Abstract
Objective
Weight, physical activity, and sleep are modifiable lifestyle factors that impact cognitive functioning in non-cancer populations, but have yet to be examined in cancer survivors. The aim of the study was to assess the relationship of obesity, physical activity, and sleep, with cognitive functioning among breast cancer survivors.
Methods
Participants were 136 early-stage post-menopausal breast cancer survivors who completed an assessment of neuropsychological testing, height, weight, physical activity and sleep. Linear regression models examined the associations of the seven neuropsychological domains with obesity, physical activity, and sleep. Logistic regression models examined odd of impairment in each domain. All models controlled for breast cancer treatment variables and relevant demographic and clinical variables.
Results
Obese participants had significantly worse performance (β=−5.04, SE=2.53) and were almost 3 times more likely to be impaired (OR=2.87; 95% CI:1.02–8.10) on the Information Processing domain. The highest tertile of physical activity was significantly related to better performance on the Executive Functioning domain (β=5.13, SE=2.42) and Attention domain (β=4.26, SE=2.07). The middle tertile of physical activity was significantly related to better performance (β=9.00, SE=3.09) and decreased odds of impairment (OR=0.89, 95% CI:0.07–0.91) on the Visual Spatial domain. More hours of sleep per night was significantly associated with better performance (β = 2.69, SE=0.98) and decreased odds of impairment (OR=0.52; 95% CI:0.33–0.82) on the Verbal Functioning domain.
Conclusions
These findings suggest that obesity, physical activity, and sleep are related to cognitive functioning among breast cancer survivors and have potential to be intervention targets to improve cognitive functioning.
Keywords: Breast Cancer, Oncology, Obesity, Physical Activity, Sleep, Cognitive Functioning
Background
An estimated 2.4 million breast cancer survivors are alive today and this number is projected to continue to rise. Deficits in cognitive functioning is an important survivorship concern that effects up to 75% of breast cancer patients and can last for years after the end of treatment.1–3 To date, research on cognitive functioning among breast cancer survivors has mainly focused on examining the impact of the breast cancer treatments (e.g., chemotherapy and endocrine therapy).1–6
Research in non-cancer populations indicates that modifiable lifestyle factors including physical activity, obesity, and sleep duration impact cognitive functioning. These lifestyle factors may play an important role in breast cancer survivors’ cognitive functioning, as many breast cancer survivors experience decreases in physical activity,7,8 increases in weight,9–11 and disturbances in sleep following their cancer treatments.12–14 However, to our knowledge, no studies have examined the relationship of these lifestyle factors to objective cognitive functioning in cancer patients.
Evidence for associations between physical activity and cognitive functioning are well documented in both healthy and cognitively impaired populations.15–18 A Cochrane review found a robust effect of physical activity on cognitive functioning, indicating that regular physical activity can slow down or prevent functional decline associated with aging.15 A 2011 meta-analysis also reported that regular physical activity, in conjunction with weight loss interventions, improves cognitive functioning.19 Although few human studies have explored associations between physical activity and cognition in cancer populations, data from animal studies suggest that physical activity may also have favorable effects on cognitive functioning among adults who have received adjuvant therapies for cancer. For example, one recent animal study reported that physical activity improved cognitive functioning in mice who had received chemotherapy.20
A growing body of literature also suggests that obesity and sleep are associated with cognitive functioning. For example, middle-aged obese (but otherwise healthy) individuals have higher rates of cognitive deficits than normal weight individuals19,21,22; and a prospective study of 109 obese patients undergoing bariatric surgery demonstrated significant improvements in the memory domain, compared to obese controls not receiving surgery (n=41).23 Evidence for associations among sleep and cognitive ability is also well-documented in the general population. A review of the literature found that in controlled experiments of sleep restriction in healthy adults, sleeping less than 7 hours of sleep per night was associated with significant cognitive deficits in psychomotor vigilance and working memory.24 Additionally, a study of 5,177 healthy adults reported that participants who slept less than 7 hours a night performed worse on measures of verbal fluency and memory than participants who slept 7 or 8 hours a night.
It is unclear whether breast cancer survivors have the same benefits to their cognitive functioning from engaging in healthy lifestyle behaviors as other populations. The impact of having cancer and undergoing cancer treatments may outweigh the benefits of healthy behaviors. Alternatively, the combination of poor lifestyle with cancer treatments may increase the risk of cognitive dysfunction for cancer survivors. The purpose of this study was to examine if the associations of lifestyle factors with cognitive functioning seen in non-cancer populations also holds true for breast cancer patients. The primary aim was to examine the associations of lifestyle factors with objectively measured cognitive functioning in early-stage breast cancer survivors. We hypothesized that greater physical activity, and greater hours of sleep per night would be related to higher scores on the neuropsychological tests; and that obese participants would have lower scores on the neuropsychological tests compared to non-obese participants, while controlling for breast cancer treatment variables. The secondary aim was to explore the extent to which physical activity, obesity, and sleep, would predict cognitive impairment.
Methods
Participants
Participants were women who were post-menopausal when diagnosed with breast cancer within the past 5 years. Additional eligibility criteria included primary operable invasive breast carcinoma categorized as Stage I, II or III, and not scheduled for or currently undergoing chemotherapy. Exclusion criteria included: Renal insufficiency; liver impairment or congestive heart failure; diabetes being treated with other than diet and lifestyle; hormone therapy; and other primary or recurrent invasive cancer within the last 10 years (other than nonmelanomic skin cancer or carcinoma of the cervix in situ).
Participants were from two studies within the UCSD Transdisciplinary Research in Energetics and Cancer (TREC) center, a multi-study program project examining the role of insulin resistance and inflammation in breast cancer risk.25 Ninety-six women with a BMI ≥25 kg/m2 were recruited for a randomized trial examining the impact of weight loss on biomarkers associated with breast cancer outcomes. We recruited an additional 40 women with a BMI < 25 kg/m2 to enrich the baseline dataset of the randomized trial so that breast cancer survivors across the entire BMI continuum could be examined when assessing the relationship of lifestyle factors to cognitive functioning. Recruitment of all participants occurred simultaneously with the TREC trial, using the same recruitment methods and staff. Specifically, women were recruited through flyers at community events (e.g., breast cancer charity walks, breast cancer support groups), through physician referral, and cancer patient registries.
Of the 1157 women who were contacted about the study, 166 were eligible and 136 completed the clinic visit. The most frequent reasons for ineligibility were not being post-menopausal at diagnosis, and diagnosed more than 5 years ago. All participants attended a clinic visit at the Moores UCSD Cancer Center to complete the study measures. The UCSD institutional review board approved all study procedures and all participants signed informed consent.
Measures
Demographic data
Demographic data were obtained through use of a standard self-report questionnaire. Variables assessed included age, race/ethnicity, primary language spoken, and educational level.
Clinical data
Medical charts were reviewed to obtain information about the original breast cancer and treatment characteristics. Variables assessed included date of diagnosis, disease stage, type of breast surgery, chemotherapy, and use of endocrine therapy.
Lifestyle Factors
Body Mass Index (BMI) was calculated from weight and height measured at the clinic visit. Recreational physical activity was assessed using the Global Physical Activity Questionnaire (GPAQ).26 The GPAQ calculates metabolic equivalents (METs) to express the intensity of self-reported physical activities. The GPAQ has adequate reliability (Kappa=0.67 to 0.73; Spearman's rho=0.67 to 0.81) and has been validated with the International Physical Activity Questionnaire in the general population,27 and against objective accelerometer measures in population sub-groups.28 For the purpose of this paper, we focused on recreational activity only. Sleep duration was assessed with the single-item: “On average, how many hours of sleep do you get per night?”.
Cognitive functioning
Cognitive functioning was assessed using a computerized battery of neuropsychological tests.29 This battery was specifically designed to detect mild cognitive impairment. This software consists of interactive cognitive tests, providing accuracy and reaction time (millisecond timescale) data. This battery consists of 10 tests providing a total score and 7 domain scores: memory, executive function, visual spatial processing, verbal function, attention, information processing speed, and motor skills. Tests were adaptive, meaning that the level of difficulty of each test is adjusted to the user’s performance. All scores were normalized for age and education level with a mean of 100 and standard deviation of 15. Higher test scores represent better cognitive functioning. The test also provides classifications of impairment level: Abnormal (≤ 85), Probably Abnormal (>85 and ≤ 96.25), Probably Normal (>96.25 and ≤ 103.75), Normal (>103.75). This battery also contains a measure of general intelligence (IQ) that is designed to be used as a covariate to estimate overall intellectual ability.
Statistical Methods
Demographic characteristics of participants, breast cancer disease and treatment information, lifestyle factors and neuropsychological testing scores were summarized. Variables are presented as means (standard deviations) for continuous variables and percentages for categorical variables. Obesity status was grouped according to the World Health Organization International Classification for obesity30: women with a BMI ≥ 30 kg/m2 were classified as obese and a BMI < 30kg/m2 was classified as normal/overweight. Due to the skewed distribution of recreational physical activity METs per week, physical activity was divided into tertiles.
Using linear regression models, we examined the association of each of the seven neuropsychological domain scores and the total score with lifestyle factors (obesity, physical activity, and sleep) controlling for breast cancer treatment variables (chemotherapy, endocrine therapy) and relevant demographic and (primary language, IQ) clinical variables (time since diagnosis). Regression coefficients and standardized errors are given for all models. There were no differences in results when the lifestyle variables were put in the models together or individually; therefore, models with all lifestyle factors entered together are presented. Rates of impairment for each domain were calculated using the four pre-defined impairment categories from the neuropsychological battery. Participants were then classified as “Impaired”, by combining Abnormal and Probably Abnormal scores, or “Not Impaired”, combining Probably Normal and Normal scores. Logistic regression analyses were used to calculate the odds of being classified as “Impaired” based on lifestyle factors, controlling for breast cancer treatment variables and relevant demographic and clinical variables. All statistical tests were two-sided (alpha=0.05), and all analyses were conducted in SAS 9.3 (Cary N.C.).
Results
Table 1 presents demographic and clinical data from the 136 participants. Women in the study were a mean of 62.6 years old (SD=6.6; range: 49.7–81.1 years) and were predominantly native English speakers (93%). About half had been diagnosed with Stage 1, 35% with Stage 2 and 15% with Stage 3 breast cancer. Forty-nine percent had received chemotherapy and 70% were taking endocrine therapy (i.e., aromatase inhibitor or tamoxifen). On average, the interval between breast cancer diagnosis and study assessment was 2.1 years (SD=1.3) years. Thirty-two percent of women were classified as obese (BMI ≥ 30 kg/m2) and 68% were classified as normal/overweight (BMI < 30 kg/m2). The women reported sleeping an average of 6.90 hours per night (SD=1.23).
Table 1.
Demographic, Clinical, and Lifestyle Factors in a Sample of Postmenopausal Breast Cancer Survivors (N = 136)
| Age mean (SD) (range: 49.7–81.1) | 62.6 (6.6) |
| White, non-Hispanic n (%) | 107 (78.7) |
| Primary Language: English n (%) | 127 (93.4) |
| Has college degree n (%) | 80 (58.8) |
| IQ | 102.7 (13.8) |
| Years since diagnosis mean (SD) | 2.1 (1.3) |
| Cancer Stage n (%) | |
| 1 | 67 (49.6) |
| 2 | 48 (35.6) |
| 3 | 20 (14.8) |
| Received Chemotherapy n (%) | 65 (48.5) |
| Taking Endocrine Therapy n (%) | 94 (70.2) |
| Obese (BMI > 30mg/k2) n (%) | 44 (32.4) |
| Recreational PA1 METs/week (tertiles)2 | |
| 1st (0 to 240 METs/wk) n (%) | 45 (33.6) |
| 2nd (241 to 1399 METs/wk) n (%) | 43 (32.1) |
| 3rd (≥ 1400 METs/wk) n (%) | 46 (34.3) |
| Sleep (hours/night) mean (SD) | 6.9 (1.2) |
Physical Activity
Missing physical activity data for 2 study participants (N=134)
Overall, scores on the neuropsychological test were slightly higher than the average normed score of 100. Mean scores ranged from M=108.13, SD = 14.70 in the Visual Spatial domain to M = 100.25, SD=15.23 in the Verbal Functioning domain. Rates of being classified as “Impaired” (i.e., Abnormal or Probably Abnormal score) on any of the seven neuropsychological domains ranged from 12.1% to 24.3%. Rates of impairment were highest for the Visual Spatial (24.3%), Information processing speed (23.0%), Executive functioning (22.8%), and Verbal functioning (22.8%) domains. See Table 2 for rates based on the four levels of impairment for each neuropsychological domain.
Table 2.
Distribution of Cognitive Impairment Within Neuropsychological Domains in a Sample of Postmenopausal Breast Cancer Survivors (n=136)
| Information Processing1 |
Memory | Executive Functioning |
Visual Spatial |
Verbal Function |
Attention | Motor Skills2 |
|
|---|---|---|---|---|---|---|---|
|
Impairment Categories3 |
|||||||
| Abnormal | 7 (5%) | 5 (4%) | 9 (7%) | 11 (8%) | 11 (8%) | 8 (6%) | 3 (2%) |
| Probably Abnormal | 24 (18%) | 20 (14%) | 22 (16%) | 22 (16%) | 20 (15%) | 17 (13%) | 13 (10%) |
| Probably Normal | 25 (19%) | 22 (16%) | 37 (28%) | 6 (4%) | 33 (25%) | 41 (31%) | 40 (30%) |
| Normal | 77 (57%) | 87 (65%) | 66 (49%) | 95 (71%) | 70 (52%) | 68 (51%) | 74 (55%) |
missing data n=1
missing data n=4
Abnormal(≤ 85); Probably Abnormal (>85 ≤ 96.25); Probably Normal (> 96.25 ≤ 103.75); Normal (> 103.75)
Table 3 presents the relationships between cognitive functioning and lifestyle factors. Significant associations were observed among each of the lifestyle factors and 5 of the 7 neuropsychological testing domains, after adjusting for breast cancer treatment variables and other covariates. Specifically, obese participants had significantly worse performance than non-obese participants on the Information Processing domain (β=−5.04, SE=2.53, p=.049). The highest tertile of physical activity was significantly related to better performance on the Executive Functioning domain (β=5.13, SE=2.42, p=.036) and Attention domain (β=4.26, SE=2.07, p=.042). The middle tertile of physical activity was significantly related to better performance on the Visual Spatial domain (β=9.00, SE=3.09, p=.004). More hours of sleep per night was significantly associated with better performance on the Verbal Functioning domain (β = 2.69, SE=0.98, p=.007). Having received chemotherapy was significantly related to worse performance in the Visual spatial domain (β = −6.01, SE=2.53, p=.017). Endocrine therapy was not significantly related to any neuropsychological domain score. The Memory domain and the Motor Skills domain and the Total Score were not significantly related to any of the lifestyle or cancer treatment variables. No significant interactions were found between any of the lifestyle factors and cancer treatment factors on cognitive functioning (data not shown).
Table 3.
Separate Linear Regression Models1 of the Association of Lifestyle/Treatment Factors With Neurocognitive Domain Scores2 in a Sample of Post-menopausal Breast Cancer Survivors (n=136)
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | MODEL 5 | MODEL 6 | MODEL 7 | |
|---|---|---|---|---|---|---|---|
| Executive Functioning |
Visual Spatial |
Verbal Functioning |
Attention | Information Processing |
Memory | Motor Skills |
|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Treatment Factors | |||||||
| Chemotherapy | 0.43 (1.82) | −5.95* (2.45) | −0.06 (2.37) | 2.03 (1.54) | 2.14 (2.16) | −1.01 (1.56) | −0.77 (1.34) |
| Endocrine Therapy | −2.21 (1.96) | 1.34 (2.64) | −2.51 (2.55) | −1.97 (1.67) | −2.00 (2.33) | −0.37 (1.68) | 1.15 (1.44) |
| Lifestyle Factors | |||||||
| Obese3 | 0.55 (2.11) | 1.88 (2.86) | 1.94 (2.76) | 0.13 (1.80) | −5.04* (2.53) | 0.45 (1.82) | 0.69 (1.57) |
| High PA4 | 5.13* (2.42) | 2.60 (3.28) | 0.11 (3.16) | 4.26* (2.07) | 3.87 (3.89) | −0.99 (2.09) | 0.80 (1.82) |
| Middle PA4 | 2.09 (2.29) | 9.00**(3.09) | 3.64 (2.98) | 1.76 (1.95) | 0.49 (2.72) | 1.12 (1.97) | 1.06 (1.71) |
| Sleep | −0.48 (0.75) | 0.70 (1.04) | 2.69** (0.98) | −0.95 (0.64) | −1.09 (0.89) | −0.27 (0.65) | −0.61 (0.56) |
p < .05
p < .01
All models were adjusted for primary language (English vs. other), IQ, and time since diagnosis.
Domain scores were normalized for age and education
Obese defined as BMI ≥ 30kg/m2
Recreatonal Physical Activity (PA) metabolic equivalents (METs) per week, categorized into tertiles (low, middle, high)
Figure 1 presents the odds of being impaired on a neuropsychological domain by lifestyle predictors, while controlling for breast cancer treatment variables, and covariates. We only present relationships for the lifestyle factors we identified as significant predictors in the linear regression models. Obese women were almost 3 times more likely to be impaired on the Information processing speed domain than non-obese women (OR=2.87; 95%CI:1.02–8.10, p=.046). More sleep per night was associated with significantly lower odds of impairment on the Verbal functioning domain (OR=0.52; 95%CI:0.33–0.82, p=.005). Women in the middle tertile of physical activity were significantly less likely to be impaired in the Visual Spatial domain (OR=0.89, 95%CI:0.07–0.91, p=.035). Women in the highest tertile of physical activity were less likely to be impaired in the Attention (OR=0.46, 95%CI:0.13–1.60) and Executive functioning domains (OR=0.42, 95%CI:0.12–1.46), however these associations were not statistically significant (p>.05) in logistic regression models.
Figure 1.
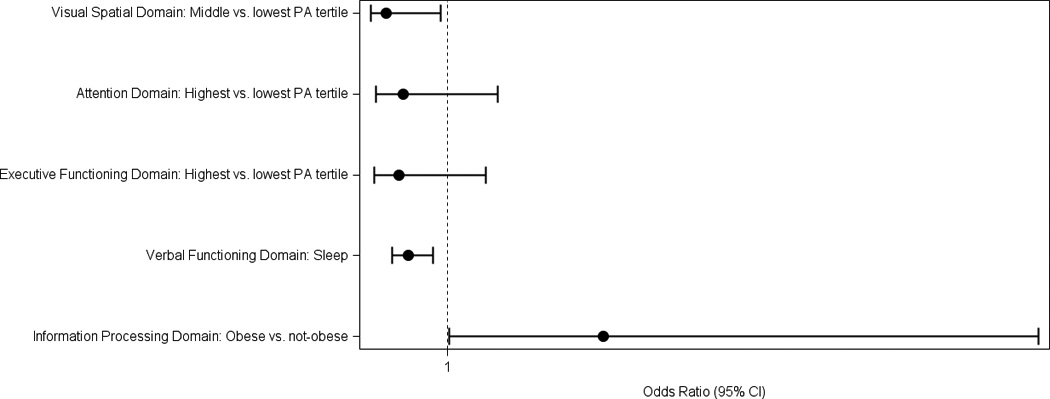
Odds Ratios of Domain Specific Impairements by Lifestyle Factors in a Sample of Postmenopausal Breast Cancer Survivors (n=136)
Conclusions
To our knowledge, this is the first study to examine associations of physical activity, obesity, and sleep with cognitive functioning in a cancer population. As hypothesized, we found significant associations between lifestyle factors and cognitive functioning among breast cancer survivors. Specifically, physical activity, obesity, and sleep were independently associated with performance on a standardized test of cognitive functioning. These associations included adjustment for clinical variables related to breast cancer diagnosis and treatment.
Consistent with the literature in non-cancer populations, women who reported the highest levels of physical activity had significantly better performance on the Attention and Executive Functioning domains.31 Women in the middle tertile of activity performed better than women in the lowest tertile of activity on the Visual spatial domain, but surprisingly there was no benefit for women in the highest tertile of activity. This unexpected finding with the Visual spatial domain may suggest that the optimal dose of physical activity varies by neuropsychological domain, or alternatively, may be a function of the relatively small sample size. More research is needed to determine the optimal amount of physical activity to improve cognitive functioning.
Obese women had almost 3-fold higher odds of exhibiting information processing speed impairments, relative to non-obese participants. This magnitude of association between body mass and processing-speed is comparable to results in non-cancer populations.32 Data from studies conducted in non-cancer populations also suggest that obesity is associated with executive functioning. However, the extent to which obesity affects executive functioning may vary based on age.32 Thus, it is possible that the age range of our study participants (50–81 years) was too narrow to observe such associations.
Consistent with our findings, sleep deprivation affects areas of the brain involved in verbal functioning.33,34 However, sleep deprivation previously has exerted a wide range of effects on cognitive functioning including attention, memory, and psychomotor speed.24,35,36 There is also evidence that there may be a U-shape relationship between sleep and cognition with 6 to 8 hours of sleep as ideal, and more than 8 hours and less than 6 hours related to cognitive deficits.34,37 Unfortunately due to the distribution of sleep for the current study, with few women reporting 9 or more hours of sleep per night, we were unable to test for a U-shaped relationship with cognitive functioning.
Exposure to chemotherapy also appears to have deleterious effects on cognition. A recent meta-analysis of published studies that explored the long-term impact of chemotherapy on cognitive functioning suggests that deficits due to chemotherapy are modest, and the impact may be isolated to domains of visual spatial ability and verbal functioning.4 Consistent with this meta-analysis, we found chemotherapy was only significantly associated with visual spatial abilities (p<0.05). However we found no association between chemotherapy and verbal functioning in this sample of breast cancer survivors who were (on average) two years post-diagnosis.
Findings from this study may be limited by the small sample size. However, we were able to see associations between lifestyle variables and cognitive functioning; therefore it is plausible that the overall trends found here would continue or even strengthen if evaluated in a larger sample. Other study limitations include the cross-sectional study design, and use of self-reported data for physical activity and sleep. Although sleep was measured with a single question, single-item assessments have been used in numerous studies, such as NHANES 2011, the Nurses Health Study, and the 2007 National Youth Risk Behavior Survey. A validation study in the Nurses Health Study reported that self-reported sleep duration was highly correlated (r=0.79) with sleep duration estimated from a one-week log.38 The CARDIA sleep study found a correlation of 0.45 between self-reported sleep duration and actigraphy-measured sleep duration39 and the investigators noted that correlations between actigraphy and polysomnography for sleep duration were over 0.9 in healthy adults.40 Nonetheless, this measure did not address issues of sleep quality such as awakenings in the night and did not take into account the amount of sleep participants received the night before the cognitive test was administered. It is possible that acute sleep loss may have attenuated the association between average sleep duration and cognitive functioning, with the effect of reducing the magnitude of association. We also used BMI as a proxy for overall adiposity. BMI does not distinguish lean muscle mass from fat mass; nor does it enable us to assess body-fat distribution, which may modulate the effect of elevated BMI on cognition.32
This was a pilot study whose purpose was exploratory and hypothesis generating and therefore we did not adjust for multiple statistical comparisons. We were interested in eliciting evidence of associations between lifestyle factors and each of the cognitive functioning domains. Individual domains, rather than a composite score, are more sensitive for evaluating mild cognitive deficits, and we were interested in learning if lifestyle factors impacted the same or different domains. Given the paucity of data on this topic, we felt it was important to present any suggestive findings in order to inform future studies and guide the design of future studies. Larger studies of cancer survivors using sensitive assessments of mild cognitive study should be conducted to replicate our findings in a more robust fashion.
In summary, results of the current study provide initial evidence that engaging in a healthy lifestyle may enhance cognitive functioning in breast cancer survivors. In cancer patients, the most frequently reported cognitive deficits are related to executive function, attention, processing speed, and memory.41 All but one of these domains was associated with a modifiable lifestyle factor in the current study. This suggests that in order to address the issue of cognitive deficits in cancer patients, we need to look beyond cancer treatments and examine the role of lifestyle. Although the magnitude of associations between lifestyle variables and performance on aspects of the neuropsychological tests were modest, the clinical implications of these findings may be substantive. Deficits in cognitive functioning are a significant issue and concern for many breast cancer survivors and can impact quality of life and daily functioning. Future research needs to examine the therapeutic potential of intervening on these modifiable lifestyle factors to improve cognitive functioning in cancer survivors.
Acknowledgement
Research support was provided by funding from the National Cancer Institute (U54 CA155435-01).
Footnotes
Financial Disclosures: There are no financial disclosures from any author.
References
- 1.Vardy J. Cognitive function in breast cancer survivors. Cancer treatment and research. 2009;151:387–419. doi: 10.1007/978-0-387-75115-3_24. [DOI] [PubMed] [Google Scholar]
- 2.Vodermaier A. Breast cancer treatment and cognitive function: the current state of evidence, underlying mechanisms and potential treatments. Women's health (London, England) 2009 Sep;5(5):503–516. doi: 10.2217/whe.09.36. [DOI] [PubMed] [Google Scholar]
- 3.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain and cognition. 2005 Oct;59(1):60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Oct 10;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast cancer research and treatment. 2009 Jul;116(1):113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast cancer research and treatment. 2008 Jul;110(1):143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000 Feb 1;88(3):674–684. [PubMed] [Google Scholar]
- 8.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Aug 20;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001 May 1;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 10.Saquib N, Flatt SW, Natarajan L, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women's healthy eating and living (WHEL) study. Breast cancer research and treatment. 2007 Oct;105(2):177–186. doi: 10.1007/s10549-006-9442-2. [DOI] [PubMed] [Google Scholar]
- 11.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011 Apr;12(4):282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 12.Alfano CM, Lichstein KL, Vander Wal GS, et al. Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast cancer research and treatment. 2011 Nov;130(1):243–254. doi: 10.1007/s10549-011-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors--a systematic review. International journal of psychiatry in medicine. 2010;40(2):163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 14.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Feb 10;26(5):768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane database of systematic reviews (Online) 2008;3 doi: 10.1002/14651858.CD005381.pub3. CD005381. [DOI] [PubMed] [Google Scholar]
- 16.Foster PP, Rosenblatt KP, Kuljis RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer's disease. Frontiers in neurology. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolland Y, Abellan van Kan G, Vellas B. Healthy brain aging: role of exercise and physical activity. Clinics in geriatric medicine. 2010 Feb;26(1):75–87. doi: 10.1016/j.cger.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine. 2010 Apr;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siervo M, Arnold R, Wells JC, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011 Jul 18; doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- 20.Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology. 2012 Mar;220(1):183–193. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- 21.Kerwin DR, Gaussoin SA, Chlebowski RT, et al. Interaction between body mass index and central adiposity and risk of incident cognitive impairment and dementia: results from the Women's Health Initiative Memory Study. Journal of the American Geriatrics Society. 2011 Jan;59(1):107–112. doi: 10.1111/j.1532-5415.2010.03219.x. [DOI] [PubMed] [Google Scholar]
- 22.van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica et biophysica acta. 2009 May;1792(5):470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Gunstad J, Strain G, Devlin MJ, et al. Improved memory function 12 weeks after bariatric surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2011 Jul-Aug;7(4):465–472. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007 Aug 15;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson RE, Colditz GA, Hu FB, et al. The 2011–2016 Transdisciplinary Research on Energetics and Cancer (TREC) initiative: rationale and design. Cancer causes & control : CCC. 2013 Apr;24(4):695–704. doi: 10.1007/s10552-013-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ) Journal of Public Health. 2006;14:66–70. [Google Scholar]
- 27.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. Journal of physical activity & health. 2009 Nov;6(6):790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 28.Hoos T, Espinoza N, Marshall S, Arredondo EM. Validity of the Global Physical Activity Questionnaire (GPAQ) in adult Latinas. Journal of physical activity & health. 2012 Jul;9(5):698–705. doi: 10.1123/jpah.9.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of the Mindstreams computerized cognitive battery for mild cognitive impairment. Journal of molecular neuroscience : MN. 2004;24(1):33–44. doi: 10.1385/jmn:24:1:033. [DOI] [PubMed] [Google Scholar]
- 30.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 31.Guiney H, Machado L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychonomic bulletin & review. 2013 Feb;20(1):73–86. doi: 10.3758/s13423-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 32.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. International journal of obesity. 2006 Jan;30(1):201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 33.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993 Mar;162:413–419. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 34.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011 May;34(5):565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in neurology. 2005 Mar;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 36.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in neurology. 2009 Sep;29(4):320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. Journal of sleep research. 2009 Dec;18(4):436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 38.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004 May 1;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 39.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008 Nov;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003 May 1;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 41.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An Update on Cancer- and Chemotherapy-Related Cognitive Dysfunction: Current Status. Seminars in Oncology. 2011;38(3):431–438. doi: 10.1053/j.seminoncol.2011.03.014. 6// [DOI] [PMC free article] [PubMed] [Google Scholar]


