Abstract
Objective
We assessed the occurrence of acute coronary syndrome (ACS) in patients with giant cell arteritis (GCA) compared to subjects without GCA.
Methods
We retrospectively reviewed a population-based incidence cohort of Olmsted County, Minnesota residents with GCA diagnosed in 1950-2009. We compared this cohort with a cohort of patients without GCA of similar age, sex and calendar year from the same population.
Results
The study included 245 patients with GCA and 245 non-GCA subjects. Mean Framingham cardiovascular risk score was 30% (SD 19%) in GCA and 34% (SD 23%) in non-GCA (p=0.096) at incidence/index date. Diabetes mellitus was significantly less common in GCA than non-GCA at index date. Mean high-density lipoprotein was higher and triglycerides were lower and fewer patients were using lipid-lowering medications in the GCA cohort compared to the non-GCA at index date. During follow-up, no difference between the two cohorts was noted in overall rate of ACS events [hazard ratio (HR) 0.74; 95% confidence interval (CI) 0.44, 1.26]. Overall thrombosis in myocardial infarction (TIMI) scores were similar in both cohorts. Revascularization procedures were done less frequently in GCA than non-GCA subjects (19% vs. 50%; p=0.015). Post ACS hospital length of stays and complications were similar in both cohorts.
Conclusion
Multiple cardiovascular risk factors are more favorable at incidence of GCA. There is no overall increased risk of acute coronary syndromes in patients with GCA.
Indexing terms: Acute Coronary Syndrome, Giant Cell Arteritis
Introduction
Coronary heart disease is a major cause of death and disability in the general population. (1) Giant cell arteritis (GCA) is the most common type of systemic vasculitis which predominantly affects females of northern European origin greater than 70 years of age (2). Inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus and psoriatic arthritis are associated with an increased risk for coronary heart disease(3) (4, 5).
Because GCA is a systemic inflammatory condition affecting the elderly and treated with chronic glucocorticoid therapy, patients with this disease may be at an increased risk of coronary artery disease. However, it is unclear if there is an increased risk of acute coronary syndrome in these patients. Population-based data on the association between GCA and coronary artery disease are available but population-based data on the association between GCA and acute coronary events are lacking (6). We aimed at assessing the occurrence of acute coronary syndrome (ACS) in patients with GCA.
Patients and Methods
This is a retrospective population-based study performed using resources of the Rochester Epidemiology Project (REP) medical record linkage system (7). The REP allows virtually complete access to medical records from all community medical providers including Mayo Clinic, Olmsted Medical Center and their affiliated hospitals, local nursing homes, and the few private practitioners in Olmsted County, MN. The uniqueness of the REP and its advantages in performing population-based studies in rheumatic diseases has been previously described (7).
We retrospectively reviewed the incidence cohort of patients with GCA diagnosed between 1950 and 2009 based on American College of Rheumatology 1990 GCA classification criteria (8). We also included patients diagnosed with GCA ≥ 50 years of age with elevation of erythrocyte sedimentation rate or C-reactive protein and computed tomography, magnetic resonance imaging or positron emission tomography evidence of large vessel vasculitis involving ascending aorta and its branches. We compared this cohort with an age, sex and calendar year matched cohort from the same population without GCA. Each comparator subject in the non-GCA cohort was assigned an index date corresponding to the GCA incidence date of the matched patient in the GCA cohort. All subjects were longitudinally followed through all available community medical records until death, migration from Olmsted County or April 30, 2013.
Data was collected in both cohorts around the index date on traditional cardiovascular risk factors such as age, sex, chronic kidney disease, obesity, current smoking history, diabetes mellitus and family history of coronary heart disease of premature onset. Chronic kidney disease and diabetes mellitus were defined as per physician diagnosis in medical records. The systolic and diastolic blood pressure (BP) readings closest to the index date or use of any antihypertensive medication recorded in medical records in the 12 month period prior to or 3 month post index date was collected. Total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL) and triglyceride levels up to 12 months prior to or 3 month post index date were collected. We also collected data on use of statins during this period. Body mass index (BMI) was calculated based on height and weight data available up to 12 months prior to index date and 3 months post-index date.
Obesity was defined as BMI ≥ 30kg/m2. Smoking history was collected based on the data entered in the patient charts. Family history of premature coronary heart disease was collected based on physician entered data in the medical record. The 2008 Framingham ten year general cardiovascular risk score was used to calculate the cardiovascular risk at the time of GCA incidence/index date (9). Non-lab version (using BMI instead of lipids) was used when lipid data were not available especially in the subjects with the index date prior to 1980s.
We also collected data regarding all documented episodes of ACS including Thrombolysis in Myocardial Infarction (TIMI) score for unstable angina and non-ST elevation myocardial infarction (NSTEMI) (10). Acute coronary syndrome was defined based on physician diagnosis of unstable angina, myocardial infarction, NSTEMI or STEMI requiring hospitalization, antiplatelet and anticoagulation therapy. Unstable angina was defined as a physician diagnosis of acute coronary syndrome with no elevation in troponin or creatine kinase-MB, with or without electrocardiogram (ECG) changes indicative of ischemia. To calculate the TIMI score, a value of 1 is assigned when each of the following variable was present and 0 when it was absent: Age ≥65 years, presence of at least three risk factors for coronary heart disease (CHD), prior coronary stenosis of ≥50 percent, presence of ST segment deviation on admission ECG, at least two anginal episodes in prior 24 hours, elevated serum cardiac biomarkers, use of aspirin in prior seven days.
Statistical Methods
Descriptive statistics (means, percentages, etc.) were used to summarize the characteristics of the 2 cohorts. Comparisons of baseline characteristics in the 2 cohorts were performed using chi-square and rank-sum tests. The cumulative incidence of ACS was calculated using Kaplan-Meier methods. Patients with history of prior ACS were excluded from these analyses because they were not at risk of developing incident ACS. Cox models were used to compare the development of ACS between the GCA and non-GCA cohorts. Poisson regression models were used to examine trends in the rate of ACS over time (for both calendar time and disease duration). The possibility of differing trends over time in the GCA compared to the non-GCA cohort was examined using interaction terms. Cox regression model was used to identify predictors of ACS among patients with GCA.
Results
Demographics
We identified 245 subjects in the GCA cohort and 245 age, sex and calendar year matched subjects in the non-GCA cohort. Baseline characteristics at GCA incidence/index date are compared between the two cohorts in Table 1. Mean age was about 76 years in both cohorts. 79% of the patients were females in each cohort. The mean length of follow-up was about 10 years in both cohorts.
Table 1. Baseline characteristics of Giant Cell Arteritis (GCA) and non-GCA cohorts at index dates.
| GCA (N=245) | Non-GCA (N=245) | p value | |
|---|---|---|---|
| Age, years (SD) | 76.2 (8.3) | 75.9 (8.5) | 0.743 |
| Females | 194 (79.2%) | 194 (79.2%) | 1.000 |
| Length of follow-up, years (SD) | 9.7 (6.7) | 10.4 (7.8) | |
| Alive at the date of last follow-up (%) | 74 (30.2%) | 66 (26.9%) | |
| Blood pressure Mean (SD) | |||
| [N=240 in GCA cohort; 241 in non-GCA cohort] | |||
| Systolic | 139.7 (20.5) | 140.8 (20.3) | 0.72 |
| Diastolic | 75.9 (11.2) | 76.2 (12.0) | 0.98 |
| Antihypertensive medication (%) | 107 (44.4%) | 127 (52.3%) | 0.08 |
| [N=241 in GCA cohort and 243 in non-GCA cohort] | |||
| Lipid Profile: | |||
| Total Cholesterol Mean (SD) | 209.1 (49.3) | 207.5 (42.3) | 0.88 |
| [N=84 in GCA cohort and 96 in non-GCA cohort] | |||
| HDL (SD) | 61.5 (17.1) | 55.3 (17.9) | 0.034 |
| [N=62 in GCA cohort and 65 in non-GCA cohort] | |||
| LDL (SD) | 113.1 (36.1) | 108.0 (31.8) | 0.45 |
| [N=59 in GCA cohort and 56 in non-GCA cohort] | |||
| Triglycerides (SD) | 124.2 (59.6) | 148.8 (77.5) | 0.029 |
| [N=76 in GCA cohort and 91 in non-GCA cohort] | |||
| Lipid-lowering medication (%) | 23 (9.6%) | 39 (16.1%) | 0.032 |
| [N=240 in GCA cohort and 242 in non-GCA cohort] | |||
| Chronic kidney disease (physician diagnosis) (%) | 12 (5.0%) | 7 (2.9%) | 0.24 |
| [N=242 in GCA cohort and 242 in non-GCA cohort] | |||
| Body mass index, kg/m2 (SD) | 25.2 (5.1) | 26.0 (5.3) | 0.12 |
| Obesity (BMI≥30 kg/m2) | 38 (15.5%) | 41 (16.7%) | 0.71 |
| Smoking status, ever | 97 (42.5%) | 103 (44.8%) | 0.63 |
| [N=228 in GCA cohort and 230 in non-GCA cohort] | |||
| Diabetes Mellitus | 17 (6.9%) | 40 (16.3%) | 0.001 |
| Framingham Risk Score % (SD) | 30.0 (18.6) | 34.1 (22.6) | 0.10 |
| [N=240 in GCA cohort and 241 in non-GCA cohort] | |||
| Framingham Risk Score (categorized) | 0.48 | ||
| Missing | 5 | 4 | |
| <6% | 6 (2.5%) | 4 (1.7%) | |
| 6-10% | 13 (5.4%) | 16 (6.6%) | |
| 10-20% | 65 (27.1%) | 54 (22.4%) | |
| 20-50% | 115 (47.9%) | 113 (46.9%) | |
| >50% | 41 (17.1%) | 54 (22.4%) | |
| Any Prior ACS | 18 (7.4%) | 17 (6.9%) | 0.86 |
Cardiovascular risk factors
Mean systolic and diastolic blood pressures were similar between the two cohorts. 107 (44%) GCA subjects were on antihypertensives as opposed to 127 (52%) non-GCA subjects (p=0.08). Mean high density lipoprotein (HDL) was higher in the GCA cohort [61.5 mg/dl; standard deviation (SD) 17.1] than non-GCA cohort (55.3 mg/dl; SD 17.9) (p=0.034). Mean triglyceride levels were lower in GCA cohort (124.2 mg/dl; SD 59.6) than in the non-GCA cohort (148.8 mg/dl; SD 77.5) (p=0.029). Mean low density lipoprotein (LDL) and total cholesterol were not different between the two cohorts. 23 (9.6%) subjects were on lipid lowering medication in the GCA cohort as opposed to 39 (16.1%) in the non-GCA cohort (p=0.032).
No difference was noted in smoking status, obesity or chronic kidney disease between the two cohorts. Diabetes mellitus was present in 17 (6.9%) GCA subjects and 40 (16.3%) non-GCA subjects (p=0.001). Mean overall Framingham 10 year cardiovascular risk score (11) was 30% (SD 19) in GCA subjects and 34% (SD 23) in non-GCA subjects (p=0.096) at incidence/index date. Framingham score >50% was present in 41 (17.1%) GCA subjects as opposed to 54 (22.4%) non-GCA subjects.
Acute Coronary Syndrome events
Prior to GCA incidence/index date, the occurrence of acute coronary syndrome (ACS) was similar between the two cohorts: 18 subjects (7.4%) in the GCA cohort versus 17 subjects (6.9%) in the non-GCA cohort (p=0.86). These subjects had at least one episode of ACS prior to GCA incidence/index date. Among those without ACS prior to GCA incidence/index date, ACS developed during follow-up in 26 patients with GCA and 32 patients without GCA. The cumulative incidence of ACS was 7.9% (standard error [SE]: 2.2%) at 10 years after GCA incidence in patients with GCA compared to 11.5% (SE: 2.5%) among non-GCA subjects. No difference between the two cohorts was noted in ACS event rates [hazard ratio (HR) 0.74; confidence interval (95% CI) 0.44, 1.26] (Table 2). There was also no difference in types of ACS such as unstable angina [HR 0.41; 95% CI (0.16, 1.09)], non-ST elevation myocardial infarction (NSTEMI) [HR 0.85; 95% CI (0.39, 1.85)] and ST elevation myocardial infarction (STEMI) [HR 1.03; 95% CI (0.41, 2.62)].
Table 2. Comparison of first Acute Coronary Syndrome (ACS) events in Giant Cell Arteritis (GCA) and non-GCA cohorts.
| ACS event | N events in GCA / Non-GCA | GCA Cumulative incidence at 10 years, % (± SE) | Non-GCA Cumulative incidence at 10 years, % (± SE) | Hazard ratio* (95% CI) | p-value |
|---|---|---|---|---|---|
| Any ACS | 26 / 32 | 7.9 ± 2.2 | 11.5 ± 2.5 | 0.74 (0.44, 1.26) | 0.26 |
| Unstable angina | 6 / 13 | 2.9 ± 1.3 | 6.5 ± 2.0 | 0.41 (0.16, 1.09) | 0.073 |
| Non-ST elevation MI | 12 / 15 | 2.0 ± 1.0 | 5.3 ± 2.8 | 0.85 (0.39, 1.85) | 0.68 |
| ST elevation MI | 9 / 9 | 2.9 ± 1.5 | 3.1 ± 1.2 | 1.03 (0.41, 2.62) | 0.95 |
| Any MI | 24 / 26 | 6.4 ± 1.9 | 9.1 ± 2.2 | 0.90 (0.51, 1.60) | 0.73 |
adjusted for age, sex and calendar year of GCA incidence/index date
MI = Myocardial infarction
We then compared characteristics of the subjects who developed ACS after the index date (Table 3). Age at first ACS event after index date was similar in both cohorts. Time from index date to first ACS event was also similar in both cohorts. Overall TIMI scores for unstable angina and NSTEMI were similar in both cohorts.
Table 3. Comparison of subjects who developed acute coronary syndrome after Giant Cell Arteritis (GCA) incidence/index date.
| GCA (N=26) | Non-GCA (N=32) | p value | |
|---|---|---|---|
| Age at first ACS (SD)* | 85.0 (8.6) | 82.7 (7.3) | 0.17 |
| Females (%) | 21 (81%) | 25 (78%) | 0.80 |
| Mean time from index date to first ACS, years (SD) | 10.5 (7.3) | 8.0 (6.5) | 0.16 |
| Median length of first ACS hospitalization, days (Q1, Q3) | 4.5 | 4.0 | 0.84 |
| [N= 16 in GCA cohort; 24 in non-GCA cohort] | (2.0, 7.5) | (2.5, 8.0) | |
| Positive family history of early MI (%)* | 2 (13%) | 10 (48%) | 0.024 |
| [N= 16 in GCA cohort; 23 in non-GCA cohort] | |||
| Prior coronary stenosis ≥50% (ever) (%)* | 6 (23%) | 10 (31%) | 0.49 |
| ST segment deviation on admission (ever) (%)* | 20 (77%) | 28 (88%) | 0.29 |
| At least 2 anginal episodes in 24hrs (ever) (%)* | 11 (42%) | 24 (75%) | 0.011 |
| Elevated cardiac biomarkers (ever) (%)* | 19 (73%) | 21 (66%) | 0.54 |
| Use of aspirin in prior 7 days (ever) (%)* | 7 (27%) | 13 (41%) | 0.28 |
| TIMI score ≥5 (in patients with UA or NSTEMI) | 5 (33%) | 9 (39%) | 0.72 |
| Management of ACS: | |||
| Coronary angiography (ever) (%) | 10 (38%) | 19 (59%) | 0.11 |
| Fibrinolysis (ever) (%) | 1 (4%) | 1 (3%) | 0.88 |
| PCI (ever) (%) | 5 (19%) | 12 (38%) | 0.13 |
| CABG (ever) (%) | 0 (0%) | 5 (16%) | 0.035 |
| Revascularization procedure (ever) (%) | 5 (19%) | 16 (50%) | 0.015 |
| Complications of ACS: | |||
| Hemodynamic instability or cardiogenic shock (ever) (%) | 4 (15%) | 2 (6%) | 0.26 |
| Recurrent or persistent rest angina despite intervention (ever) (%) | 3 (12%) | 1 (3%) | 0.21 |
| New or worsening mitral regurgitation or new VSD (ever) (%) | 0 (0%) | 1 (3%) | 0.36 |
| Sustained ventricular arrhythmias (ever) (%) | 0 (0%) | 0 (0%) | - |
used to calculate TIMI score
TIMI score = Thrombolysis in Myocardial Infarction score
With regard to management of the ACS episodes, 10 of 26 GCA subjects (38%) underwent coronary angiography compared to 19 of 32 non-GCA subjects (59%) (p=0.11). Revascularization procedures including percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) were done less frequently in GCA [5 of 26 (19%)] than non-GCA subjects [16 of 32 (50%)] (p=0.015). Median length of hospital stay of first ACS event after index date was similar in both cohorts (4.5 vs 4 days). Post-ACS complications including cardiogenic shock, persistent rest angina despite intervention, ventricular septal defect, mitral regurgitation and sustained ventricular arrhythmias were similar in both cohorts. 6 of 26 GCA subjects who had ACS experienced 10 recurrent events during 83 person-years of observation after the first episode compared with 6 of 32 non-GCA subjects who experienced 17 recurrent events during 138 person-years (rate ratio 1.00; 95% CI 0.44, 2.10).
We looked for any predictors of ACS in patients with GCA at the time of incidence (Table 4). None of the listed symptoms of GCA, comorbidities or medication use was associated with increased risk of ACS in GCA cohort.
Table 4. Identifying predictors of acute coronary syndrome in patients with GCA at incidence.
| Variables | Hazard ratio (95% CI) | P-value |
|---|---|---|
| GCA symptoms and signs: | ||
| PMR symptoms | 1.53 (0.66, 3.51) | 0.318 |
| Fever >100 | 1.80 (0.71, 4.57) | 0.218 |
| Weight loss | 0.41 (0.10, 1.74) | 0.225 |
| Jaw claudication | 0.73 (0.32, 1.65) | 0.444 |
| Scalp tenderness | 0.76 (0.33, 1.75) | 0.515 |
| Tender temporal artery | 0.64 (0.22, 1.82) | 0.400 |
| Temporal artery biopsy positivity# | 0.87 (0.19, 3.88) | 0.85 |
| Transient vision loss | 1.16 (0.15, 8.79) | 0.888 |
| Permanent vision loss* | - | - |
| Bruit | 1.06 (0.14, 8.17) | 0.953 |
| Aortitis* | - | - |
| ESR, mm/hr | 1.01 (0.99, 1.02) | 0.245 |
| Comorbidities: | ||
| Smoking | 0.45 (0.16, 1.28) | 0.136 |
| Hypertension before GCA incidence | 0.88 (0.36, 2.16) | 0.778 |
| Diabetes mellitus before GCA incidence | 0.00 (0.00,) | 0.990 |
| Coronary artery disease before GCA incidence | 0.31 (0.04, 2.39) | 0.262 |
| Transient ischemic attack/stroke before GCA incidence | 1.92 (0.57, 6.49) | 0.293 |
| Medications: | ||
| Steroid sparing disease modifying agent use (time-dependent) | 1.36 (0.53, 3.53) | 0.524 |
| Starting dose of glucocorticoid | 0.99 (0.96, 1.02) | 0.476 |
| Cumulative glucocorticoid dose in the first year (per 1 gm increase) | 1.03 (0.86, 1.22) | 0.749 |
No patients with permanent vision loss or aortitis had acute coronary syndrome
89% of the GCA cohort was temporal artery biopsy positive
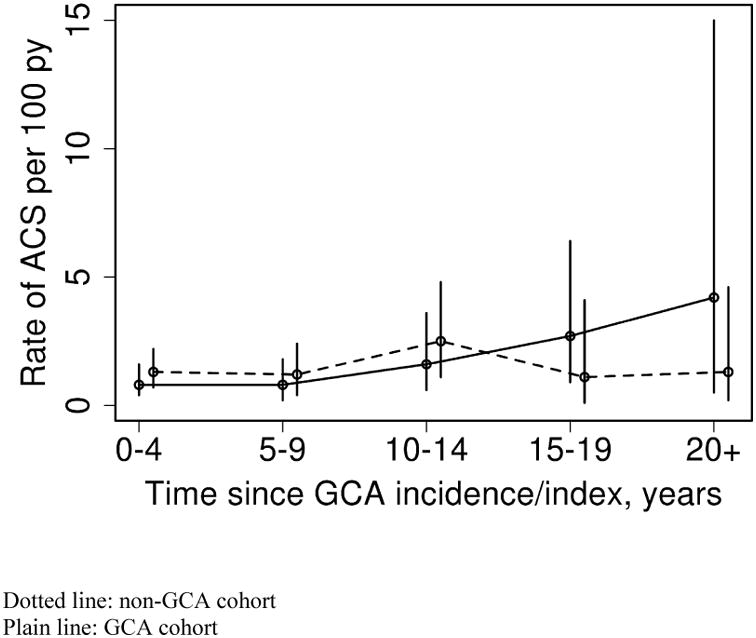
The rate of ACS increased with age in both groups (GCA and non-GCA). The rate of increase in ACS events by age did not differ for GCA compared to non-GCA subjects (interaction p=0.31). As well, examination of calendar year trends did not reveal any differences in the rate of ACS according to calendar year of follow-up between GCA and non-GCA subjects (interaction p=0.18). Examination of trends in rate of ACS according to disease duration revealed a significant interaction between years since GCA incidence/index date and cohort (GCA vs. non-GCA)(p=0.033) indicating that the rate of ACS over disease duration differed in patients with GCA compared to non-GCA as shown in Figure 1. In other words, the rate of ACS according to years after index date was constant among the non-GCA subjects (p=0.72), but increased over disease duration among the patients with GCA (p=0.037).
Figure 1. Rate of acute coronary syndrome (ACS) in Giant Cell Arteritis (GCA) and non-GCA cohorts according to time since GCA incidence/index.
Discussion
Inflammatory rheumatic diseases such as rheumatoid arthritis, lupus and psoriatic arthritis have been associated with increased risk of coronary artery disease (3-5). However GCA, although an inflammatory condition treated with chronic glucocorticoid therapy was not found to be associated with increased risk of acute coronary syndromes. In a similar population-based study in rheumatoid arthritis (RA) in the same population, increased occurrence of ACS was noted in RA prior to incidence date when compared to controls however no difference was noted after the incidence date (4).
This is the only study to date that has evaluated the experience of patients with GCA over such a prolonged period (six decades); mean length of follow-up was about 10 years. Since lipid studies were more consistently performed since about 1990, we did a subgroup analysis of the baseline characteristics between the two cohorts from 1990-2009 which showed similar results with respect to ACS occurrence.
Patients aged ≥50 years of age with chest imaging studies suggestive of inflammation in vessel walls and elevated inflammatory markers were also included in the study. Since there were only 5 such subjects, subgroup analysis excluding these subjects was not done. We did not assess sudden death in the cohorts occurring during the study period. These were not included as acute coronary events in our study as the cause of sudden death in those patients in whom it occurred was not clear.
With a mean age of onset around 70-75 years, GCA affects an older population of patients. The question is whether a possible “healthy cohort” bias affects the risk of patients with GCA for developing ACS. We believe that this is less likely since the GCA patients were age and sex-matched to the non GCA cohort.
The incidence of cardiovascular events in a French GCA cohort was assessed after controlling the traditional cardiovascular risk factors (12). Cardiovascular events all combined were significantly increased in patients with GCA though each subset of cerebrovascular accident or ischemic heart disease did not reach statistical significance individually. A population-based retrospective study of cardiovascular diseases in patients who had an ICD code of GCA in the province of Ontario, Canada showed that the incidence of the composite cardiovascular events was higher in GCA group versus patients with osteoarthritis or neither condition (13). A non-significant trend remained after adjustment for all covariates except prescribed corticosteroids. Of note, only 25% of the patients with ICD code of GCA temporal artery biopsy within 30 days of the diagnosis of GCA. Less than half of the patients in the GCA group were prescribed corticosteroids. It is possible that some of the patients in this cohort might have been misclassified. The primary cardiovascular disease outcome assessed in this study was a composite diagnosis or surgical treatment of any one of the following: coronary artery disease, stroke, peripheral arterial disease, or aneurysm or dissection of the aorta.
Interestingly, many cardiovascular risk factors were found to be favorable in the GCA cohort when compared to the non-GCA cohort in our study. Diabetes mellitus was significantly less common in GCA cohort at index date. Lower numbers of subjects in the GCA cohort were on antihypertensives (not statistically significant) and lipid-lowering medications at the index date. The lower prevalence of diabetes is also noted in other GCA cohorts (14-16). In all these cohorts less than 10% of the biopsy-positive GCA patients had diabetes at the time of biopsy. In one of these cohorts, a history of diabetes mellitus was associated with a 50% reduction in the odds of developing GCA in women (14).
A prospective study conducted in France comparing the cardiovascular risk factors between cases and controls revealed diabetes mellitus and hypercholesterolemia to be less common in women with GCA at the time of diagnosis (17). It is not clear why diabetes is less prevalent in patients with GCA.
A recent retrospective study has also shown lower frequency of history of hypertension, smoking, myocardial infarction, overweight, statin use among patients with biopsy-proven GCA when compared to population-based controls (16). In our study, triglyceride level was significantly lower in GCA cohort when compared to non-GCA cohort. HDL was significantly higher in the GCA cohort at index date. Overall Framingham Risk Score was lower in GCA cohort when compared to non-GCA cohort at the index date though this did not reach statistical significance.
Revascularization procedures were performed less frequently in ACS patients with history of GCA. The reason is not clear if revascularization was less needed or if procedures were avoided for other reasons. The total number of days of hospitalization for ACS was not different in the GCA cohort compared to the non GCA subjects.
We found a significant increase in ACS over disease duration in GCA, but the rates were constant in non-GCA cohort. However, because the confidence intervals are wide and the number of events is small, no strong conclusions can be drawn from this finding.
The strengths of this study are that it is population-based and targets an understudied problem in patients with GCA. It is not administrative data, as complete medical record information is available for all subjects. With the exception of a higher proportion of the working population employed in the health care industry, and correspondingly higher education levels, on the whole, results of this study utilizing the population of Olmsted County, Minnesota are generalizable to the populations of interest elsewhere (18). Study limitations are those inherent in the retrospective design. Only those persons who had a medical encounter during an acute coronary event be identified and included. Some acute coronary events that happened outside Olmsted County, MN may not have been included, however these events are usually recorded upon return to the primary care provider in Olmsted County, and it is not likely that event rates would have been different between patients with and those without GCA. Sudden deaths with no clear cause of death were not included.
In conclusion, there is no overall increased risk of acute coronary syndromes in patients with GCA. Multiple cardiovascular risk factors are less frequent at incidence of GCA when compared to non-GCA subjects of similar age and gender. Anti-hypertensives and lipid lowering medications are less frequently used in GCA subjects at incidence. Framingham overall 10 year cardiovascular risk may be lower at GCA incidence although this did not achieve statistical significance in our study. Revascularization procedures are performed less frequently in patients with giant cell arteritis. Overall length of hospital stay is not different in patients with giant cell arteritis who develop acute coronary syndrome.
Significance and innovation.
This is the first US population-based study to report the occurrence of acute coronary syndrome in patients with giant cell arteritis (GCA) when compared to patients without GCA.
This is the first study that covers a period of six decades for retrospective data on acute coronary syndromes in GCA.
Contrary to what one would expect, multiple cardiovascular risk factors are found to be less frequent at incidence of GCA. There is no overall increased risk of acute coronary syndromes in patients with giant cell arteritis
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61(10):1454–61. doi: 10.1002/art.24459. [DOI] [PubMed] [Google Scholar]
- 3.Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93(2):198–200. doi: 10.1016/j.amjcard.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–11. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 5.Tobin AM, Veale DJ, Fitzgerald O, Rogers S, Collins P, O'Shea D, et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol. 2010;37(7):1386–94. doi: 10.3899/jrheum.090822. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Gay MA, Rubiera G, Pineiro A, Garcia-Porrua C, Pego-Reigosa R, Gonzalez-Juanatey C, et al. Ischemic heart disease in patients from Northwest Spain with biopsy proven giant cell arteritis. A population based study. J Rheumatol. 2005;32(3):502–6. [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 9.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. Jama. 2000;284(7):835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 12.Le Page L, Duhaut P, Seydoux D, Bosshard S, Ecochard R, Abbas F, et al. Incidence of cardiovascular events in giant cell arteritis: preliminary results of a prospective double cohort study (GRACG) Rev Med Interne. 2006;27(2):98–105. doi: 10.1016/j.revmed.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Ray JG, Mamdani MM, Geerts WH. Giant cell arteritis and cardiovascular disease in older adults. Heart. 2005;91(3):324–8. doi: 10.1136/hrt.2004.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews JL, Gilbert DN, Farris BK, Siatkowski RM. Prevalence of diabetes mellitus in biopsy-positive giant cell arteritis. J Neuroophthalmol. 2012;32(3):202–6. doi: 10.1097/WNO.0b013e31825103cb. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Gay MA, Pineiro A, Gomez-Gigirey A, Garcia-Porrua C, Pego-Reigosa R, Dierssen-Sotos T, et al. Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteritis. Medicine (Baltimore) 2004;83(6):342–7. doi: 10.1097/01.md.0000145369.25558.b5. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt J, Kermani TA, Muratore F, Crowson CS, Matteson EL, Warrington KJ. Statin use in giant cell arteritis: a retrospective study. J Rheumatol. 2013;40(6):910–5. doi: 10.3899/jrheum.121150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duhaut P, Pinede L, Demolombe-Rague S, Loire R, Seydoux D, Ninet J, et al. Giant cell arteritis and cardiovascular risk factors: a multicenter, prospective case-control study. Groupe de Recherche sur l'Arterite a Cellules Geantes. Arthritis Rheum. 1998;41(11):1960–5. doi: 10.1002/1529-0131(199811)41:11<1960::AID-ART10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


