Abstract
Purpose
Research has shown that recent post-treatment breast cancer survivors face significant challenges around physical activity as they transition to recovery. This review examined randomized controlled trials targeting physical activity behavior change in breast cancer survivors <5 years post-treatment and describes 1) characteristics of interventions for breast cancer survivors as well as 2) effect size estimates for these studies.
Methods
A systematic search was conducted following PRISMA guidelines with Medline, PubMed, PsycINFO, CINAHL, and Scopus databases. Data were abstracted for primary intervention strategies and other details (e.g., setting, duration, theory use). A subgroup analysis was conducted to assess intensity of exercise supervision/monitoring and intervention effectiveness.
Results
The search produced 14 unique behavior intervention trials from the US and abroad published 2005-2013. The mean sample size was 153 participants per study. All interventions included moderate-intensity activities plus various behavioral change strategies. Most interventions were partially or entirely home-based. The overall standardized mean difference was 0.47 (0.23, 0.67) with p < 0.001.
Conclusion
Most interventions were effective in producing short-term behavior changes in physical activity, but varied greatly relative to intervention strategies and intensity of supervision/monitoring. Highly structured interventions tended to produce larger behavior change effects overall, but many larger effect sizes came from interventions supported by phone counseling or email. We observed that ‘more’ may not be better in terms of direct supervision/monitoring in physical activity behavior interventions. This may be important in exploring less resource-intensive options for effective behavior change strategies for recent post-treatment survivors.
Keywords: breast cancer, survivorship, physical activity, behavior change, cancer rehabilitation
Introduction
The completion of primary treatment for breast cancer is a major milestone for its survivors. However, studies have shown that the time post-treatment represents a new period of vulnerability, one in which breast cancer survivors (BCS) face important health promotion challenges and often get “lost in the transition” from patient to survivor, the theme of the Institute of Medicine's sentinel report on survivorship care [1]. Physical activity initiation, reinitiation and maintenance are particular challenges in the post-treatment population. Authorities from the Institute of Medicine (IOM), American Cancer Society, American College of Sports Medicine and others [2-4] have recommended physical activity (PA) as a critical, safe and effective part of survivorship planning. Substantial benefits of PA may include reducing recurrence risk, mitigating cancer treatment side effects and enhancing quality of life outcomes [5-7]. Despite these benefits, surveillance data have suggested that only 10% of BCS meet PA recommendations [8, 9].
Physical activity in survivorship has generated significant research interest, but much of the literature focuses on physiological and psychosocial outcomes, rather than behavior change. Several recent reviews and meta-analyses have examined a variety of PA benefits related to health outcomes in the survivor population, most notably in quality of life [10-13], but the specific intervention components that facilitate behavior change in post-treatment BCS require further study. Assessing research conducted following the release of the 2005 IOM report [1] is also useful in assessing recent contributions to evidence specific to behavior change in this population.
The purpose of this systematic review and meta-analysis is to rigorously assess the effectiveness of PA interventions among recent, post-treatment BCS using data from experimental and quasi-experimental studies. Given evidence for the timeliness of interventions in the post-treatment period [14-16], this study included BCS who were five years or less from completion of active treatment, and thus transitioning to lifestyle changes that may impact long-term recovery. The aims are to: 1) describe the characteristics of PA behavior interventions for BCS, including targeted populations, intervention features, and use of behavior theory and to 2) determine effect size estimates for behavior change from these PA interventions.
For the purposes of this paper, intervention is defined as a strategy or set of strategies, often derived from behavior change theories, to influence health behaviors, such as PA [17]. We chose the term PA, rather than exercise, to include interventions that target moderate- to vigorous-intensity physical activity (MVPA) but may not require access to exercise facilities or equipment. As PA is the behavior of interest, the terms “behavior intervention” and “PA intervention” are equivalent descriptors and will be used interchangeably.
Methods
Search Strategy
A systematic search was conducted based on the Preferred Reporting of Systematic Reviews and Meta-Analysis (PRISMA) guidelines [18] and with assistance from a trained public health librarian. Four databases were used in the search: Medline (via Ovid; 1948 to September Week 2 2013; In-Process & Other Non-Indexed Citations; searched September 19, 2013); PubMed (National Library of Medicine; searched September 20, 2013); PsycINFO (via Ovid; 2002 to September Week 4 2013; searched October 1, 2013); and CINAHL Plus with Full Text (via Ebsco; 2001 to present; searched October 4, 2013).
Several general database-adapted concepts were utilized in the initial search, including: breast cancer/breast neoplasm, PA, exercise, movement, motor activity, interventions, RCTs and survivors. A detailed listing of search terms is available in Figure 1. Strategies were pretested and refined through an iterative process in which abstracts and citations were screened for fulfillment of eligibility criteria. As an additional measure, reference lists of relevant articles were hand searched and compared to lists produced by the four databases. Scopus (via Elsevier) was searched as a final step to ensure that no eligible articles had been previously overlooked.
Figure 1. Search Terms Used with Medline Database.
All eligible studies were 1) published in English in a peer-reviewed journal January 2005-October 2013; 2) utilized a randomized controlled trial or quasi-experimental design with comparison groups; 3) studied BCS who were approximately five years or less from completion of active cancer treatment (i.e., transitioning from completion of treatment to long-term recovery); 4) reported a PA behavior intervention (including language that describes strategies and an intent to change lifestyle behaviors) and 5) reported PA behavior change outcomes.
To verify that the intent of interventions was PA behavior change, we searched first for PA behavior as a stated trial outcome, and second, for key terms in the study description, such as changes in “active lifestyle,” “exercise habits” and “PA patterns,” which confirmed that broader lifestyle changes were central to study goals. Studies that did not meet these or the aforementioned eligibility criteria were excluded.
Study Selection
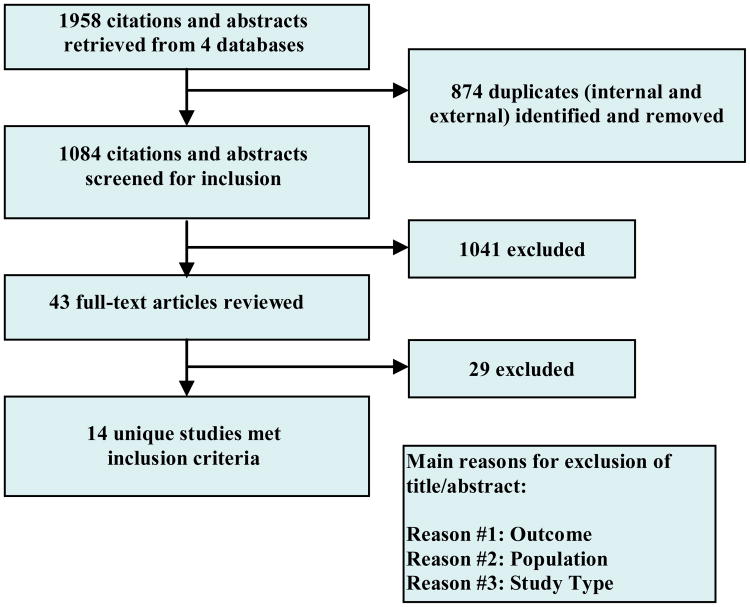
Studies were chosen through a multi-step screening strategy. First, citations and abstracts were screened by the lead author for fulfillment of eligibility criteria. Any study that did not meet one or more criterion was excluded, assigned a reason for exclusion (e.g., population, study type or outcome) and reported back to the other co-authors. Remaining articles were given a “maybe” rating and referred to co-authors for independent review. Discrepancies in ratings across co-authors occurred in less than five percent of abstracts reviewed; all disagreements were resolved through discussion until consensus was reached. Final selection of studies was based on full-text review of potential studies by the authors. All authors agreed with the final choice of studies for this review. A PRISMA-based study selection flowchart is shown in Figure 2.
Figure 2. PRISMA-based Study Selection Flowchart.
Data Abstraction
Data were collected using coding procedures described by Lipsey and Wilson [19]. Two codebooks were created for study-level outcomes and effect-size information. The original coding instruments were previewed by two authors, revised and presented to all co-authors for approval prior to data collection. Two independent coders abstracted data. Any discrepancies were compared against the full-text articles and resolved through discussion. For one study [20], outcomes specific to BCS were obtained through the lead author.
Data abstraction was completed through review of 28 coded study-level descriptors of sample, research design and intervention characteristics and 18 pieces of data describing measurement and effect size information for each study. Meta-analysis parameters were calculated using the “metan” function in Stata 13.0 software (StataCorp, College Station, Texas).
Additionally, to evaluate study quality, ten criteria adapted from the CONSORT checklist for reporting of RCTs (www.consort-statement.org) were applied and customized to PA interventions. These criteria addressed details about intervention implementation, determination of sample size, details about randomization procedures, attrition and response rates post-intervention, behavior measurement tools and summary results with estimated effect sizes. A similar procedure for quality assessment in PA interventions was recently published in a separate review [21].
Data Analysis
Post-intervention change values (typically either mean minutes of MVPA or mean MET hours per week) were used to calculate standardized mean differences (SMD). The Hedge's g method was applied to reduce positive bias [22]. Where possible, samples were analyzed on an intent-to-treat basis, regardless of the original study method, to provide a more conservative estimate of the effect in the population [23]. Based on evidence of heterogeneity (assessed using the I-squared statistic) [24], weighted effect sizes were analyzed. Meta-analytic assumptions followed a random effects model [25]. Meta-funnel procedures were used to statistically assess publication bias [26].
Because high variability was noted for intensity of exercise supervision/monitoring in these studies, an exploratory subgroup analysis was added to meta-analytic procedures, in which studies were stratified by low, medium and high levels of supervision/monitoring intensity during the intervention. High levels of supervision/monitoring included multi-component, structured interactions with participants; medium supervision/monitoring included counseling but not supervised exercise sessions; low supervision/monitoring included little or no individual PA oversight.
Results
Based on our search strategy (Figure 2), 14 unique RCTs described over 24 articles fully met the eligibility criteria. Four studies were conducted internationally (in the United Kingdom, Finland, Canada and South Korea). The remaining studies were conducted in the United States. A summary of participant and intervention characteristics is provided in Table 1.
Table 1. Characteristics of Participants and Physical Activity Interventions.
| Study First author (year) |
Year(s) of trial f |
Sample Size |
Mean age of Participants (in years)b |
Main Race/Ethnicity |
Primary Intervention Components |
Intervention Setting |
Intervention Duration (in weeks) |
Number (Frequency) of Intervention Sessions |
Theory Use Mentioned (Y/N) |
Primary Method of PA Behavior Assessment d | Quality Scoree (n/10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basen-Engquist (2006) | 2003-2004 | N = 60 | 55 | Mixedc | Workshops, group exercise, walking (pedometers provided) | Clinic, community (local church), home-based | 24 | 21 (group meeting 1× per wk for 16 wks, alternating wks for 8 wks) | Y | Researcher-administered questionnaire (7-DPARQ) | 8/10 |
| Bloom (2008) | NR g | N= 404 | 45 | White | Workshops, group exercise | Community, home-based | 12 | 3 (1 6-hour workshop per month) | Y | Self-administered survey (created by authors) | 4/10 |
| Daley (2007 a, 2007) | 2005 | N = 108 | 51 | White | Behavioral counseling (in person), group exercise | Clinic (university center) | 8 | 24 (3 supervised exercise sessions per wk) | Y | Self-administered survey (GLTEQ) | 9/10 |
| Kim (2011) | NR | N = 45 | 46 | Asian | Behavioral counseling (by phone), walking (pedometer s provided) | Home-based | 12 | 12 (1 phone counseling session per wk) | Y | Researcher-administered questionnaire (IPAQ) | 8/10 |
| Greenlee (2013) | 2007-2008 | N = 42 | 50.6 | Latino | Behavioral counseling (in person and by phone), walking or similar activity | Community (health club) | 26 | 78 (3 supervised exercise sessions per wk) | N | Self-administered survey (Kaiser PA Survey) | 7/10 |
| Hatchett (2012) | NR | N = 74 | NR | White | Behavioral counseling (by e-mail), any aerobic activity | Home-based | 12 | 8 (1 e-mail per wk for 5 wks, alternating wks for 6 wks) | Y | Researcher -administered questionnaire (7 day PAR) | 6/10 |
| Irwin (2008 a), Latka (2009) | 2006 | N = 75 | 55.8 | White | Behavioral counseling (in group), group exercise, walking or similar activity | Community (health club), home-based | 26 | 78 (3supervised exercise sessions per wk) | Y | Researcher-administered Questionnaire (PAQ) | 9/10 |
| Ligibel (2011) | 2009 | N = 100 | 54.3 | White | Behavioral counseling (by phone), walking (pedometer provided) | Home-based | 16 | 10 (1 phone counseling session per 1-1.5 wks) | Y | Researcher-administered questionnaire (7-day PAR) | 7/10 |
| Matthews (2007) | NR | N = 36 | 54.1 | White | Behavioral counseling (in person and by phone), walking (accelerometer provided) | Home-based | 12 | 6 (1 in-person, 5 phone counseling sessions spread over 12 wks) | Y | Self-administered survey (CHAMP S) | 8/10 |
| Pinto (2005 a, 2008 and 2009) | NR | N = 86 | 53.14 | White | Behavioral counseling (by phone and in person), walking or similar activity (pedometer provided) | Home-based | 12 | 13 (1 in-person PA demo, 12 wkly phone counseling sessions) | Y | Researcher -administered questionnaire (7-day PAR) | 7/10 |
| Pinto (2013) | NR | N = 192 | 60 | White | Behavioral counseling (by health provider and by phone), walking or similar activity (pedometer provided) | Clinic, home-based | 12 | 14 (1 doctor contact, 1 in-person PA demo, 8 phone counseling sessions, wkly for 4 wks, alternating wks for 4 wks) | Y | Researcher -administered questionnaire (7-day PAR) | 9/10 |
| Rogers (2009) | 2007 | N = 41 | 53 | White | Behavioral counseling (group and individual), group exercise, walking (accelerometer provided) | Clinic, home-based | 12 | 21 (6 discussion groups, 12 supervised exercise sessions, 3 in-person counseling, spread over 12 wks) | Y | Self-administered survey (GLTEQ) | 9/10 |
| Nikander (2007), Penttinen (2009), Saarto (2012 a) | 2005-2008 | N = 500 | 52 | White | Behavioral counseling (in person), group exercise, walking or similar activity | Clinic (university center), home-based | 52 | 52 (1 supervised exercise session per week) | N | Self-administered log (7-day PA record) | 10/10 |
| Vallance (2007 a, 2008 and 2010) | 2006 | N = 377 | 58 | White | PA guidebook, walking (pedometer provided) | Home-based | 12 | No structured intervention sessions | Y | Self-administered survey (GLTEQ) | 9/10 |
Indicates main outcomes paper used for analysis, in addition to related papers.
Where not explicitly reported (e.g., provided mean age by treatment arm), overall mean age was estimated based on other data provided.
Predominant racial/ethnic descriptors applied when participants from a specific group represented 60% or more of the sample. In cases where less than 60% of any one group was represented, the study population was described as a “mix” of racial/ethnic groups.
Assessment tool listed provided appropriate data to calculate effect size estimates for PA behavior change.
A quality score for each study was calculated using 10 criteria derived from CONSORT, as described in the methods section.
Years trial was conducted. If not directly reported, timeframe estimated based on reported completion of trial recruitment.
Items not reported listed as “NR.”
Study Population Description
The total number of participants across the 14 studies was 2,140 with a mean sample size of 153 post-treatment BCS per study. The majority of participants in the review were white with a mean age of 49 years. Two studies included only non-white participants [27, 28], while the remaining studies included white or ethnically mixed populations (Table 1). Based on reported data, the estimated mean time since diagnosis across studies was 2.6 years.
Most participants in the 14 studies reported receiving an early cancer diagnosis at Stage I or Stage II of disease. Most studies excluded women diagnosed at Stage IV. The majority (> 60%) of participants in all studies had received multiple treatment modalities, including surgery plus one or more forms of adjuvant therapy. The participants across the studies in the review reported high levels of education; most (65%) had attended some college, received a college degree and/or pursued graduate level education. Income level was specified as a coding item, but insufficient data was available to report on this descriptor.
Intervention Design
Intervention design was assessed by examining the primary mode(s) of intervention (e.g., PA type, method), level of supervision, as well as intervention setting, duration, number of treatment sessions and behavioral theory use.
Consistent with literature that supports walking as an acceptable form of PA for BCS [29], most studies used a walking component in their intervention design. Seven studies provided either a pedometer or accelerometer to participants. In seven cases, some form of behavior counseling was offered by phone [20, 27], by e-mail [30], in person [31, 32] or a combination of these [33-35]. Coaching strategies varied, but included activities such as in-person PA demonstrations [34, 35], counseling from a healthcare provider [35], supervised exercise sessions combined with cognitive-behavioral therapy [31], tailored counseling sessions [27] and group discussions about perceived barriers and goals related to PA [32, 36].
Five studies [31, 32, 37-39] offered group exercise options and alternatives to walking in their intervention design. For example, Irwin (2008) reported that, in the home-based portion of the study, participants were asked to walk or do another aerobic activity of their choice. Bloom (2008) offered three group workshops, of which group exercise (Qigong, yoga or walking) was a part. Greenlee (2013) reported an intervention offered entirely in a commercial exercise studio (Curves for Women), though walking or similar MVPA using exercise equipment was still the focus.
Despite the similarities in PA type and intervention methods, the level of interaction with the research team and other intervention characteristics (duration, setting and use of theory) varied highly across the studies. The mean duration of the RCTs was 17 weeks, but the supervised exercise and/or counseling sessions associated with each intervention ranged from 3 sessions (1×/month over 12 weeks) [37] to 78 sessions (3×/week for 26 weeks) [28, 38, 40]. Most studies reported sessions that were 45 minutes or less.
The majority of the intervention designs relied on a home-based setting, in whole or in part. Half of the interventions were entirely home-based [20, 27, 30, 33, 34, 41]; two took place exclusively in a clinic or research-setting [31, 37]; the remaining studies took place in mixed settings, combining clinical/research sites with community or home-based environments. Though most of the home-based designs involved some form of ongoing interaction or counseling with the research team, one study [41] used only printed materials and pedometers to guide participants on PA.
Finally, theory use was assessed via identification of a specific behavior theory in program development. Twelve studies cited at least one theory or theoretical construct that was used in intervention design. For example, Kim (2011) used the Transtheoretical Model [42] to develop a stage-matched intervention using counseling and stage-based workbooks. Hatchett (2013) and Rogers (2009) also described the use of Social Cognitive Theory [43] to match intervention methods to theoretical constructs. But, most other studies mentioned relevant theories or theoretical constructs without extensive discussion of theory application.
Heterogeneity, Measures and Quality Assessment
Statistical testing revealed a moderate to high level of variability in effect size attributable to heterogeneity (I-squared = 76.6%) but a relatively low estimate of between-study variance (Tau-squared = 0.10). The heterogeneity may be due to the differences in population and intervention design previously described or methods of measurement for PA behavior change. For example, seven studies used a researcher-administered PA recall questionnaire, such as the 7-day PA Recall Questionnaire [44]. The remaining studies used another validated self-report instrument, such as the Godin Leisure-time Questionnaire [45] or CHAMPS Survey [46] to collect data on PA performed by participants. These surveys vary on several factors, such as units to calculate PA summary estimates (e.g., METs per week or kcal per week) and recall strategies (e.g., prompts) or time frame, which may also affect estimates.
Based on the previously described quality assessment procedures derived from CONSORT criteria, a quality score (n/10) was assigned to each study (Table 1). Most studies (k=10) achieved 80% or more of 10 quality indicators. However, there was some variation in transparency of reporting, especially related to randomization procedures and between-group differences, which may have affected how effect sizes were calculated.
Synthesis of Results
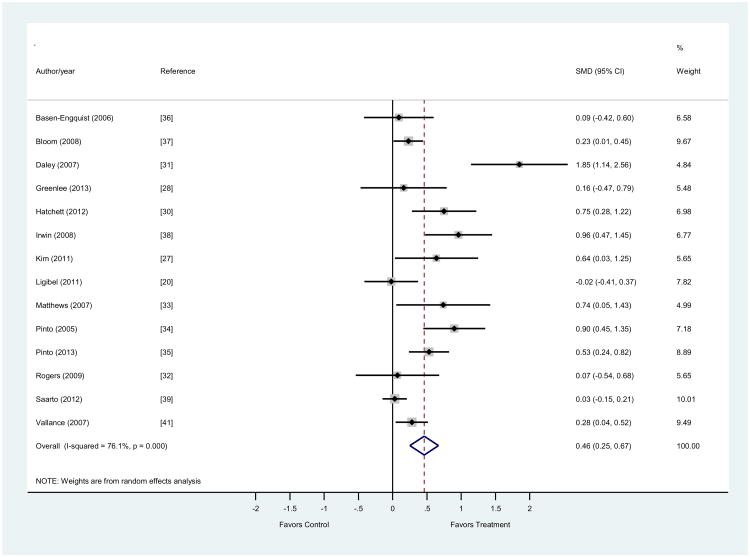
The overall SMD was 0.47 (0.23, 0.67) with a p < 0.001. The range for effect sizes was -0.02 (-0.41, 0.37) to 1.85 (1.15, 2.6) at the 95% confidence level. Out of 14 studies, all but one [20] positively favored the treatment group and eight were statistically significant. The effect sizes for the four studies with non-significant results all approached zero, as illustrated in Figure 3.
Figure 3. Forest plot for effects of physical activity interventions on behavior change.
Studies (n=14) measured changes in physical activity behavior comparing results from control to treatment groups. The size of the shaded boxes around each study represents the relative weight assigned to the study in calculating an overall estimate of standardized mean difference (SMD), which in this case was calculated using Hedge's g to reduce positive bias. Larger boxes indicate that greater weight was assigned to each study. Lines going through each box represent the confidence interval for each estimate.
To isolate variability related to interaction with the research team, an additional exploratory analysis was conducted in which studies were stratified by low, medium and high intensity levels of supervision/monitoring during the intervention, as previously described. The studies in the high intensity group had the largest effect, with an overall SMD of 0.69 (-0.08, 1.5), p=0.08 (not significant). The low intensity group had the lowest overall SMD of 0.23 (0.09, 0.38), p <0.002. The details of this subgroup analysis are reported in Table 2.
Table 2. Results of Sub-group Analysis by Intensity of Direct Supervision/Monitoring.
| Low Supervision/Monitoringa | ||||
|---|---|---|---|---|
| Author (year) | SMD (g)b | [95% CI] | % Weight | |
| Basen-Engquist (2006) | 0.09 | --0.42 | 0.59 | 8.45 |
| Bloom (2008) | 0.23 | 0.01 | 0.45 | 46.14 |
| Greenlee (2013) | 0.16 | -0.47 | 0.8 | 5.32 |
| Vallance (2007) | 0.23 | 0.09 | 0.38 | 100 |
| Pooled Effect Size | 0.23 | 0.09 | 0.38 | 100 |
| Medium Supervision/Monitoring | ||||
| Author (year) | SMD (g) | [95% CI] | % Weight | |
| Hatchett (2012) | 0.75 | 0.28 | 1.22 | 16.48 |
| Kim (2011) | 0.64 | 0.04 | 1.24 | 12.91 |
| Ligibel (2011) | -0.02 | -0.41 | 0.37 | 19.24 |
| Matthews (2007) | 0.74 | 0.05 | 1.42 | 10.87 |
| Pinto (2005) | 0.90 | 0.46 | 1.35 | 17.39 |
| Pinto (2013) | 0.53 | 0.24 | 0.82 | 23.12 |
| Pooled Effect Size | 0.56 | 0.28 | 0.85 | 100 |
| High Supervision/Monitoring | ||||
| Author (year) | SMD (g) | [95% CI] | % Weight | |
| Daley (2007) | 1.85 | 1.14 | 2.55 | 22.79 |
| Irwin (2008) | 0.96 | 0.48 | 1.44 | 25.43 |
| Rogers (2009) | 0.07 | -0.55 | 0.68 | 23.92 |
| Saarto (2012) | 0.03 | -0.15 | 0.2 | 27.86 |
| Pooled Effect Size | 0.69 | -0.08 | 1.45 | 100 |
Low = minimal/no individual oversight
Medium = counseling but no supervised exercise sessions
High = highly structured program with in-person supervision
Standardized mean differences (SMD) for behavior change effects calculated using Hedge's g
Discussion
This review is one of the first to evaluate evidence for PA behavior change interventions generated exclusively from RCTs, looking only at recent post-treatment BCS. The purpose was to describe the features of PA behavior change interventions for post-treatment BCS and to assess the impact of these interventions in effecting behavior change. With a statistically significant overall SMD of 0.47 (0.26, 0.67), it can be inferred using conventional criteria that the interventions collectively suggest a moderate but positive effect on behavior change in participating survivors.
There were many similarities in intervention designs that appeared to enhance their success. Most interventions used self-monitoring or coaching techniques in various combinations, with varying media. For example, several studies supplied research-grade pedometers or accelerometers to participants, in addition to self-report tools, a strategy known to support measurement validity and help participants monitor their progress [47]. Half of the studies gathered survivors in workshops or peer support groups of some kind during the intervention. Additionally, individual counseling was frequently used to motivate participants and address barriers to PA. Both strategies are well-supported in PA literature with older women and with cancer survivors [48, 49]. The home-based environment and walking as the primary type of exercise were emphasized in all the interventions in some form, which has also been a successful component in lifestyle studies with similar populations [50-53].
A notable finding in this review was the wide variation across studies in terms of direct supervision/monitoring of the participants during the intervention. Based on this finding, the studies were stratified by supervision/monitoring level, using procedures previously described. From this, it was observed that more intense supervision (i.e., in-person, frequent interactions) tended to produce larger effects on behavior change. However, it should also be noted that five of the six largest effect sizes came from trials in the “medium” intensity supervision/monitoring group, which included home-based programs and counseling delivered by telephone or e-mail. This may be an important insight in exploring less resource-intensive options for effective behavior change strategies for recent post-treatment survivors. Similar behavioral interventions delivered by telephone were found to be moderately effective in increasing PA in recent systematic review papers for various chronic health conditions [54, 55]. Telephone-based delivery of PA counseling may also be advantageous in outreach to older adults and underserved populations [56, 57].
It should be noted that the largest individual intervention effect sizes in our review did not generally come from the studies with the largest sample size or the longest exposure to the intervention. In fact, the largest (n=500) and longest trial (12 months) reviewed [39, 58, 59] showed almost no effect on PA behavior in participants post-intervention. This information is instructive in planning behavior interventions that balance the benefits of program structure and supervision with other components that may stimulate behavior change.
A few questions remain after this literature review. As most of these studies captured only PA changes during the intervention period, more information about the sustainability of these behavior changes in real world settings, outside of carefully controlled trials, is needed [60]. A few trials in this review [31, 31, 61-63] included additional follow-up measures post-intervention (e.g. 6 month). In these cases, modest gains in PA post-intervention were generally not sustained. In most other cases, the long-term effects of these interventions could not be evaluated based on available data. Sustainability of behavior changes remains a critical question in behavioral research. Additionally, many of the studies recruited BCS who were identified as sedentary at the start of the trial. It has been argued that the change mechanism in active versus inactive survivors is different and should be thoughtfully addressed as part of intervention design [64]. Finally, cancer events such as diagnosis and completion of primary treatment have been suggested as unique windows of opportunity or ‘teachable moments’ to influence behavior [65]. Most women in the studies reviewed were 3 years or less from initial diagnosis and demonstrated largely similar PA patterns. However, there may be additional opportunities to investigate the role of time since diagnosis and teachable moments relative to health behaviors in cancer survivors.
Another important unanswered question is the role of behavior theory in intervention development. In this review, studies were evaluated only on whether or not behavior theory was cited in relation to intervention design. Most of the studies mentioned a specific theory or theoretical construct in intervention development. However, experts suggest that researchers frequently describe behavior interventions as theory-based, though theory application in development of interventions for complex behaviors requires careful planning beyond simply identifying an appropriate theory [66]. Further investigation of theory application using a systematic coding framework could be beneficial [67, 68].
This review had several limitations. The search strategy included only articles published in peer-reviewed journals, in English, and which focused on BCS who had recently completed cancer treatment. There may be additional studies published in other languages or in journals not available through the chosen databases that may have contributed to knowledge of behavior change interventions for BCS within five years of completing cancer treatment. Additionally, although several steps were taken to reduce bias and increase estimate accuracy, the reported effects are estimates only and are a reflection of the accuracy and consistency of the data as originally reported. In at least one case [31], it is believed that the incomplete reporting of trial data may have inflated the effect size estimate, but lack of data impeded additional adjustments. Incomplete or inconsistent reporting in RCTs has been identified as a common barrier in similar translational research, especially with PA studies [69].
Many studies used self-reported PA measures which may be subject to reporting bias [70], but half of the studies also included objective measurement methods (e.g., accelerometer or pedometer) to validate or confirm self-reported measures. Matthews et al, for example, found a strong correlation (rho=0.65) between measures obtained by accelerometer and those self-reported in the CHAMPS survey in the Breast Cancer Walking Study [33]. Others [32] found similar improvement trends in objective and self-reported measures, but noted that estimated PA counts were higher in self-reported data. But, in order to calculate a pooled effect size for our meta-analysis, we were limited to the common measure (self-report) that was provided in all studies. Self-reported data measures are also especially useful in establishing context for physical activity behavior, which is valuable to understanding behavioral factors but not always well represented by mechanical devices [71].
More generally, this review highlights challenges of the literature itself on this subject. There were surprisingly few RCTs that have tested PA interventions with post-treatment BCS in the eight-year time frame covered by our literature search. Of these studies, most included only white women, in their 40s or 50s, diagnosed at an early stage of disease and living in medium or large metropolitan areas. Findings within these populations may not be generalizable to older or younger survivors, women diagnosed at later stages of disease, or to survivors that live in rural or inner-city settings [72]. It was also observed that study quality, even for a group of RCTs, varied widely. While some studies carefully reported randomization procedures and between-group differences, for example, others were less transparent in how some of the effect size estimates were made.
Despite these challenges, this analysis represents one of few studies of its kind to comprehensively and rigorously assess experimental studies testing PA behavioral interventions in recent post-treatment BCS. It was also observed that many of the developments in this literature have occurred in the last few years. In fact, although many of the trials started earlier, nearly half were not published until 2011-2013. As such, this paper offers specific and timely insights on PA behavior research that will contribute in a meaningful way to the growing survivorship literature.
Conclusion
The 2005 IOM survivorship report challenged the medical and scientific community to change the experience of cancer survivors, especially as they transition to long-term recovery. This review and meta-analysis systematically captured results from domestic and international RCTs from 2005 to the present, offering insight on progress in developing effective interventions to help BCS adopt a physically active lifestyle as part of the path to recovery. Data from these studies suggest that they produced modest but positive effects on PA levels in post-treatment BCS. They also offer timely and relevant information about the features and success of behavior interventions in changing PA in BCS in the critical period following active treatment.
Despite the promising results, these findings reveal a lack of literature on empirically tested interventions for this vulnerable population. PA is a complex behavior even for healthy adults. For post-treatment BCS, the prospect of initiating, re-initiating or maintaining an active lifestyle following primary cancer treatment is fraught with challenges that are not yet fully understood. More research, especially in large experimental studies, is needed to better understand the needs of post-treatment BCS and to facilitate sustainable lifestyle changes.
Acknowledgments
Dr. Bluethmann was supported by the Susan G. Komen Foundation (KG111378) and the Cancer Education and Career Development Program at the School of Public Health, University of Texas Health Science Center at Houston, funded by the National Cancer Institute (R25T 2R25CA57712). The findings and conclusions in this presentation are those of the authors and do not necessarily represent the official positions of the Susan G. Komen Foundation or the National Cancer Institute.
The authors would also like to thank the following individuals for their assistance with this review: Gilbert Ramirez, Patricia Dolan Mullen, Helena Vonville and Sohini Dhar.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Contributor Information
Shirley M. Bluethmann, Email: shirley.m.bluethmann@uth.tmc.edu.
Sally W. Vernon, Email: sally.w.vernon@uth.tmc.edu.
Kelley Pettee Gabriel, Email: kelley.p.gabriel@uth.tmc.edu.
Caitlin C. Murphy, Email: caitlin_murphy@med.unc.edu.
L. Kay Bartholomew, Email: leona.k.bartholomew@uth.tmc.edu.
References
- 1.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. National Academies Press; 2005. [Google Scholar]
- 2.Ganz PA. Quality of care and cancer survivorship: the challenge of implementing the institute of medicine recommendations. J Oncol Pract. 2009;5:101–105. doi: 10.1200/JOP.0934402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians. 2012 doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 4.Physical Activity and Public Health: Updated Recommendation for Adults From the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 5.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz KH, Holtzman J, Courneya KS, et al. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 7.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Underwood JM, Townsend JS, Stewart SL, et al. Surveillance of Demographic Characteristics and Health Behaviors Among Adult Cancer Survivors: Behavioral Risk Factor Surveillance System, United States, 2009. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [PubMed] [Google Scholar]
- 9.Smith SG, Chagpar AB. Adherence to Physical Activity Guidelines in Breast Cancer Survivors. Am Surg. 2010;76:962–965. [PubMed] [Google Scholar]
- 10.Sabiston CM, Brunet J. Reviewing the benefits of physical activity during cancer survivorship. American Journal of Lifestyle Medicine. 2012;6:167–177. [Google Scholar]
- 11.Speck RM, Courneya KS, Masse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of Cancer Survivorship. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz K. Physical activity and breast cancer survivorship. Recent Results in Cancer Research. 2011;186:189–215. doi: 10.1007/978-3-642-04231-7_8. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer RA, Huedo-Medina T, Johnson BT, et al. Exercise Interventions for Cancer Survivors: A Meta-Analysis of Quality of Life Outcomes. Annals of Behavioral Medicine. 2011;41:32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfano CM, Molfino A, Muscaritoli M. Interventions to promote energy balance and cancer survivorship Interventions to promote energy balance and cancer survivorship: European and North American priorities for research and care. Cancer. 2013;119:2143–2150. doi: 10.1002/cncr.28062. 0008543X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database of Systematic Reviews. 2010:005211. doi: 10.1002/14651858.CD005211.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Rabin C. Promoting Lifestyle Change Among Cancer Survivors: When Is the Teachable Moment? American Journal of Lifestyle Medicine. 2009 Sep-Oct;3:369–378. [Google Scholar]
- 17.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Lipsey MW, Wilson DB. Practical Meta-analysis. Sage Publications; 2001. [Google Scholar]
- 20.Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spark LC, Reeves MM, Fjeldsoe BS, et al. Physical activity and/or dietary interventions in breast cancer survivors: a systematic review of the maintenance of outcomes. Journal of Cancer Survivorship. 2013;7:74–82. doi: 10.1007/s11764-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 22.Hedges LV ERIC Clearinghouse. Statistical Methodology in Meta-Analysis 1982 [Google Scholar]
- 23.Gupta SK. Intention-to-treat concept: A review. Perspectives in Clinical Research. 2011;2:109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal (International Edition) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Smith GD. Bias in meta-analysis detected by a simple, graphical test. BMJ: British Medical Journal (International Edition) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Shin MS, Lee HS, et al. Randomized pilot test of a simultaneous stage-matched exercise and diet intervention for breast cancer survivors. Oncol Nurs Forum. 2011;38:E97–106. doi: 10.1188/11.ONF.E97-E106. [DOI] [PubMed] [Google Scholar]
- 28.Greenlee HA, Crew KD, Mata JM, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity. 2013;21:65–76. doi: 10.1002/oby.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 30.Hatchett A, Hallam JS, Ford MA. Evaluation of a social cognitive theory-based email intervention designed to influence the physical activity of survivors of breast cancer. Psychooncology. 2013;22:829–836. doi: 10.1002/pon.3082. [DOI] [PubMed] [Google Scholar]
- 31.Daley AJ, Crank H, Saxton JM, et al. Randomized trial of exercise therapy in women treated for breast cancer. Journal of Clinical Oncology. 2007;25:1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 32.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Medicine & Science in Sports & Exercise. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 33.Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Supportive Care in Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 34.Pinto BM, Frierson GM, Rabin C, et al. Home-based physical activity intervention for breast cancer patients. Journal of Clinical Oncology. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 35.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychology. 2013;32:616–626. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 36.Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Education & Counseling. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Bloom JR, Stewart SL, D'Onofrio CN, et al. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term, low intensity intervention produce change? Journal of Cancer Survivorship. 2008;2:190–204. doi: 10.1007/s11764-008-0058-x. [DOI] [PubMed] [Google Scholar]
- 38.Irwin ML, Cadmus L, Alvarez-Reeves M, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial. Cancer. 2008;112:2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saarto T, Penttinen HM, Sievanen H, et al. Effectiveness of a 12-month Exercise Program on Physical Performance and Quality of Life of Breast Cancer Survivors. Anticancer Res. 2012;32:3875–3884. [PubMed] [Google Scholar]
- 40.Latka RN, Alvarez-Reeves M, Cadmus L, et al. Adherence to a randomized controlled trial of aerobic exercise in breast cancer survivors: the Yale exercise and survivorship study. Journal of Cancer Survivorship. 2009;3:148–157. doi: 10.1007/s11764-009-0088-z. [DOI] [PubMed] [Google Scholar]
- 41.Vallance JK, Courneya KS, Plotnikoff RC, et al. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. Journal of Clinical Oncology. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 42.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American journal of health promotion. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 43.Bandura A. Social cognitive theory: An agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Hayden HA. Validation of the Telephone and In-Person Interview Versions of the 7-Day PAR. Medicine & Science in Sports & Exercise. 2003;35:801–809. doi: 10.1249/01.MSS.0000064941.43869.4E. [DOI] [PubMed] [Google Scholar]
- 45.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Journal canadien des sciences appliquees au sport. 1985;10:141. [PubMed] [Google Scholar]
- 46.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Murphy SL. Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Prev Med. 2009;48:108–114. doi: 10.1016/j.ypmed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ottenbacher AJ, Day RS, Taylor WC, et al. Exercise among breast and prostate cancer survivors-what are their barriers? J Cancer Surviv. 2011 doi: 10.1007/s11764-011-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers LQ, Vicari S, Courneya KS. Lessons learned in the trenches: facilitating exercise adherence among breast cancer survivors in a group setting. Cancer Nurs. 2010;33:E10–7. doi: 10.1097/NCC.0b013e3181db699d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ottenbacher AJ, Day RS, Taylor WC, et al. Long-term physical activity outcomes of home-based lifestyle interventions among breast and prostate cancer survivors. Support Care Cancer. 2012 doi: 10.1007/s00520-011-1370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36:185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Knols RH, de Bruin ED, Shirato K, et al. Physical activity interventions to improve daily walking activity in cancer survivors. BMC Cancer. 2010;10:406. doi: 10.1186/1471-2407-10-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eakin EG, Lawler SP, Vandelanotte C, et al. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med. 2007;32:419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: an updated systematic review. Am J Prev Med. 2012;42:81–88. doi: 10.1016/j.amepre.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 56.Marcus BH, Williams DM, Dubbert PM, et al. Physical Activity Intervention Studies: What We Know and What We Need to Know: A Scientific Statement From the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114:2739–2752. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 57.Gustafson DH, McTavish FM, Stengle W, et al. Reducing the digital divide for low-income women with breast cancer: a feasibility study of a population-based intervention. J Health Commun. 2005;10:173–193. doi: 10.1080/10810730500263281. [DOI] [PubMed] [Google Scholar]
- 58.Penttinen H, Nikander R, Blomqvist C, et al. Recruitment of breast cancer survivors into a 12-month supervised exercise intervention is feasible. Contemporary Clinical Trials. 2009;30:457–463. doi: 10.1016/j.cct.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Nikander R, Sievanen H, Ojala K, et al. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients--a 12-month RCT. Journal of Musculoskeletal Neuronal Interactions. 2012;12:127–135. [PubMed] [Google Scholar]
- 60.Courneya KS. Efficacy, effectiveness, and behavior change trials in exercise research. Int J Behav Nutr Phys Act. 2010;7:81. doi: 10.1186/1479-5868-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daley AJ, Crank H, Mutrie N, et al. Determinants of adherence to exercise in women treated for breast cancer. European Journal of Oncology Nursing. 2007;11:392–399. doi: 10.1016/j.ejon.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Vallance JK, Courneya KS, Plotnikoff RC, et al. Maintenance of physical activity in breast cancer survivors after a randomized trial. Medicine & Science in Sports & Exercise. 2008;40:173–180. doi: 10.1249/mss.0b013e3181586b41. [DOI] [PubMed] [Google Scholar]
- 63.Pinto BM, Rabin C, Papandonatos GD, et al. Maintenance of effects of a home-based physical activity program among breast cancer survivors. Supportive Care in Cancer. 2008;16:1279–1289. doi: 10.1007/s00520-008-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabin CS, Pinto BM, Trunzo JJ, et al. Physical activity among breast cancer survivors: regular exercisers vs participants in a physical activity intervention. Psychooncology. 2006;15:344–354. doi: 10.1002/pon.961. [DOI] [PubMed] [Google Scholar]
- 65.Demark-Wahnefried W, Aziz NM, Rowland JH, et al. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartholomew LK, Parcel GS, Kok G, et al. Planning health promotion programs: an intervention mapping approach. Jossey-Bass; 2011. [Google Scholar]
- 67.French SD, Green SE, O'Connor DA, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implementation Science. 2012;7:38. doi: 10.1186/1748-5908-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychology. 2010;29:1. doi: 10.1037/a0016939. [DOI] [PubMed] [Google Scholar]
- 69.White SM, McAuley E, Estabrooks PA, et al. Translating physical activity interventions for breast cancer survivors into practice: an evaluation of randomized controlled trials. Annals of Behavioral Medicine. 2009;37:10–19. doi: 10.1007/s12160-009-9084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 71.Sallis JF, Cervero RB, Ascher W, et al. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 72.Weaver KE, Palmer N, Lu L, et al. Rural-urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control. 2013;24:1481–1490. doi: 10.1007/s10552-013-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]