Abstract
Purpose
Perturbations of transforming growth factor-beta (TGF-β) signaling are pivotal to tumorigenesis and tumor progression through their effects on cell proliferation and cell invasion. This study aims to evaluate the association of TGF-βRII and pSmad2 protein expressions in breast tissue with clinicopathological factors and prognosis of breast cancer.
Methods
Expression of the TGF-βRII and pSmad2 proteins was assessed in breast tissue of 1,045 breast cancer cases in the Shanghai Breast Cancer Study using a double immunofluorescence staining method, which was validated with standard single immunostains.
Results
TGF-βRII expression intensity was positively associated with younger age at diagnosis (P=0.03), pre-menopausal status (P=0.03), and lower TNM stage (P=0.04). Cytoplasmic predominant expression pattern of TGF-βRII was associated with older age at diagnosis (P=0.04) and invasive histological type (P=0.03). Increased pSmad2 expression was associated with higher breast cancer grade (P<0.01). Higher pSmad2 expression (HR (95%CI):1.48 (1.07–2.04), P=0.02) and cytoplasmic predominant TGF-βRII expression (HR (95%CI): 1.80 (1.08–3.00), P=0.02) were significantly associated with reduced cancer-free survival.
Conclusions
Our data suggest that TGF-βRII and pSmad2 expressions are associated with certain clinical and pathologic features of breast cancer. A cytoplasmic predominant TGF-βRII expression pattern and a higher pSmad2 expression were associated with decreased breast cancer survival. Our study provides additional evidence to support the important role of TGF-β signaling in breast cancer prognosis.
Keywords: TGF-β signaling, TGF-βRII, pSmad2, Breast cancer, Prognosis
Introduction
Perturbations of transforming growth factor-beta (TGF-β) signaling are central to tumorigenesis and tumor progression via their effects on cell proliferation and cell invasion [1]. The physiological consequences of TGF-β signaling are highly contextual with different or even opposite TGF-β functions in cancerous and normal cells [2]. TGF-β family members signal through a heteromeric complex of transmembrane serine/threonine kinases, the type I and II receptors (TGF-βRI and TGF-βRII), which subsequently phosphorylate receptor-regulated Smad proteins (R-Smads). R-Smads usually translocate to the nucleus together with the common mediator, Smad4, where they regulate gene transcription by binding to the promoter of target genes [3–8]. Loss of TGF-β responsiveness frequently occurs at the level of TGF-βRII in many tumors, including breast cancer, colon cancer, and glioma [9–12]. Smad2 is a major receptor-activated Smad downstream of TGF-β signaling. Phospho-Smad2 (pSmad2) is translocated into the nucleus to modulate the transcription of target genes involved in many cell functions [13–18]. Our previous data indicated that high circulating levels of TGF-β1 are associated with worse survival independent of disease stage [19], implying that TGF-β signaling may play an important role in breast cancer prognosis. However, no large studies have examined TGF-β signaling protein expression in both breast tumor and adjacent normal tissues [20]. In this study, we evaluated the correlation of TGF-βRII and pSmad2 protein expression in 1,045 breast cancer cases with clinicopathological factors in human breast cancer tissue and prognosis of breast cancer from the Shanghai Breast Cancer Study.
Materials and Methods
Study populations
The study population is from the second phase of the Shanghai Breast Cancer Study (SBCS-II), a population-based case-control study being conducted in Shanghai, China [21–23]. Briefly, breast cancer cases were identified via the Shanghai Cancer Registry. Recruitment occurred between April 2002 and February 2005. Cancer diagnoses were reviewed and confirmed by two senior pathologists. A structured questionnaire was used to elicit detailed information on demographic factors for breast cancer. Trained interviewers measured all participants for weight, height, and circumferences of the waist and hips. All interviews were tape-recorded and reviewed by the field supervisor and quality control staff. The study was approved by the institutional review boards at all participating institutes, and all participants provided written, informed consent before participating in the study.
Clinicopathological data
Pathological slides for 1,045 cases were available for this study. The slides were collected from the diagnosis hospitals according to a standard protocol. Clinical information collected included cancer stage, tumor ERα and progesterone receptor (PR) status, and primary treatments. The HER2 status of cancer cases was evaluated previously by a centralized laboratory [23]. The diagnoses and clinicopathological data were confirmed by a combination of medical chart review and a centralized review of pathology slides. The histological types of breast cancer were confirmed according to the criteria of the World Health Organization classification [24] by the research pathologist (Su). The histologic grade of all cancer slides was determined using the Nottingham histologic grading system.
Double-label fluorescent immunohistochemistry staining for TGF-βRII and pSmad2
Pathological sections of breast cancer samples were deparaffinized, and a sequential double immunofluorescence staining was performed using a Dako automated immunostainer (Dako Colorado, Inc., U.S.A). In brief, the slides were put in citrate buffer (pH6, ZyMed, Cat# 00-5000), heated with a programmed pressure cooker (PickCell Laboratories B.V., Amsterdam, the Netherlands) for 2 hours for antigen retrieval. After blocking steps with 3% H2O2, 5% normal goat serum, biotin solution, and avidin D solution (Vector, Cat# SP-2001), the slides were incubated with polyclonal rabbit antibody anti-TGFβRII (Spring, Cat# E11244, 1:100) overnight at 4°C; biotin conjugated goat anti-rabbit (Vector, Cat# BA-1000, 1:300) for 30 minutes at 37°C; and streptavidin-Cy3 (Zymed, Cat# 43-8315, 1:100) for 15 minutes at 37°C. The slides were then incubated with polyclonal rabbit antibody anti-pSmad2 (Ser465/467) (Cell Signaling, Cat# 9510, 1:200) for 30 minutes at 37°C; biotin conjugated goat anti-rabbit for 30 minutes at 37°C; and streptavidin-FITC (Zymed, Cat# 43-8311, 1:100) for 30 minutes at 37°C. Slides were washed thoroughly, the coverslip was mounted with ProLong Gold antifade reagent with DAPI (Invitrogen, Cat# P36935), and slides were stored in the dark at 4°C. The double immunofluorescence staining protocol was validated by comparing it with a single standard staining method by the DAKO Envision™ kit (DAKO, Cat#K4011) using the control slides freshly cut from a lab-constructed tissue microarray (TMA) block which included one placenta tissue and three breast cancer tissues with tumor grades 1, 2, and 3 (Figure 1). The TMA slides were also used as quality controls. Each batch of staining samples included two TMA slides as positive and negative controls. Before formal staining of the study samples, four TMA blocks including 182 valid cases of breast cancer made by our centralized laboratory were stained as a training set. Consistent staining results were observed with our system by comparing builtin control tissues and cell lines (Supplementary Figure 1A).
Figure 1.
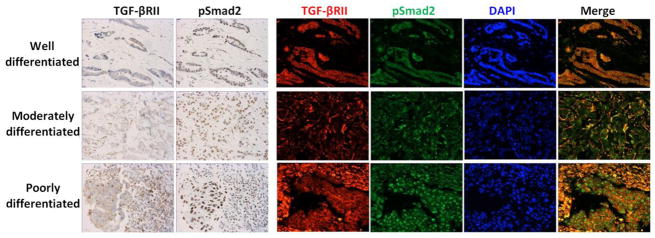
Comparison of double immunofluorescence staining (right four columns) and single standard staining (left two columns) for TGF-βRII and pSmad2 expression in breast cancer tissue, using lab-constructed tissue microarray slides as positive controls. The nuclear pSmad staining was identical between two methods, and positive signal of TGF-βRII was stronger with double immunofluorescent staining method than DAKO single staining kit.
TGF-βRII and pSmad2 were semi-quantified using a modified four-scale Allred Scoring System [25] in which the proportion of positive cells and staining intensity are taken into account: 0 (negative), no positive staining or less than 1/3 cells with weak fluorescent signal which is difficult to be identified under X100 field (A-score ≤ 4); 1 (weak positive), 1/3 – 2/3 cells with weak fluorescent signal (A-score 5); 2 (moderate positive), more than 1/3 cells with moderate fluorescent signal which is easily identified under X100 field or less than 2/3 cells with strong fluorescent signal (A-score 6 –7); and 3 (strong positive), more than 2/3 cells with strong fluorescent signal (A-score 8)(Supplementary Figure 1B). The staining pattern of TGF-βRII was classified into two groups: 1) membranous predominant as beehive-like appearance and 2) cytoplasmic or membranous cytoplasmic as cloudy appearance in the cells (Supplementary Figure 1C). The analysis was carried out independently by two observers (Su and Qiu) and the samples were scored blinded with respect to clinical patient data. All the slides with inconsistent results were jointly evaluated again by the two investigators, and a consensus score was used.
Statistical analysis
Chi-square test and ANOVA were used in the analysis for differences of characteristics and clinicopathological parameters among TGF-βRII and pSmad2 expressions. Fisher’s exact test was used for the data of histological type because histological data was sparse. The primary outcome for this study was disease-free survival (DFS). For the DFS analysis, follow-up time was calculated as the number of days between the date of cancer diagnosis and disease recurrence or date of last survey for women who did not have disease recurrence or died of breast cancer. For women who died of breast cancer but were missing information on disease recurrence, we imputed the date for recurrence on the basis of the tumor–node–metastasis (TNM) stage-specific recurrence rate estimated for the current study. Multivariate Cox proportional hazards models were employed to evaluate the expressions of TGF-βRII and pSmad2 in association with breast cancer survival after adjusting for age at diagnosis, BMI, tumor size, grade, TNM stage, ER/PR status, radiotherapy, chemotherapy, and tamoxifen treatment. Adjusted survival curves, based on a stratified Cox regression model, were applied to compare the breast cancer survival rate among breast cancer patients with different TGF-βRII and pSmad2 expression [26]. All the tests were performed using SAS (version 9.3; SAS Institute, Inc., Cary, North Carolina). The significance levels were set at P< 0.05 and based on two-sided probability.
Results
Table 1 presents the characteristics of study participants. In this study, 1,045 breast cancer patients were included. The mean age was 51.4 years. There were 2.4% breast cancer patients diagnosed at stage 0, 31.9% at stage I, 32.9% at stage IIa, 21.5% at stage IIb, 10.5% at stage III, and 0.69% at stage IV. All patients received surgical treatment (100%) and a vast majority received chemotherapy (94.4%). Radiotherapy was given to 32.1% of patients, whereas 54.2% received tamoxifen therapy. TNM stage (P<0.01), histological grade (P<0.01), tumor size (P<0.01), PR status (P=0.05), and radiotherapy (P<0.01) were significantly associated with DFS.
Table 1.
Characteristics of study participants, Shanghai Breast Cancer Study, Phase II
| Participant characteristics | No. of subjects | Percentage | 5-yr DFS | P |
|---|---|---|---|---|
| Age, y (N=1,045) | ||||
| <45 | 213 | 20.4 | 0.81 | |
| 45–49 | 321 | 30.7 | 0.88 | |
| 50–59 | 323 | 30.9 | 0.82 | |
| ≥ 60 | 188 | 18.0 | 0.83 | 0.16 |
| Mean ± SD = 51.4 ± 8.3 | ||||
| Menopausal status (N=1,045) | ||||
| Pre-menopause | 570 | 54.6 | 0.84 | |
| Post-menopause | 475 | 45.5 | 0.84 | 0.66 |
| Family history of BC (N=1,045) | ||||
| No | 992 | 94.9 | 0.84 | |
| Yes | 53 | 5.1 | 0.77 | 0.31 |
| TNM stage (N=1,015) | ||||
| 0 | 24 | 2.4 | 0.96 | |
| I | 324 | 31.9 | 0.95 | |
| IIa | 334 | 32.9 | 0.89 | |
| IIb | 219 | 21.6 | 0.76 | |
| III | 107 | 10.5 | 0.55 | |
| IV | 7 | 0.7 | 0.29 | <0.01 |
| Histological grade (N=1,038) | ||||
| I | 175 | 16.9 | 0.91 | |
| II | 528 | 50.9 | 0.85 | |
| III | 335 | 32.3 | 0.78 | <0.01 |
| Tumor size (N=981) | ||||
| <=2 cm | 437 | 44.6 | 0.91 | |
| >2 cm | 544 | 55.5 | 0.78 | <0.01 |
| ER/PR/HER2 status (N=1,045) | ||||
| ER Positive | 655 | 62.7 | 0.86 | 0.08 |
| PR Positive | 642 | 61.4 | 0.86 | 0.05 |
| HER2 Positive | 313 | 30.4 | 0.80 | 0.12 |
| Molecular type (N=845) | ||||
| Luminal A | 443 | 52.4 | 0.88 | |
| Luminal B | 150 | 17.8 | 0.83 | |
| HER2 | 125 | 14.8 | 079 | |
| Triple negative | 127 | 15.0 | 0.80 | 0.07 |
| Cancer therapy received (N=1,044) | ||||
| Chemotherapy | 986 | 94.4 | 0.84 | 0.14 |
| Radiotherapy | 335 | 32.1 | 0.77 | <0.01 |
| Tamoxifen | 566 | 54.2 | 0.86 | 0.16 |
Positive TGF-βRII expression was associated with younger age at diagnosis (P=0.03), pre-menopausal status (P=0.03), positive PR status (P=0.03), and lower TNM stage (P=0.04) (Table 2). The cytoplasmic predominant expression pattern of TGF-βRII was associated with older age at diagnosis (P=0.04) and invasive histological type (P=0.03). TGF-βRII protein expression was unrelated to other prognostic factors, such as family history of breast cancer, tumor size, histological grade, ER status, HER2 status, and molecular type. pSmad2 expression was positively associated with higher breast cancer grade (P<0.01) but unrelated to ER, PR, and HER2 expression and other clinicopathological factors (Table 2).
Table 2.
Correlation of TGF-βRII and pSmad2 expressions with clinicopathological parameters of breast cancer, Shanghai Breast Cancer Study, Phase II
| TGFβ-RII intensity (No. of cases/%)
|
TGFβ-RII subcellular pattern (No. of cases/%)
|
pSmad2 intensity (No. of cases/%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | P | 1c | 2d | P | 0–1 | 2 | 3 | P | |
| Age at diagnosis (mean±SD)a | 53.5±8.8 | 50.9±8.1 | 51.6±8.5 | 0.03 | 50.3±7.9 | 51.7±8.5 | 0.04 | 51.5±8.3 | 51.3±8.6 | 51.6± − 8.3 | 0.93 |
| Menopausal status | |||||||||||
| Pre-menopause | 39/7.5 | 224/43.2 | 256/49.3 | 97/18.7 | 422/81.3 | 97/18.7 | 144/27.7 | 278/53.6 | |||
| Post-menopause | 55/12.6 | 176/40.6 | 204/46.8 | 0.03 | 62/14.2 | 374/85.8 | 0.06 | 83/19.0 | 133/30.5 | 220/50.5 | 0.58 |
| Family history (BC) | |||||||||||
| No | 88/9.7 | 380/41.8 | 441/48.5 | 156/17.2 | 753/82.8 | 171/18.8 | 265/29.2 | 473/52.1 | |||
| Yes | 6/13.0 | 21/45.7 | 19/41.3 | 0.57 | 3/6.5 | 43/93.5 | 0.06 | 9/19.6 | 12/26.1 | 25/54.3 | 0.90 |
| BMI (mean±SD) a | 24.3±3.3 | 24.3±3.1 | 24.1±3.4 | 0.57 | 25.1±3.7 | 24.0±3.1 | <0.01 | 24.1±3.4 | 24.2±3.4 | 24.2±3.1 | 0.92 |
| Tumor size | |||||||||||
| <=2 cm | 38/9.6 | 161/40.6 | 198/49.9 | 62/15.6 | 335/84.4 | 82/20.7 | 119/30.0 | 196/49.4 | |||
| >2 cm | 50/9.9 | 214/42.3 | 242/47.8 | 0.83 | 87/17.2 | 419/82.8 | 0.53 | 88/17.4 | 138/27.3 | 280/55.3 | 0.19 |
| Histological type | |||||||||||
| Non-invasive | 2/11.8 | 4/23.5 | 11/64.7 | 7/41.2 | 10/58.8 | 5/29.4 | 6/35.3 | 6/35.3 | |||
| IDC | 71/9.7 | 303/41.5 | 356/48.8 | 119/16.3 | 611/83.7 | 133/18.2 | 205/28.1 | 392/53.7 | |||
| ILC | 4/10.3 | 24/61.5 | 11/28.2 | 3/7.7 | 36/92.3 | 4/10.3 | 13/33.3 | 22/56.4 | |||
| Other | 14/9.2 | 64/42.1 | 74/48.7 | 0.16b | 26/17.1 | 126/82.9 | 0.03b | 36/23.7 | 44/28.9 | 72/47.4 | 0.27b |
| Histological grade | |||||||||||
| I | 7/4.6 | 68/44.4 | 78/51.0 | 27/17.7 | 125/82.3 | 47/30.7 | 40/26.1 | 66/43.1 | |||
| II | 48/10.1 | 200/42.0 | 228/47.9 | 77/16.2 | 399/83.8 | 86/18.1 | 143/30.0 | 247/51.9 | |||
| III | 38/11.8 | 132/41.1 | 151/47.0 | 0.18 | 54/16.8 | 267/83.2 | 0.91 | 45/14.0 | 94/29.3 | 182/56.7 | <0.01 |
| TNM stage | |||||||||||
| 0–I | 25/8.2 | 132/43.4 | 147/48.4 | 54/17.7 | 250/82.2 | 70/23.0 | 91/29.9 | 143/47.0 | |||
| IIa | 24/7.8 | 129/41.7 | 156/50.5 | 54/17.5 | 255/82.5 | 53/17.2 | 89/28.8 | 167/54.0 | |||
| IIb | 32/15.2 | 78/37.0 | 101/47.9 | 33/15.6 | 178/84.4 | 37/17.5 | 55/26.1 | 119/56.4 | |||
| III–IV | 9/8.0 | 56/50.0 | 47/42.0 | 0.04 | 14/12.5 | 98/87.5 | 0.58 | 16/14.3 | 34/30.4 | 62/55.4 | 0.23 |
| ER | |||||||||||
| Positive | 52/8.6 | 256/42.4 | 296/49.0 | 111/18.4 | 493/81.6 | 115/19.0 | 178/29.5 | 311/51.5 | |||
| Negative | 42/12.1 | 144/41.4 | 162/46.6 | 0.22 | 47/13.5 | 301/86.5 | 0.05 | 65/18.7 | 98/28.2 | 185/53.2 | 0.88 |
| PR | |||||||||||
| Positive | 47/7.9 | 254/42.7 | 294/49.4 | 109/18.3 | 486/81.7 | 116/19.5 | 164/27.6 | 315/53.0 | |||
| Negative | 47/13.3 | 144/40.8 | 162/45.9 | 0.03 | 48/13.6 | 305/86.4 | 0.06 | 64/18.1 | 110/31.2 | 179/50.7 | 0.49 |
| HER2 | |||||||||||
| Positive | 28/10.1 | 107/38.6 | 142/51.3 | 39/14.1 | 238/85.9 | 52/18.8 | 86/31.0 | 139/50.2 | |||
| Negative | 59/9.9 | 257/43.0 | 282/47.2 | 0.47 | 105/17.6 | 493/82.4 | 0.20 | 110/18.4 | 168/28.1 | 320/53.5 | 0.61 |
| Molecular type | |||||||||||
| Luminal A | 38/9.2 | 174/42.2 | 200/48.5 | 79/19.2 | 333/80.8 | 73/17.7 | 119/28.9 | 220/53.4 | |||
| Luminal B | 11/8.2 | 53/39.3 | 71/52.6 | 23/17.0 | 112/83.0 | 30/22.2 | 43/31.9 | 62/45.9 | |||
| HER2 | 16/15.0 | 39/36.5 | 52/48.6 | 13/12.1 | 94/87.9 | 13/12.2 | 33/30.8 | 61/57.0 | |||
| TN | 14/12.1 | 46/39.7 | 56/48.3 | 0.56 | 14/12.1 | 102/87.9 | 0.16 | 25/21.6 | 30/25.9 | 61/52.6 | 0.36 |
Age-adjusted results are presented.
Monte Carlo simulation
Membranous predominant
Cytoplasmic or membranous cytoplasmic
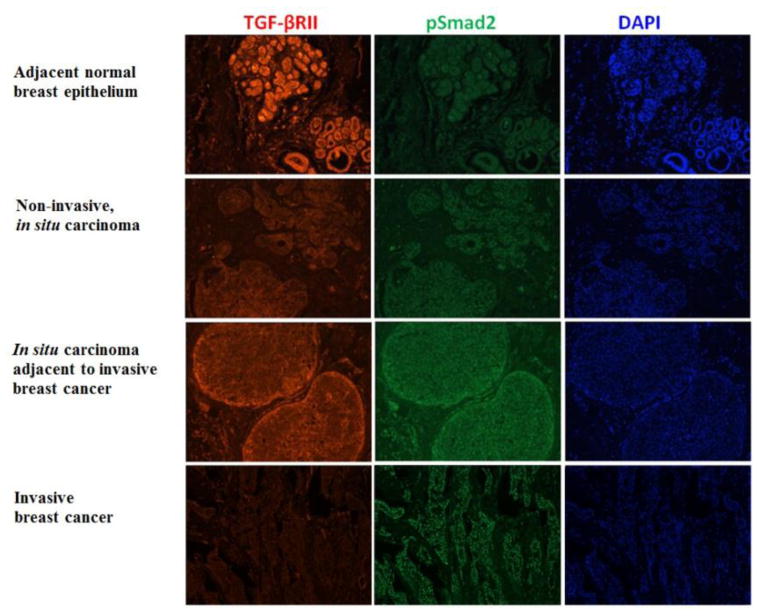
Positive TGF-βRII expression (score 2–3) in breast cancer and adjacent normal breast epithelium were 88.2% and 97.0%, respectively. TGF-βRII protein exhibited both cytoplasmic and membranous immunostaining patterns. Strong Positive TGF-βRII expression was more frequent observed in adjacent normal breast epithelium and early-stage breast cancer tissue (in situ carcinoma) than in invasive breast cancer tissue (P<0.01) (Table 3, Figure 2). The cytoplasmic predominant expression pattern of TGF-βRII was more frequently observed in breast cancer than in adjacent normal breast epithelium on the same pathological section (P<0.01). A total of 17.6% of the adjacent normal breast epithelium and 84.8% of the invasive breast carcinomas had a cytoplasmic expression pattern. Adjacent normal breast epithelium had stronger TGF-βRII expression intensity than that of the invasive breast carcinomas (70.7% vs. 44.1%, score 3) (Table 3). pSmad2 expression was restricted to the nucleus. pSmad2 expression intensity was stronger in adjacent normal breast epithelium, in situ carcinoma, and in situ carcinoma tissue component within invasive breast carcinoma than in invasive breast carcinoma tissue (P<0.01) (Table 3 and Figure 2). The correlation between pSmad2 intensity and TGF-β RII intensity is low (kappa = 0.12).
Table 3.
Correlation of TGF-βRII and pSmad2 expression with invasiveness of breast cancer, Shanghai Breast Cancer Study, Phase II
| TGF-βRII intensity
|
TGFβ-RII subcellular pattern
|
pSmad2 intensity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | P | 1c | 2d | P | 0–1 | 2 | 3 | P | |
| Adjacent normal breast tissue | |||||||||||
| Number | 24 | 212 | 570 | 664 | 142 | 312 | 231 | 261 | |||
| % | 3.0 | 26.3 | 70.7 | 82.4 | 17.6 | 38.8 | 28.7 | 32.5 | |||
| Non-invasive, in situ carcinomaa | |||||||||||
| Number | 3 | 28 | 59 | 24 | 66 | 17 | 34 | 39 | |||
| % | 3.3 | 31.1 | 65.6 | 26.7 | 73.3 | 18.9 | 37.8 | 43.3 | |||
| In situ with invasive carcinomaa | |||||||||||
| In situ component | |||||||||||
| Number | 18 | 93 | 220 | 120 | 211 | 61 | 109 | 161 | |||
| % | 5.4 | 28.1 | 66.5 | 36.3 | 63.8 | 18.4 | 32.9 | 48.6 | |||
| Invasive component | |||||||||||
| Number | 20 | 126 | 185 | 64 | 267 | 56 | 95 | 180 | |||
| % | 6.0 | 38.1 | 55.9 | 19.3 | 80.7 | 16.9 | 28.7 | 54.4 | |||
| Invasive carcinomab | |||||||||||
| Number | 74 | 275 | 275 | 95 | 529 | 124 | 182 | 318 | |||
| % | 11.9 | 44.1 | 44.1 | <0.01 | 15.2 | 84.8 | <0.01 | 19.9 | 29.2 | 51.1 | <0.01 |
Including ISDC and ISLC
Including IDC, ILC and Others
Membranous predominant
Cytoplasmic or membranous cytoplasmic
Figure 2.
Representative images of TGF-βRII and pSmad2 expression in adjacent normal breast epithelium and different stages of breast cancer.
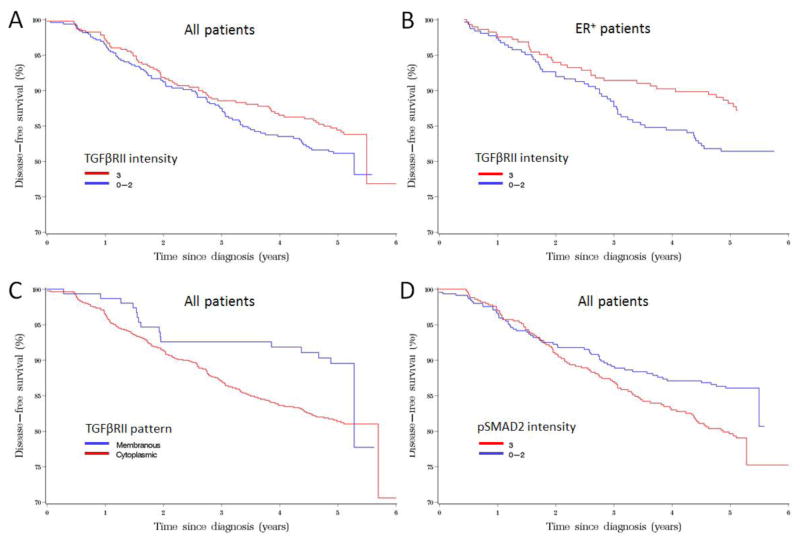
Five-year DFS in patients expressing high pSmad2 was 80% compared with 86% in low (0–2) pSmad2 patients. Higher pSmad2 expression was significantly associated with lower DFS of breast cancer (HR [95%CI]:1.48 [1.07–2.04], P=0.02, Table 4 and Figure 3) adjusted for age at diagnosis and BMI. Further adjustment of tumor characteristics and therapy did not significantly change the HR estimate for breast cancer survival with pSmad2 intensity. The association of pSmad2 intensity with breast cancer survival was more pronounced in the ER-positive patients (Table 4). Five-year cancer-free survival in patients expressing cytoplasmic predominant TGF-βRII was 82% compared with 90% in membranous predominant TGF-βRII expression. Cytoplasmic predominant TGF-βRII was significantly associated with cancer-free survival of breast cancer (HR [95%CI]: 1.80 [1.08–3.00], P=0.02, Table 4 and Figure 3). However, the association of TGFβRII pattern with breast cancer survival was borderline significant after adjusting for tumor characteristics and cancer therapy. The association of TGFβRII pattern with breast cancer survival was not modified by ER status (Table 4).
Table 4.
Expression of TGF-βRII and pSmad2 in association with breast cancer disease-free survival, Shanghai Breast Cancer Study, Phase II
| No. of Cases | No. of Events | % | 5-yr DFS | HR (95% CI)c | Pc | HR (95% CI)d | Pd | HR (95% CI)e | Pe | |
|---|---|---|---|---|---|---|---|---|---|---|
|
All patients
|
||||||||||
| pSmad2 intensity | ||||||||||
| 0–2 | 457 | 62 | 13.6 | 0.86 | reference | reference | reference | |||
| 3 | 498 | 96 | 19.3 | 0.80 | 1.48 (1.07–2.04) | 0.02 | 1.49 (1.07–2.06) | 0.02 | 1.40 (1.00–1.94) | 0.04 |
| TGFβRII intensity | ||||||||||
| 0–2 | 495 | 89 | 18.0 | 0.81 | reference | reference | reference | |||
| 3 | 460 | 69 | 15.0 | 0.85 | 0.81 (0.59–1.11) | 0.19 | 0.86 (0.63–1.19) | 0.37 | 0.87 (0.64–1.20) | 0.35 |
| TGFβRII pattern | ||||||||||
| 1a | 159 | 17 | 10.7 | 0.90 | reference | reference | reference | |||
| 2b | 796 | 141 | 17.7 | 0.82 | 1.80 (1.08–3.00) | 0.02 | 1.70 (1.02–2.83) | 0.04 | 1.65 (0.99–2.76) | 0.07 |
|
Patients with ER-positive Breast Cancer
|
||||||||||
| pSmad2 intensity | ||||||||||
| 0–2 | 293 | 35 | 11.9 | 0.88 | reference | reference | reference | |||
| 3 | 311 | 55 | 17.7 | 0.82 | 1.53(1.00–2.33) | 0.05 | 1.57 (1.02–2.44) | 0.04 | 1.48 (0.96–2.30) | 0.08 |
| TGFβRII intensity | ||||||||||
| 0–2 | 308 | 55 | 17.9 | 0.81 | reference | reference | reference | |||
| 3 | 296 | 35 | 11.8 | 0.89 | 0.65 (0.42–0.98) | 0.04 | 0.71 (0.46–1.10) | 0.21 | 0.71 (0.46–1.11) | 0.12 |
| TGFβRII pattern | ||||||||||
| 1a | 111 | 11 | 9.9 | 0.90 | reference | reference | reference | |||
| 2b | 493 | 79 | 16.0 | 0.84 | 1.80 (0.95–3.40) | 0.07 | 1.59 (0.83–3.03) | 0.16 | 1.45 (0.76–2.78) | 0.28 |
|
Patients with ER-negative Breast Cancer
|
||||||||||
| pSmad2 intensity | ||||||||||
| 0–2 | 163 | 26 | 15.9 | 0.83 | reference | reference | reference | |||
| 3 | 185 | 41 | 22.2 | 0.76 | 1.47(0.89–2.42) | 0.13 | 1.31 (0.79–2.18) | 0.30 | 1.27 (0.76–2.13) | 0.36 |
| TGFβRII intensity | ||||||||||
| 0–2 | 186 | 33 | 17.7 | 0.81 | reference | reference | reference | |||
| 3 | 162 | 34 | 21.0 | 0.78 | 1.15 (0.71–1.87) | 0.04 | 1.13 (0.69–1.84) | 0.62 | 1.10 (0.67–1.80) | 0.70 |
| TGFβRII pattern | ||||||||||
| 1a | 47 | 6 | 12.8 | 0.88 | reference | reference | reference | |||
| 2b | 301 | 61 | 20.3 | 0.78 | 1.63 (0.70–3.79) | 0.25 | 1.80 (0.76–4.25) | 0.18 | 1.70 (0.71–4.04) | 0.23 |
Membranous predominant
Cytoplasmic or membranous cytoplasmic
Adjusted for age at diagnosis (continuous), BMI (continuous).
Adjusted for age at diagnosis (continuous), BMI (continuous), tumor size, grade, TNM stage, ER, PR.
Adjusted for age at diagnosis (continuous), BMI (continuous), tumor size, grade, TNM stage, ER, PR, radiotherapy, Chemotherapy, immunotherapy, and Tamoxifen use.
Figure 3.
Disease free survival curves based on a stratified Cox regression model to compare the breast cancer survival rate among breast cancer patients with different TGF-βRII and pSmad2 expressions.
Five-year overall survival rate in patients expressing cytoplasmic predominant TGF-βRII was 86% compared to 92% (P=0.03) in membranous predominant TGF-βRII expression. Five-year overall survival rate in patients expressing high pSmad2 was 84% compared with 89 % in low pSmad2 expression patients (P=0.01) (data not shown in table).
Discussion
In this study, we found that breast cancer tissues had a lower TGF-βRII protein expression, a cytoplasmic predominant TGF-βRII expression pattern, and a higher pSmad2 expression compared to adjacent normal breast epithelium. Lower TGF-βRII protein expression, a cytoplasmic predominant TGF-βRII expression pattern, and a higher pSmad2 expression were associated with a decreased DFS. These results indicate that loss of TGF-βRII expression in the membranes and translocation of TGF-βRII to cytoplasm, which may lead to increasing TGF-β downstream signaling by activating Smad2, was related to prognosis of breast cancers. It has been reported that TGF-βRII was only detected in the cytoplasm in breast cancer MCF7 cells and predominantly presented in MDA-MB-231 cells [27]. Translocation of TGF-βRII to cytoplasm may be a potential mechanism for loss of TGF-β-mediated autocrine growth control and tumorigenicity in human breast cancer cells [27]. TGF-β acts as a tumor suppressor in normal epithelia by inhibiting cell proliferation and inducing apoptosis, but it accelerates progression of established cancers by autocrine and paracrine mechanisms [28;29]. In transformed cells, signaling of TGF-β loses its tumor-suppressor effects and begins to function as a cancer-promoting agent that synergizes with transforming oncogenes [30].
For the association between TGF-βRII/pSmad2 expression and clinicopathological factors, we found that loss of TGF-βRII expression occurs more frequently in patients with older age at diagnosis, post-menopausal status, negative PR status, and higher TNM stage. Cytoplasmic predominant TGF-βRII expression is associated with older age at diagnosis and invasive histological type. Higher pSmad2 expression is associated with higher tumor grade. Buck et al [31] reported that TGF-βRII expression was correlated with a reduced overall survival in ER-negative patients. In our study, TGF-βRII expression was not associated with breast cancer survival. However, cytoplasmic predominant TGF-βRII expression and higher pSmad2 expression were associated statistically significant reduced cancer-free survival. Loss of TGF-βRII expression and increased pSmad2 were inversely and significantly associated with breast cancer disease-free survival even after adjusting for known clinical predictors. These data indicate that increased pSmad2, cytoplasmic predominant TGF-βRII expression, and reduced TGF-βRII, may be independent predictors for poor prognosis of breast cancer.
In a study conducted among 178 breast biopsies, Gobbi et al. [32] observed a significant inverse correlation between loss of TGF-βRII expression and tumor grade within both ductal carcinoma in situ and invasive mammary carcinomas. Two studies using tissue microarray have evaluated the association of TGF-βRII and pSmad2 expressions with breast cancer pathological factors and outcome. In a study conducted among 324 Brazilian breast cancer cases, Paiva et al [33] showed that TGF-βRII positivity was associated with increased DFS in HER2 negative patients. However, no significant association between TGF-βRII and tumor stage was found [33]. A population-based, case-control study conducted in 842 Polish women found that TGF-βRII and pSmad2 expression were strongly associated with earlier age at onset independent of ER status, which supports our findings. In addition, it showed that negative TGF-βRII expression was associated with larger tumor size while high pSmad2 expression was associated with positive axillary node metastasis [34].
To the best of our knowledge, this study is the largest to evaluate the correlation of TGF-β signaling with clinicopathological factors in both human breast cancer and adjacent normal breast tissue. This study has several notable strengths. The population-based study design and high overall response rate (80%) minimized potential selection bias. The pathological diagnoses and histological grading were reviewed and confirmed by a centralized laboratory. The stained slides were scored separately by two investigators blinded to clinical data, and all the slides with inconsistent results were re-evaluated jointly to get a consensus score. We used whole tissue sections in this study, which may provide more accurate information than a biopsy [32–34].
This study also has some limitations. A major limitation is that the pathological tissue slides were collected from multiple hospitals and stored for about 10 years before staining. Degradation of protein antigenicity may vary despite use of a standard protocol to collect, process, and store tissue sections to maximally preserve tissue antigens. Tissue antigen degradation might have reduced the statistical power of this study. In addition, the follow-up period of this cohort is relatively short. Our ongoing follow-up with the cohort would overcome this limitation and allow an examination of the long-term associations between TGF-βRII/pSmad2 expression and breast cancer prognosis.
In summary, our findings suggest that increased pSmad2 expression, reduced TGF-βRII expression, and cytoplasmic presence of TGF-βRII may be independent predictors of breast cancer prognosis. These findings provide additional evidence to support the important role of TGF-β signaling in breast cancer prognosis.
Supplementary Material
Acknowledgments
The authors thank study participants and research staff for their contributions and commitment to this project. We thank Regina Courtney for technical assistance and Jacqueline Stern for assistance with editing and manuscript preparation. The imunofluorescence staining was performed at the Survey and Biospecimen Shared Resource, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485).
Grant Support
This research was supported by research grants from the National Institutes of health (R01CA064277, R01CA090899, R01CA118229, and R01CA122756).
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- DFS
disease-free survival
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- PR
Progesterone receptor
- pSmad2
Phospho-Smad2
- SBCS
Shanghai Breast Cancer Study
- TGF-β
Transforming growth factor-beta
- TGFβ-RI
transforming growth factor beta receptor I
- TGFβ-RII
transforming growth factor beta receptor II
- TMA
tissue microarray
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Standards
All data collection was conducted with approval of appropriate institutional review boards to protect human subjects with consent and data protection systems in place. Data analysis for this manuscript was conducted on de-identified data sets.
Reference List
- 1.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuchida K, Lewis KA, Mathews LS, Vale WW. Molecular characterization of rat transforming growth factor-beta type II receptor. Biochem Biophys Res Commun. 1993;191:790–795. doi: 10.1006/bbrc.1993.1286. [DOI] [PubMed] [Google Scholar]
- 4.Takumi T, Moustakas A, Lin HY, Lodish HF. Molecular characterization of a type I serine-threonine kinase receptor for TGF-beta and activin in the rat pituitary tumor cell line GH3. Exp Cell Res. 1995;216:208–214. doi: 10.1006/excr.1995.1026. [DOI] [PubMed] [Google Scholar]
- 5.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 6.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 7.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 8.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury S, Ammanamanchi S, Howell GM. Epigenetic Targeting of Transforming Growth Factor beta Receptor II and Implications for Cancer Therapy. Mol Cell Pharmacol. 2009;1:57–70. doi: 10.4255/mcpharmacol.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, Kinzler KW, Vogelstein B, Willson JK, Markowitz S. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 11.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 12.Izumoto S, Arita N, Ohnishi T, Hiraga S, Taki T, Tomita N, Ohue M, Hayakawa T. Microsatellite instability and mutated type II transforming growth factor-beta receptor gene in gliomas. Cancer Lett. 1997;112:251–256. doi: 10.1016/s0304-3835(96)04583-1. [DOI] [PubMed] [Google Scholar]
- 13.Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten DP. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd RD, Kos SM, Rinker KD. Flow-dependent Smad2 phosphorylation and TGIF nuclear localization in human aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301:H98–H107. doi: 10.1152/ajpheart.00668.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G, Chen YG, Ozdamar B, Gyuricza CA, Chong PA, Wrana JL, Massague J, Shi Y. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287:92–97. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- 17.Wu JW, Hu M, Chai J, Seoane J, Huse M, Li C, Rigotti DJ, Kyin S, Muir TW, Fairman R, Massague J, Shi Y. Crystal structure of a phosphorylated Smad2. Recognition of phosphoserine by the MH2 domain and insights on Smad function in TGF-beta signaling. Mol Cell. 2001;8:1277–1289. doi: 10.1016/s1097-2765(01)00421-x. [DOI] [PubMed] [Google Scholar]
- 18.Inman GJ, Nicolas FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell. 2002;10:283–294. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 19.Grau AM, Wen W, Ramroopsingh DS, Gao YT, Zi J, Cai Q, Shu XO, Zheng W. Circulating transforming growth factor-beta-1 and breast cancer prognosis: results from the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng W. Genetic polymorphisms in the transforming growth factor-beta signaling pathways and breast cancer risk and survival. Methods Mol Biol. 2009;472:265–277. doi: 10.1007/978-1-60327-492-0_11. [DOI] [PubMed] [Google Scholar]
- 21.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, Sellers TA, Kushi LH, Ruan Z, Bostick RM, Jin F, Zheng W. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87:295–300. doi: 10.1002/1097-0215(20000715)87:2<295::aid-ijc23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Zheng W, Long J, Gao YT, Li C, Zheng Y, Xiang YB, Wen W, Levy S, Deming SL, Haines JL, Gu K, Fair AM, Cai Q, Lu W, Shu XO. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009;41:324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y, Zheng Y, Zheng W, Gu K, Chen Z, Li G, Cai Q, Lu W, Shu XO. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer. 2011;11:292. doi: 10.1186/1471-2407-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakhani S, Ellis I, Schnitt S, Tan P, van de Vijver M. WHO Classification of Tumours of Breast. International Agency for Research on Cancer; 2012. [Google Scholar]
- 25.Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanh lD, To TV, Qian Z, Love RR, Allred DC. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol. 2004;17:1545–1554. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Koli KM, Arteaga CL. Predominant cytosolic localization of type II transforming growth factor beta receptors in human breast carcinoma cells. Cancer Res. 1997;57:970–977. [PubMed] [Google Scholar]
- 28.Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 30.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C. Prognostic significance of transforming growth factor beta receptor II in estrogen receptor-negative breast cancer patients. Clin Cancer Res. 2004;10:491–498. doi: 10.1158/1078-0432.ccr-0320-03. [DOI] [PubMed] [Google Scholar]
- 32.Gobbi H, Arteaga CL, Jensen RA, Simpson JF, Dupont WD, Olson SJ, Schuyler PA, Plummer WD, Jr, Page DL. Loss of expression of transforming growth factor beta type II receptor correlates with high tumour grade in human breast in-situ and invasive carcinomas. Histopathology. 2000;36:168–177. doi: 10.1046/j.1365-2559.2000.00841.x. [DOI] [PubMed] [Google Scholar]
- 33.Paiva CE, Drigo SA, Rosa FE, Moraes Neto FA, Caldeira JR, Soares FA, Domingues MA, Rogatto SR. Absence of transforming growth factor-beta type II receptor is associated with poorer prognosis in HER2-negative breast tumours. Ann Oncol. 2010;21:734–740. doi: 10.1093/annonc/mdp518. [DOI] [PubMed] [Google Scholar]
- 34.Figueroa JD, Flanders KC, Garcia-Closas M, Anderson WF, Yang XR, Matsuno RK, Duggan MA, Pfeiffer RM, Ooshima A, Cornelison R, Gierach GL, Brinton LA, Lissowska J, Peplonska B, Wakefield LM, Sherman ME. Expression of TGF-beta signaling factors in invasive breast cancers: relationships with age at diagnosis and tumor characteristics. Breast Cancer Res Treat. 2010;121:727–735. doi: 10.1007/s10549-009-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.