > Correct Go | R Ant. Insula | 27884 | 6.7 | 34 | 22 | −6 |
| dmPFC | 6.12 | 4 | 24 | 42 | ||
| R MFG | 5.79 | 32 | 4 | 58 | ||
| R IFG | 5.56 | 44 | 14 | 28 | ||
| R IPL | 17067 | 6.51 | 38 | −56 | 52 | |
| R Supramarginal | 6.24 | 50 | −40 | 46 | ||
| R SPL | 6 | 34 | −64 | 52 | ||
| PCC | 499 | 3.89 | 6 | −30 | 24 | |
| Incorrect NoGo > Correct Go | dmPFC | 10881 | 6 | 4 | 22 | 40 |
| dACC | 5.82 | 6 | 32 | 28 | ||
| R Ant. Insula | 5.65 | 38 | 18 | 0 | ||
| R SFG | 5.38 | 10 | 18 | 62 | ||
| R Temp Pole | 5.35 | 46 | 16 | −10 | ||
| R IFG | 5.19 | 52 | 26 | 4 | ||
| R Supramarginal | 9102 | 5.51 | 52 | −32 | 40 | |
| R IPL | 5.32 | 50 | −42 | 44 | ||
| R SPL | 5.19 | 42 | −56 | 52 | ||
| L OFC | 3607 | 5.73 | −32 | 16 | −14 | |
| L Ant Insula | 5.3 | −40 | 12 | −2 | ||
| L Insula | 5.14 | −38 | 10 | 2 | ||
| L Temp Pole | 4.14 | −46 | 12 | −12 | ||
| R Caudate | 2681 | 5.09 | 12 | 8 | 6 | |
| R Thalamus | 4.45 | 8 | −18 | 8 | ||
| L Caudate | 4.28 | −10 | 6 | 2 | ||
| R Brainstem | 4.26 | 10 | −24 | −16 | ||
| R Thalamus | 4.26 | 8 | −6 | −2 | ||
| R Brainstem | 4.24 | 4 | −28 | −4 | ||
| L Cerebellum | 292 | 3.96 | −34 | −54 | −34 |
Notes. All clusters were corrected using a voxel threshold of p < .01 and a minimum cluster size determined by Monte Carlo simulations to meet corrected p values of .05. dmPFC – dorsomedial prefrontal cortex; MFG – middle frontal gyrus; IPL – inferior parietal lobe; SPL – superior parietal lobe; dACC – dorsal anterior cingulate cortex; SFG – superior frontal gyrus; OFC – orbitofrontal cortex.
The association of WM with behavioral measures of response inhibition was examined in two steps. First, associations of WM group with the 5 task parameters (proportion hits, proportion false alarms, d’, response time (RT) to Correct Go trials, RT to Incorrect NoGo trials) were examined using independent samples t-tests; these analyses were conducted separately by beverage condition. Individuals with higher WM performance displayed significantly lower rates of Incorrect Go trials when sober (t (12) = 2.97, p < .05) and higher d’ values (t(12) = 2.31, p <.05) when sober. No other associations were significant (all ps > .15). Second, we examined whether WM group predicted alcohol-induced impairment on response inhibition by computing difference scores (Alcohol - Control) for each Go/NoGo task parameter of interest and examining their correlation with WM performance. This analysis yielded no significant effects of WM on alcohol-induced changes in behavioral task performance (ps > .3). However, when examining RT data within each group, we found that RT for Go Correct and NoGo Incorrect showed marginal differences for the low WM group (t(6) = 2.37, p = .055 and t(6) = 2.08, p = .08, respectively) such that low WM participants responded faster in the alcohol condition compared to the control condition [mean Go RT difference = 30.4 msec (38.7); NoGo RT = 29.0 msec (32.3)]. No differences in RTs were noted for the high WM group (both ps > .4).
fMRI Results
Task Main effects
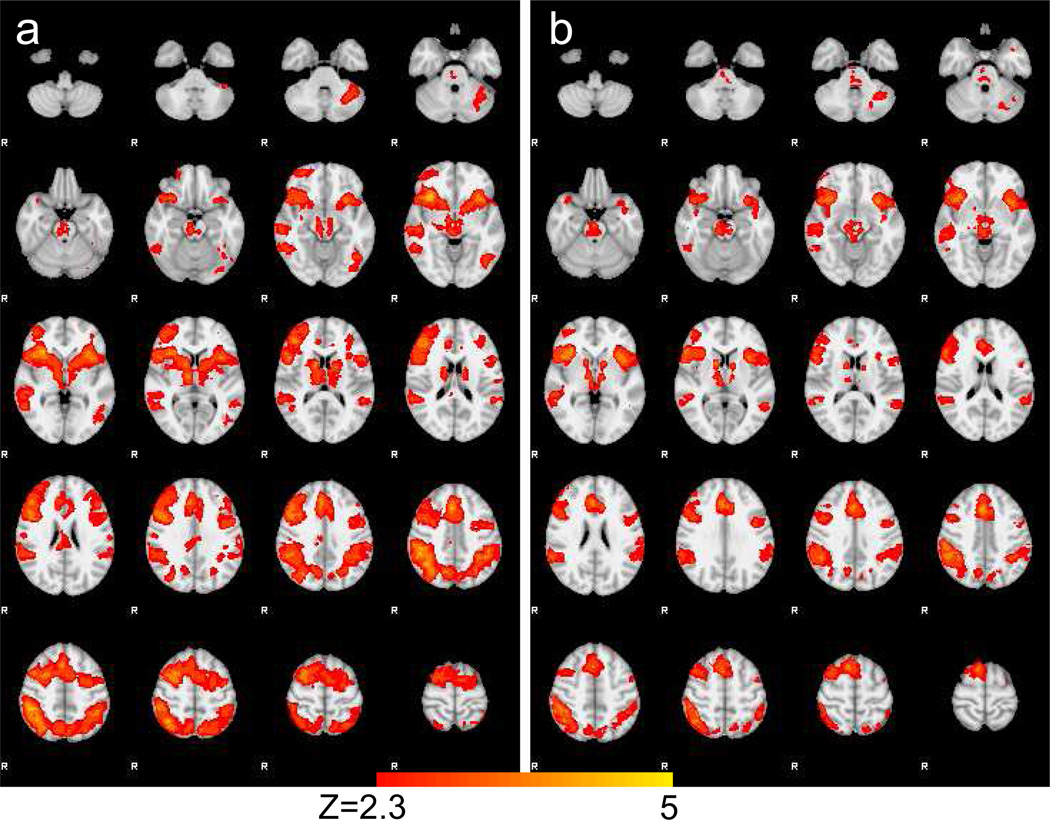
Combining both sessions across all subjects revealed main effects within our contrasts of interest. Within the Correct NoGo > Correct Go comparison, we found significant differences in bilateral inferior frontal gyrus/insula, bilateral dorsolateral prefrontal cortex, dorsal anterior cingulate cortex, striatum, bilateral inferior parietal lobe, supplemental motor area, precuneus, and right temporal pole (see Table 2 and Figure 1a). In the Incorrect NoGo > Correct Go contrast, we found increased response for Incorrect NoGo trials in bilateral IFG/insula, dorsal ACC, right middle/superior frontal gyrus, precentral gyrus, bilateral caudate, left precentral gyrus, right middle temporal gyrus, brainstem/thalamus, and bilateral IPL (see Table 2 and Figure 1b).
Figure 1.
Main effects of Correct NoGo > Correct Go contrast (a) and Incorrect NoGo > Correct Go contrast (b) across all subjects and both sessions.
WM x Task
When examining the effect of WM on response in the Correct NoGo > Correct Go contrast (across sober and intoxicated sessions), we found significantly greater response in the high WM group in occipital pole, lingual gyrus, and posterior cingulate cortex (Table 3). When examining the effect of WM on the Incorrect NoGo > Correct Go contrast, the high WM was found to have greater response in lingual/fusiform gyrus, right MFG, right frontal pole and right OFC (Table 3).
Table 3.
Main effects of WM on Correct NoGo > Go and Incorrect NoGo > Go contrasts.
| Contrast | Region | Voxels | Max z | x | y | z |
|---|---|---|---|---|---|---|
| High WM > Low WM (Correct NoGo > CorrectGo) |
Occipital Pole | 504 | 3.58 | 10 | −90 | 22 |
| PCC | 463 | 3.78 | 4 | −16 | 28 | |
| Lingual Gyrus | 250 | 3.37 | 8 | −68 | −8 | |
| High WM > Low WM (Incorrect NoGo > CorrectGo) |
Lingual Gyrus | 835 | 3.25 | 8 | −68 | −6 |
| R OFC | 559 | 4.11 | 46 | 34 | −10 | |
| Medial Frontal Pole | 421 | 3.65 | −2 | 56 | 10 | |
| R MFG | 384 | 3.24 | 34 | 44 | 32 | |
| R Fusiform Gyrus | 288 | 3.2 | 16 | −74 | −16 |
Notes. All clusters were corrected using a voxel threshold of p < .01 and a minimum cluster size determined by Monte Carlo simulations to meet corrected p values of .05. PCC – posterior cingulate cortex; OFC – orbitofrontal cortex; MFG – middle frontal gyrus.
Beverage x Task
When examining each contrast (Correct NoGo > Correct Go and Incorrect NoGo > Correct Go), we found significant differences between alcohol and juice conditions in right MFG in the Correct NoGo > Correct Go contrast only.
Beverage x Task x WM
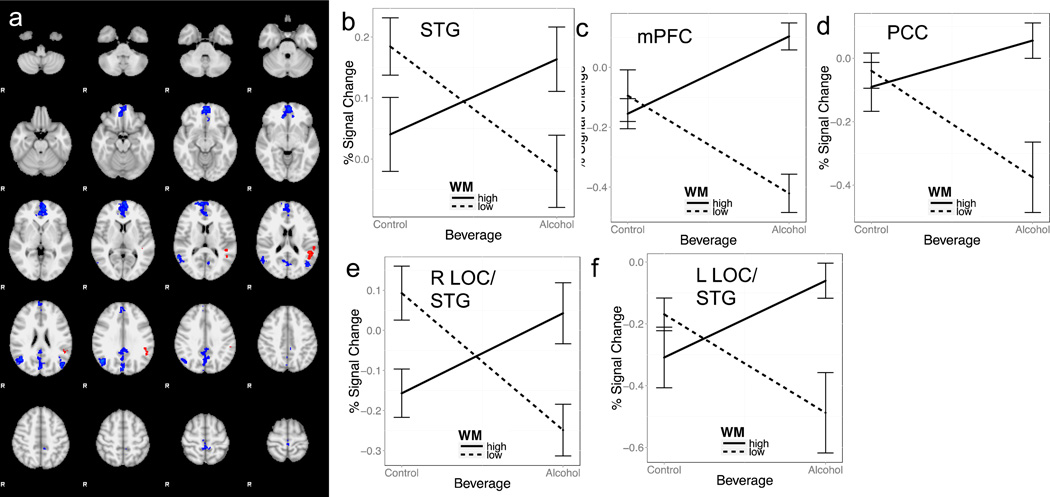
Examination of the three way interaction revealed significance in the left superior temporal gyrus within the Correct NoGo > Correct Go contrast, indicating a negative relationship between WM capacity and the difference in response within the Correct NoGo > Correct Go contrast. As seen in Figure 2a, the low WM group showed greater reduction in BOLD response when comparing alcohol to juice scan sessions than the high WM group.
Figure 2.
Moderating effect of working memory capacity on alcohol-induced changes in BOLD response during correct inhibition (orange cluster in 2a); b) and errors (blue clusters in 2a; c–f). Plots of signal change by beverage condition and working memory capacity group for each significant cluster presented in a).
Similarly, there was a significant interaction in the Incorrect NoGo > Correct Go contrast within across alcohol and juice sessions in medial PFC, posterior cingulate cortex, and bilateral lateral occipital cortex/superior temporal gyrus. In all cases, the high WM group showed increases in BOLD response during the alcohol compared to juice condition, whereas the low WM group showed decreases in BOLD response in the alcohol compared to juice condition (see Figure 2 and Table 4).
Table 4.
Interaction of WM capacity and beverage condition for the Correct NoGo > Go and Incorrect NoGo > Go contrasts.
| Contrast | Region | Voxels | Max z | x | y | z |
|---|---|---|---|---|---|---|
| Correct NoGo > Correct Go | L STG | 371 | 3.03 | −50 | −42 | 26 |
| Incorrect NoGo > Correct Go | mPFC | 2110 | 3.4 | −6 | 56 | 2 |
| Precuneus/PCC | 1302 | 3.19 | −2 | −82 | 28 | |
| R LOC/STG | 579 | 3.68 | 46 | −64 | 30 | |
| L LOC/STG | 246 | 3.38 | −48 | −72 | 20 |
Notes. All clusters were corrected using a voxel threshold of p < .01 and a minimum cluster size determined by Monte Carlo simulations to meet corrected p values of .05. STG – superior temporal gyrus; mPFC – medial prefrontal cortex; PCC – posterior cingulate cortex; LOC – lateral occipital cortex.
Discussion
The current study examined the effects of acute oral alcohol on behavioral and neural measures of response inhibition and also examined the moderating influence of working memory capacity on alcohol-induced impairment. Results suggest that individual differences in working memory capacity influenced the degree of neural change under intoxication, providing further evidence for the hypothesis that high baseline levels of working memory capacity may act to protect individuals from alcohol-induced impairment of cognitive function (Finn et al., 1999).
Effects of Acute Alcohol on Response Inhibition and Error Monitoring
Consistent with prior research (Vogel Sprott et al. 2001; Marczinski and Fillmore 2005; Fillmore and Rush 2006; Marczinski et al. 2007), a moderate dose of alcohol impaired performance on a Go/No-go task, specifically in the number of inhibition errors. Acute alcohol also affected neural responses during failed inhibition trials; examination of Incorrect NoGo trials between intoxicated and non-intoxicated conditions revealed differences in right IPL within the failed inhibition contrast. The IPL has been implicated in both successful and unsuccessful response inhibition. Specifically, it is believed that the IPL plays a critical role in maintaining stimulus-response associations in concert with prefrontal cortex and has been implicated in awareness of errors (Simmonds et al. 2008; Hester et al. 2009). The difference observed between alcohol and control conditions may reflect reduced capacity to keep these representations online during the intoxicated session, and as a result, error awareness is reduced.
The relative lack of BOLD findings between the control and alcohol sessions was unexpected, as several other studies have examined response inhibition and error-related processes using similar response inhibition tasks under sober and intoxicated conditions in an fMRI context. Prior studies have found decreased response during alcohol sessions compared to control/placebo session in medial frontal (dACC, subgenual ACC, and dorsomedial ACC), inferior frontal gyrus, and superior temporal gyrus/IPL when examining errors (Anderson et al. 2010; Gan et al. 2014; Marinkovic et al. 2011, 2013). However, one study did not appear to find any main effects of alcohol versus placebo on a stop signal task (Kareken et al. 2013), suggesting that individual differences may exist that may moderate alcohol effects of BOLD response.
Moderating Effects of Working Memory Capacity on Alcohol-induced BOLD changes
While the effects of alcohol have been studied increasingly using fMRI, no study to date has examined the moderating role of working memory on alcohol’s effects on response inhibition despite prior behavioral studies showing the importance of WM capacity as a moderator of alcohol induced disinhibition (Finn et al. 1999). In the present study, we found similar patterns of BOLD response differences in both the correct inhibition and failed inhibition contrasts. Specifically, in all cases low WM participants showed a decrease in BOLD response during the alcohol compared to juice condition whereas the high WM participants showed increases in BOLD response during the alcohol compared to control condition.
In the correct inhibition contrast, we found that alcohol induced differences in BOLD signal were moderated by WM group in left superior temporal gyrus/left inferior parietal lobe. The left STG/IPL has been reported in a meta-analysis of complex GNG tasks (Simmonds et al. 2008), and is implicated along with frontal cortex in maintaining stimulus-response associations, which would be particularly important in the current GNG task that required constant updating of the prior stimulus in order to respond or withhold appropriately (Gottlieb and Snyder 2010). These findings suggest a potential neural mechanism by which individuals with low WM capacity are particularly affected by the acute effects of alcohol, namely that they experience a reduced ability to track and update stimulus-response associations in the service of goal-directed behavior (i.e. stopping), which may put them at increased risk for participating in risky activities. In contrast, high WM capacity individuals actually increased engagement of the STG while intoxicated, possibly a compensatory mechanism that allows these individuals to avoid potential negative consequences associated with drinking. Prior work suggests that working memory capacity may act as a moderating factor in preventing problems with alcohol in high-risk individuals (Finn and Hall 2004), and the result from the inhibition contrast may be one mechanism by which WM capacity buffers individuals.
In the failed inhibition/error contrast, we found that WM capacity significantly moderated neural response in medial PFC, precuneus/posterior cingulate, and bilateral lateral occipital cortex/superior temporal gyrus. As with the correct inhibition findings, in all cases, low WM participants showed a relative decrease in BOLD response whereas high WM capacity participants demonstrated an increase in BOLD response during the alcohol compared to control session. Given that no association of WM capacity was found with the number of failed inhibitions, these results may suggest two potential mechanisms by which alcohol results in poor behavioral control. The interaction effect in medial PFC and precuneus/posterior cingulate highly overlaps with regions associated with the default mode (Raichle et al. 2001). In fact, studies have shown that while these default mode regions are typically suppressed below baseline during performance of a cognitive task, a failure to suppress this default mode of response is associated with subsequent errors (Li et al. 2007; Eichele et al. 2009). Whereas the high WM group shows a failure to suppress these regions during the alcohol session, which may be a potential cause of increased errors in inhibiting, the low WM group actually suppressed this set of regions to a greater degree during the alcohol compared to the control session. This finding, along with the faster response times on Go trials for the alcohol compared to juice session in the low WM group, may suggest increased myopia towards the prepotent “Go” response in the low WM group that is ultimately not useful for trial-specific behavioral control. The other regions observed in the failed inhibition contrast (i.e. bilateral lateral occipital cortex, superior temporal gyrus) also typically show deactivation in the contrast of NoGo Incorrect > Go correct (Hirose et al. 2012; Claus et al. 2013). The reduced suppression in the high WM group is thus consistent with prior results, whereas the low WM group’s increased suppression may point to increased myopia towards Go trials in the alcohol condition for the low WM group. Future studies with larger samples using tasks specifically designed to distinguish between inhibition failures due to enhanced prepotency versus lapses in attention will be key for determining the mechanisms that underlie different types of inhibition failure.
Clinical Implications
The findings from the current study have several clinical implications as well. For example, a prior laboratory study found that greater alcohol-induced impairment on a response inhibition task under intoxicated versus sober conditions predicted higher levels of ad libitum alcohol consumption in a subsequent laboratory session (Weafer and Fillmore 2008). The current findings suggest a basic neurobehavioral mechanism that may predict who is likely to experience deleterious outcomes after alcohol intoxication (Fillmore et al. 2008), including difficulty controlling alcohol intake once a drinking episode begins (Weafer and Fillmore 2008). These findings are also relevant for alcohol use disorders more generally. For example, in a study of detoxified alcohol-dependent patients, greater neural activation during N-back performance (i.e. working memory) in ventrolateral and rostral PFC was associated with reduced relapse risk over a 7-month follow-up period (Charlet et al. 2013). Although our study focused on baseline executive functioning, future studies should evaluate additional measures of executive function (including WM) under intoxicated and sober conditions to examine whether alcohol-induced changes in activation correlate with measures of relapse or impaired control over drinking.
Limitations
While the results of this study are compelling, some limitations must be considered. The use of a small, male-only sample limits the generalizability of the findings. However, given prior research providing evidence of the effects of alcohol on response inhibition and the moderating effects of working memory on alcohol induced disinhibition (Finn et al. 1999), we are more confident in our results which suggest a potential neural mechanism by which working memory capacity buffers some individuals from disinhibitory effects of alcohol. Future studies with larger samples will be critical for confirming the findings of the current study. One unexpected finding of the current study was a younger age of first drink and a higher number of maximum drinks (although this effect was not statistically significant) in the low WM group, which could suggest that duration of drinking history may explain some portion of the effects observed in the current study. Prior work has demonstrated that early age of onset of problem drinking is associated with reduced behavioral control (e.g. Bjork et al. 2004) and that the disinhibiting effects of alcohol are greater in binge drinkers than non-binge drinkers (Marczinkski et al. 2007). Thus, the moderating influence of WM capacity observed in the current study could be explained by these other variables; future studies should systematically examine this possibility by stratifying WM groups based on age of first drink and current drinking levels to rule out drinking history as a potential confound. Additionally, marijuana use was higher in the high WM group, and may have confounded the results. Unfortunately, because all three heavy users of marijuana were in the high WM group, it was not possible to disentangle the effects of marijuana from WM. Thus, it remains possible that at least some portion of the variance explained by WM capacity may actually be explained by marijuana use. Future studies should more carefully stratify based on marijuana usage patterns.
Another limitation is the absence of a placebo manipulation to match expectancy set across sessions. The use of a no-placebo control reflects concerns about the viability of a placebo at the current dosage (Martin & Sayette, 1993), particularly given a) the within-subjects design and lengthy assessment procedures in this study, and b) apparent difficulty inducing perceived intoxication with placebo manipulations in similar contexts (e.g., Anderson et al., 2011). The current design avoids inferential problems arising from placebo manipulation failures (Martin & Sayette, 1993) and maximizes confidence in inferring participants’ expectancy set. Also, results from this design have greater relevance to naturalistic drinking scenarios compared to a two-cell placebo-controlled design (Martin & Sayette, 1993). However, these advantages come at the expense of evaluating incremental effects of pharmacology over expectancy, which is an important consideration given evidence for compensatory behavioral (Fillmore & Blackburn, 2002; Marczinski & Fillmore, 2005) and neural (Gunderson et al., 2008) effects after placebo manipulations. Evidence for compensatory neural effects in the direction opposite of alcohol effects (Gunderson et al., 2008) suggests the present results might provide a conservative estimate of pharmacological effects. Future studies should use at least three conditions (control, alcohol, placebo) to evaluate relative effects of pharmacology and expectancy, as well as the possibility of compensatory behavioral and neural responses relevant to task performance.
Acknowledgments
Funding Source: Supported in part by R21AA020304. NIAAA did not have any input on the design, collection, analysis or interpretation of the data presented here. The authors maintain full control of all primary data and agree to review by the journal if requested.
The authors would like to thank Jesse Castillo and Kent Hutchison for assisting with the study.
Footnotes
The authors report no conflicts of interest.
References
- Anderson BM, Stevens MC, Meda SA, et al. Functional Imaging of Cognitive Control During Acute Alcohol Intoxication. Alcohol Clin Exp Res. 2010;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron A, Robbins T, Poldrack R. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Babor T, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2001 [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, et al. Alcohol effects on performance monitoring and adjustment: Affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology. 2012;121:173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–50. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Charlet K, Beck A, Jorde A, et al. Increased neural activity during high working memory load predicts low relapse risk in alcohol dependence. Addiction Biology. 2013 doi: 10.1111/adb.12103. [DOI] [PubMed] [Google Scholar]
- Chevrier A, Schachar RJ. Error detection in the stop signal task. Neuroimage. 2010;53:664–673. doi: 10.1016/j.neuroimage.2010.06.056. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, et al. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE. Behavioral control in alcohol use disorders: relationships with severity. Journal of Studies on Alcohol and Drugs. 2013;74:141–151. doi: 10.15288/jsad.2013.74.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J, Patrick C, Lang A, et al. Alcohol affects emotion through cognition. Psychological Science. 2001;12:527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried J, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, Li C-SR. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. The Journal of Neuroscience. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, et al. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Res Cogn Brain Res. 2005;25:873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Easdon CM, Vogel Sprott M. Alcohol and behavioral control: impaired response inhibition and flexibility in social drinkers. Experimental and Clinical Psychopharmacology. 2000;8:387. doi: 10.1037//1064-1297.8.3.387. [DOI] [PubMed] [Google Scholar]
- Eichele T, Calhoun VD, Debener S. Mining EEG-fMRI using independent component analysis. International Journal of Psychophysiology. 2009;73:53–61. doi: 10.1016/j.ijpsycho.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser AS, Meel CS, Snelleman M, Franken IHA. Acute effects of alcohol on feedback processing and outcome evaluation during risky decision-making: an ERP study. Psychopharmacology. 2011;217(1):111–25. doi: 10.1007/s00213-011-2264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, et al. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J Stud Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore M, Blackburn J, Harrison E. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug Alcohol Depend. 2008;95:97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M, Vogel-Sprott M, Gavrilescu D. Alcohol effects on intentional behavior: dissociating controlled and automatic influences. Experimental and Clinical Psychopharmacology. 1999;7:372–378. doi: 10.1037//1064-1297.7.4.372. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel Sprott M. Response Inhibition under Alcohol: Effects of Cognitive and Motivational Conflict. J Stud Alcohol. 2000;61:239–46. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. Journal of Psychopharmacology. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Finn P, Hall J. Cognitive Ability and Risk for Alcoholism: Short-Term Memory Capacity and Intelligence Moderate Personality Risk for Alcohol Problems. Journal of Abnormal Psychology. 2004;113:569–581. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Finn P, Justus A, Mazas C, Steinmetz J. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: Testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Gan G, Guevara A, Marxen M, et al. Alcohol-Induced Impairment of Inhibitory ControlIs Linked to Attenuated Brain Responses in RightFronto-Temporal Cortex. Biological Psychiatry. 2014:1–10. doi: 10.1016/j.biopsych.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. The Journal of Neuroscience. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WH, Davis KC, Norris J, Heiman JR, Stoner SA, Schacht RL, Hendershot CS, Kajumulo KF. Indirect effects of acute alcohol intoxication on sexual risk-taking: The roles of subjective and physiological sexual arousal. Archives of Sexual Behavior. 2009;38(4):498–415. doi: 10.1007/s10508-008-9346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WH, Stoner SA. Understanding acute alcohol effects on sexual behavior. Annual Review of Sex Research. 2000;11:92–124. [PubMed] [Google Scholar]
- Giancola PR. Executive functioning: a conceptual framework for alcohol-related aggression. Experimental and Clinical Psychopharmacology. 2000;8:576. doi: 10.1037//1064-1297.8.4.576. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Executive Functioning and Alcohol-Related Aggression. Journal of Abnormal Psychology. 2004;113:541–555. doi: 10.1037/0021-843X.113.4.541. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Corman MD. Alcohol and aggression: a test of the attention-allocation model. Psychological Science. 2007;18:649–655. doi: 10.1111/j.1467-9280.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Zeichner A. The biphasic effects of alcohol on human physical aggression. Journal of Abnormal Psychology. 1997;106:598–607. doi: 10.1037//0021-843x.106.4.598. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Snyder LH. Spatial and non-spatial functions of the parietal cortex. Current Opinion in Neurobiology. 2010;20:731–740. doi: 10.1016/j.conb.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Specht K, Grüner R, Ersland L, Hugdahl K. Separating the effects of alcohol and expectancy on brain activation: an fMRI working memory study. Neuroimage. 2008;42:1587–1596. doi: 10.1016/j.neuroimage.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S, Chikazoe J, Watanabe T, et al. Efficiency of Go/No-Go Task Performance Implemented in the Left Hemisphere. Journal of Neuroscience. 2012;32:9059–9065. doi: 10.1523/JNEUROSCI.0540-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Miller N, Pollock VE. Alcohol and aggression: a meta-analysis on the moderating effects of inhibitory cues, triggering events, and self-focused attention. Psychological Bulletin. 1996;120:60. doi: 10.1037/0033-2909.120.1.60. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, et al. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology. 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotailo PK, Egan J, Gangnon R, et al. Validity of the Alcohol Use Disorders Identification Test in College Students. Alcohol Clin Exp Res. 2006;28:914–920. doi: 10.1097/01.alc.0000128239.87611.f5. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski C, Fillmore M. Alcohol increases reliance on cues that signal acts of control. Experimental and Clinical Psychopharmacology. 2005;13:15–24. doi: 10.1037/1064-1297.13.1.15. [DOI] [PubMed] [Google Scholar]
- Marczinski C, Fillmore M. Compensating for alcohol-induced impairment of control: effects on inhibition and activation of behavior. Psychopharmacology. 2005;181:337–346. doi: 10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology. 2005;182:452–459. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychology of Addictive Behaviors. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S. Effects of Alcohol Intoxication on Response Conflict in a Flanker Task. J Addict Res Ther. 2012;S3:002. doi: 10.4172/2155-6105.S3-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Human Brain Mapping. 2011;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, et al. Effects of acute alcohol intoxication on saccadic conflict and error processing. Psychopharmacology. 2013;230:487–497. doi: 10.1007/s00213-013-3173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Sayette M. Experimental-Design in Alcohol Administration Research -Limitations and Alternatives in the Manipulation of Dosage-Set. J Stud Alcohol. 1993;54:750–761. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- Mulvihill L, Skilling T, VogelSprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Collins P, et al. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology. 2011;218:419–428. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li T. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- O’Connor S, Ramchandani V, Li T. PBPK modeling as a basis for achieving a steady BrAC of 60 +/− 5 mg% within ten minutes. Alcohol Clin Exp Res. 2000;24:426–427. [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le DA. Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: Evidence for “loss-of-control drinking” and marked individual differences. Behavioral Neuroscience. 1998;112:1247–1257. doi: 10.1037//0735-7044.112.5.1247. [DOI] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A, et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani V, Bolane J, Li T, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Rehm J, Shield KD, Joharchi N, Shuper PA. Alcohol consumption and the intention to engage in unprotected sex: systematic review and meta-analysis of experimental studies. Addiction. 2011;107:51–59. doi: 10.1111/j.1360-0443.2011.03621.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Alcohol Consumption Impairs Detection of Performance Errors in Mediofrontal Cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Schacht RL, Stoner SA, George WH, Norris J. Idiographically Determined Versus Standard Absorption Periods in Alcohol Administration Studies. Alcohol Clin Exp Res. 2010;34:925–927. doi: 10.1111/j.1530-0277.2010.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tapert S, Matthews SC, et al. fMRI Differences Between Subjects with Low and High Responses to Alcohol During a Stop Signal Task. Alcohol Clin Exp Res. 2011;36:130–140. doi: 10.1111/j.1530-0277.2011.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M, Litten R, Allen J. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: Psychosocial and biochemical methods. 1992:41–72. [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia. Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, et al. Medial frontal cortex activity and loss-related responses to errors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, et al. Complex working memory span tasks and higher-order cognition: A latent-variable analysis of the relationship between processing and storage. Memory. 2009;17:635–654. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- Vogel Sprott M, Easdon C, Fillmore M, et al. Alcohol and behavioral control: Cognitive and neural mechanisms. Alcohol Clin Exp Res. 2001;25:117–121. [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology. 2008;201:315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Milich R, Fillmore MT. Behavioral components of impulsivity predict alcohol consumption in adults with ADHD and healthy controls. Drug and Alcohol Dependence. 2010:1–8. doi: 10.1016/j.drugalcdep.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M, Behrens T, Beckmann C, et al. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]