Abstract
Telomere length, a reliable predictor of disease pathogenesis, can be affected by genetics, chronic stress, and health behaviors. Cross-sectionally, highly stressed post-menopausal women have shorter telomeres, but only if they are inactive. However, no studies have prospectively examined telomere length change over a short period, and if rate of attrition is affected by naturalistic factors such as stress and engagement in healthy behaviors. Here we followed healthy women over one year to test if major stressors that occurred over the year predicted telomere shortening, and whether engaging in healthy behaviors during this period mitigates this effect. In 239 post-menopausal, non-smoking, disease-free women, accumulation of major life stressors across a one-year period predicted telomere attrition over the same period - for every major life stressor that occurred during the year, there was a significantly greater decline in telomere length over the year of 35 base pairs (p < .05). Yet, these effects were moderated by health behaviors (interaction B = 0.19, p = .04). Women who maintained relatively higher levels of health behaviors (one standard deviation above the mean) appeared to be protected when exposed to stress. This finding has implications for understanding malleability of telomere length, as well as expectations for possible intervention effects. This is the first study to identify predictors of telomere length change over the short period of a year.
Keywords: Telomeres, Stress, Physical activity, Sleep quality, Diet
Introduction
An aging population brings with it ever-increasing risks and prevalence of diseases of aging, such as cardiovascular disease, type 2 diabetes mellitus, Alzheimer’s disease, and autoimmune disorders (1). Chronic stress is associated with many of these diseases (2) and is rooted in both the exposure to stressors experienced from birth through adulthood and in temperaments formed early in life through gene-environment interactions that predispose individuals to high threat perceptions (2,3). Stressful events across the lifespan, ranging from abuse and neglect as a child to experiences of financial difficulties and relationship breakdown during adulthood, cumulatively wear down physiological systems that accelerate individuals’ trajectories towards disease (3–5)
An impaired and aging immune system partly mediates the deleterious effects of chronic stress that drive diseases of aging (6). Adults experiencing chronic stress have impaired wound healing, weaker control of latent viruses, poorer vaccination responses, and elevated states of chronic inflammation (7). For example, caregivers of family dementia patients have a significant increase in circulating levels of interleukin-6 over a six-year period that is 4 times the increase of matched, non-caregiving controls (8). In a sample of healthy men and women, those who were exposed to any recent stressful life event over the previous 12 months were more likely to develop a cold following experimental exposure to a rhinovirus (9).
Several cellular markers indicate aging of immune cells, with significant attention paid to telomere length (10,11). Short telomeres have been linked to numerous diseases of aging and in many but not all cases, to all-cause and disease-specific mortality (12–17). In humans, telomeres consist of repeated sequences (TTAGGG repeats) of deoxyribonucleic acid nucleotides (DNA) that are thousands of nucleotides long, encapsulated and stabilized by associated proteins. Telomeres cap chromosomes in all eukaryotic cells, protecting DNA from degradation resulting from incomplete replication, exogenous and endogenous damage and detrimental fusion during DNA repair processes. With each cell cycle, the 3’ end replication problem causes telomere shortening, and when telomeres shorten to a critical length, cells typically enter senescence and undergo changes that can be harmful at the organismic level (12).
The protein-RNA complex reverse transcriptase enzyme, telomerase, significantly delays shortening to a critical length by adding repeated TTAGGG sequences onto chromosomal ends (18). Mutations in telomere maintenance genes in humans and genetic knockout of either telomerase protein (TERT −/−) or telomerase RNA (TERC −/−) components in rodent models cause a spectrum of diseases called the telomere syndromes (12). These reveal the prime mechanistic role of telomere maintenance in tissue degradation associated with early aging and disease pathogenesis (19,20). Population based genome-wide association studies also show that common sequence variants of genes known to function directly in telomere maintenance cause increased risks for cardiovascular and pulmonary disease (21).
Chronic psychological stress has been associated with shorter telomeres during childhood and adulthood, though not consistently. Children and adults with adverse and disadvantaged early life experiences (22–25) (see (26) for exception), women who provide care for a family member with a chronic health condition (27–29) (see (30) for exception), those who report high perceived stress (29,31), and women exposed to domestic violence (32) have shorter telomeres in leukocytes and varying subtypes of immune cells compared to those who have not experienced such stressors. Severity and chronicity of depression are also related to shorter telomeres (33–35). To date, no studies have prospectively examined whether the emergence of new life stressors is related to shortening of telomeres over time. It is unknown whether a combination of life stressors is potent enough to lead to greater telomere shortening in a short period of time.
The current study also sought to examine whether health behaviors moderate any effects of major life stressors on telomere shortening. In this study, we examined three specific health behaviors - physical activity, dietary intake, and sleep quality. Each behavior has been associated with telomere length cross-sectionally (36–40). Importantly, these behaviors have been independently shown to alter the relationship between stress and biological outcomes, including neural functioning and hypothalamic-pituitary-adrenal axis activation (41–44). Health behaviors, however, naturally cluster (45,46) and a combination of healthy behaviors seem to be a stronger predictor of telomere length than each individual behavior alone (47). We recently proposed that healthy lifestyle factors, alone or in combination, might mitigate the impact of chronic psychological stress on telomere length (48,49), and documented this effect cross-sectionally in chronically stressed adults and in depressed adults (31,50). Whether each behavior alone or in combination can attenuate the biological burden of stress over time remains unexamined.
The first question we asked, then, is whether major life stressors over the course of one year significantly predict leukocyte telomere shortening over the same time frame. We expected that the accumulation of major life events during the year would significantly predict accelerated shortening of telomeres. The current study also examined whether engaging in higher levels of these health behaviors, either individually or combined, can mitigate any prospective association between life stressors and telomere shortening over the course of one year.
Methods
Study Design
We recruited 263 healthy midlife women (ranging 50 to 65 years) from the San Francisco Bay Area between February and May, 2010, with online and paper advertisements for the purpose of tracking women’s health behaviors and stress over the course of one year and telomere length at the beginning and end of that year. The research objectives of the study were: (1) to determine whether changes in major life stressors and health behaviors over the course of one year predicts telomere length changes and (2) to examine the psychological impact of revealing to participants the length of their telomeres. Here, we report the findings related to the first objective of the study. Exclusionary criteria included any history of cancer within the previous 10 years, any autoimmune disease, and current smoking status.
Settings
Certified phlebotomists and nurses drew blood in the summer of 2010 at the University of California, San Francisco’s (UCSF) Department of Psychiatry, and again at one-year follow-up in the summer of 2011 at UCSF’s Clinical and Translational Science Institute Clinical Research Center. Ninety-one percent (N=239) of the women returned for follow-up blood draw. Health behaviors, including physical activity, typical food consumption, and sleep quality, were self-reported at baseline, 4 months, 8 months, and one-year follow-up. Major life stressors over the previous year were self-reported at one-year follow-up in the summer of 2011. Complete data with main study variables were available for 231 women. Study design was approved by UCSF’s Institutional Review Board and informed consent was obtained from all participants.
Measures
Telomere length
We measured leukocyte telomere length (LTL) in all women. Genomic DNA was purified from whole blood stored at −80°C with the QIAamp mini DNA kit (QIAGEN cat# 51106). Mean LTL was analyzed using a quantitative polymerase chain reaction (qPCR) method in Blackburn’s laboratory at UCSF as described in detail elsewhere (Lin et al., 2010). qPCR is the most common method of LTL measurement used in the majority of current clinical and population based studies. This method was validated for mean telomere length measurement and shown to be significantly associated with the Southern blot method (51). For the qPCR reactions to determine mean telomere length, the telomere thermal cycling profile consists of (1) Cycling for T (telomic) PCR: denature at 96°C for 1 second, anneal/extend at 54°C for 60 seconds, with fluorescence data collection, 30 cycles; and (2) Cycling for S (single copy gene) PCR: denature at 95°C for 15 seconds, anneal at 58°C for 1 second, extend at 72°C for 20 seconds, 8 cycles, denature at 96°C for 1 second, anneal at 58°C for 1 second, extend at 72°C for 20 seconds, hold at 83°C for 5 seconds with data collection, 35 cycles.
The primers for the telomere PCR are tel1b [5'-CGGTTT(GTTTGG)5GTT-3'], used at a final concentration of 100 nM, and tel2b [5'-GGCTTG(CCTTAC)5CCT-3'], used at a final concentration of 900 nM. The primers for the single-copy gene (human beta-globin) PCR are hbg1 [5' GCTTCTGACACAACTGTGTTCACTAGC-3'], used at a final concentration of 300 nM, and hbg2 [5'-CACCAACTTCATCCACGTTCACC-3'], used at a final concentration of 700 nM. The final reaction mix contains 20 mM Tris-HCl, pH 8.4; 50 mM KCl; 200 µM each dNTP; 1% DMSO; 0.4x Syber Green I; 22 ng E. coli DNA per reaction; 0.4 Units of Platinum Taq DNA polymerase (Invitrogen Inc.) per 11 microliter reaction; 0.5 – 10 ng of genomic DNA. Tubes containing 26, 8.75, 2.9, 0.97, 0.324 and 0.108ng of a reference DNA (from Hela cancer cells) are included in each PCR run so that the quantity of targeted templates in each sample can be determined relative to the reference DNA sample by the standard curve method. Each concentration of the reference DNA is run as quadruplets and samples are run as triplicates.
To control for inter-assay variability, 8 control DNA samples from cancer cell lines (including 293T, H1299, UMUC3, and UMUC3 cells infected with a lentiviral construct containing the telomerase RNA gene to extent telomeres, harvested at various population doublings after infection) are included in each run. In each batch, the T/S ratio of each control DNA is divided by the average T/S for the same DNA from 10 runs to get a normalizing factor. This is done for all 8 samples and the average normalizing factor for all 8 samples is used to correct the participant DNA samples to get the final T/S ratio. The T/S ratio for each sample is measured twice, each time in triplicate wells. When the duplicate T/S value and the initial value vary by more than 7%, the sample is run the third time and the two closest values will be reported. The inter-assay coefficient of variation for telomere length measurement was 4.3% for this study. To convert T/S ratios to basepairs, the mean telomere length of a set of genomic DNA samples from the human fibroblast primary cell line IMR90 at different population doublings, as well as with the telomerase protein subunit gene (hTERT) transfected into a lentiviral construct was measured using the above method. The mean TRF (telomeric restriction fragment) length from these DNA samples was determined using Southern blot analysis. The slope of the plot of mean TRF length versus T/S for these samples served as the conversion factor for calculation of telomere length in base pairs from the T/S ratio. The equation for conversion from T/S ratio to base pairs for this study was base pairs = 3274+2413*(T/S). The Y intercept in this equation represents the length of the subtelomeric region. For more information about the telomere length assays, including information about the primers and reaction mix, see (52,53).
Major life stressors
At follow-up, participants completed a self-reported 15-item checklist for major adverse life stressors of considerable high threat that may have happened during the previous year. Two items of occurrence of health-related stressors that are confounded with disease and can affect physiology (any major health problem, experiences of drug/alcohol dependence or addiction) were excluded, for a tally of 13. The final 13 items included (1) loss of household, (2) major financial difficulties, (3) self or (4) family member employment loss, (5) continued unemployment and job searching, (6) major interpersonal problems including divorce, (7) ongoing arguments with a spouse or (8) close family member or friend, (9) caregiving for an adult or (10) child with a serious illness, death of a (11) family member or (12) friend, and (13) sexual harassment. Items endorsed were counted as 1 and all items were summed.
Cumulative health behaviors
At three time points between baseline and one-year follow-up (at 4, 8 and 12 months), we assessed (1) leisure time physical activity, (2) typical dietary practices, and (3) sleep quality.
(1) Leisure time physical activity was assessed with the leisure activity subsection of the Stanford Brief Activity Scale (SBAS), shown to successfully discriminate different activity levels, body weight, and key metabolic markers (54). The SBAS is a self-administered questionnaire, developed for quick assessment of frequency, intensity, and type of physical activity at work and during leisure activities over the past week. Participants are provided 5 scenarios that provide a global statement of activity level and descriptors of frequencies, intensities, and type of activity. For example, the least active scenario (score of 1) is described as, “Most of my leisure time was spent without very much physical activity. I mostly did things like watching television, reading, or playing cards. If I did anything else, it was likely to be light chores around the house or yard or some easy-going game like bowling or catch. Only occasionally, no more than once or twice a month, did I do anything more vigorous, like jogging, playing tennis, or active gardening.” Each rating appropriately progresses in description of intensity, frequency, and type. Participants selected one statement from the 5 provided activities from 0 = little activity, mostly sedentary lifestyle to 4 = engaging in a regular physical fitness program at least 5 days a week. The SBAS was adjusted to ask about previous 3 months of activity. The SBAS and its revised L-CAT are well-validated and reliable, as seen in studies demonstrating matching results to the Seven Day Physical Activity Recall and to Centers for Disease Control and Prevention recommendations for physical activity (55,56) Scores from each assessment were summed for a total activity score for the year and then standardized.
(2) Typical dietary practices were self-reported with diet questions developed based on the Food Frequency Questionnaire used in the Multi-Ethnic Study of Atherosclerosis (MESA) (57). These questions measure how often participants eat foods from each of 13 food categories, including typical healthy foods, such as whole grains, fruits, vegetables, nuts, seeds, low fat dairy, and fish, and typical unhealthy foods, including starches such as white potatoes and refined grains, red meat, high-fat dairy, fried foods and sodas. Each food category was rated on a 9-point scale, including 1 ‘rarely or never’; 2 ‘once per month’; 3 ‘two to three times per month’; 4 ‘once per week’; 5 ‘twice per week’; 6 ‘three to four times per week’; 7 ‘five, six times per week’; 8 ‘once per day’; and 9 ‘twice a day or more’. Healthy and unhealthy foods (reverse scored) were summed across all time points and standardized for a total healthy foods score.
(3) Self-reported sleep quality was obtained using one question from the Pittsburgh Sleep Quality Index (PSQI) (58), “How would you rate your sleep quality overall?” rated (1) very bad, (2) fairly bad, (3) fair, (4) fairly good, and (5) very good. Again, responses were summed across the three assessments and standardized.
Cumulative health behaviors were computed as the sum of the three scores for leisure time physical activity, dietary practices, and sleep quality. Higher cumulative health behaviors designate greater engagement in healthier behaviors.
Statistical Approach
Bivariate correlations between telomere length, lifestyle behaviors, and major life events were performed. To test our hypotheses, LTL change (LTL from baseline subtracted from one year follow-up) was first regressed on major life stressors. Covariates included baseline LTL, sociodemographic factors [age, income, education (less than Bachelor’s degree/Bachelor’s or higher), ethnicity (Caucasian/other)], medication use at baseline [hypertension reducing medication (Yes/No), cholesterol reducing medication (Yes/No), hormone replacement therapy (Yes/No), antidepressants (Yes/No)], BMI at baseline, and health behaviors over the previous year. Next, a series of regression analyses were completed with (1) the interaction between major life stressors and each health behavior alone (while covarying the two other behaviors) and (2) cumulative health behaviors as a combined factor. Briefly, a significant interaction between two continuous variables, such as major life stressors and cumulative health behaviors, suggests that the relationship between major stressors and telomere length change is significantly different at varying levels of health behaviors. A significant interaction is followed up with simple slope analyses, whereby the relationship between major stressors and LTL change is tested in two different analyses: (1) at one standard deviation above and (2) one standard deviation below the mean of cumulative health behaviors. This approach is the typical, standard approach for testing significant interactions (59). We also conducted analyses for each health behavior individually.
Results
Participants
Women were highly educated (84% with a college degree or higher), had relatively high income (54% earned greater than $100,000/year), and were primarily (84%) Caucasian. Average baseline BMI for the sample was in the normal weight range at 24.19 (SD = 4.59) and minorities of women were utilizing medications (15% hypertension reducing medication; 10% statins; 17% antidepressants; 42% hormone replacement therapy).
Descriptive Statistics
At baseline, mean leukocyte telomere length was 5548 base pairs (SD = 328.9) and mean 12-month follow-up leukocyte telomere length was similar, 5584 base pairs (SD = 354.9). Baseline and follow-up telomere length were significantly related (r (229) = .74, p < .001), indicating considerable stability over time, and the average percent change was minimal (0.65%) with a standard deviation for percent change in telomere length of 4.86%. Thus, as would be expected for a short period of one year, the majority of people (68%) did not show a large increase or decrease greater than approximately 5% of their baseline telomere length. As consistently reported in other longitudinal studies of telomere length change, longer telomeres at baseline were more likely to shorten over time (B = −.31, p < .001), such that for every 100 base pairs above the average of the sample at baseline, telomeres were likely to significantly shorten by an average 24 (SE = 0.5, 95% C.I. = −33.9, −12.8) base pairs over the year.
At one year follow-up, the distribution of stressors was as follows: 32% experienced the death of a family member or close friend, 26% women reported that they experienced relationship difficulties, 20% were involved in caregiving, 17% became unemployed or experienced financial strain, 4% experienced sexual harassment, and 3% experienced loss of their house. Thirty-seven percent of women did not have any major life events over the previous year, 47% had 1 or 2, and 16% experienced 3 or more.
Women reported an average 2.29 (SD = 1.13) leisure-time physical activity over the course of the year, corresponding to a moderately active lifestyle. Furthermore, women reported eating fairly healthy diets, with a mean food frequency of 6.77 (SD = 0.67), corresponding to 2–3 times a week of eating healthy foods, and 2–3 times a month of eating less healthy foods. Finally, on average, participants reported fairly good sleep over the year (mean = 3.56, SD = 0.69). Cumulative leisure time physical activity over the year was significantly related to typical foods eaten over the year (r (229) = .34, p < .001) and to sleep quality during the year (r (229) = 0.14, p = .04). Typical foods and sleep quality were not significantly related to each other (r (229) = .10, p = .11) but the trend was in a positive direction.
Healthy behaviors were not significantly related to number of life events, though a marginal trend emerged for leisure activity, such that those who had more events during the year were less likely to be active (r (229) = −.12, p = .08).
Multivariate analyses main results
Major stressors and telomere shortening
Major stressors during the year significantly predicted accelerated telomere shortening over the same time frame (B = −.18, p = .01). These results suggest that for every one event, there was a significantly greater decline in telomere length over the year of 34.7 base pairs (SE = 14.04, 95%C.I. = −62.3, −6.9). Table 1 presents the complete model with covariates, individual health behaviors, and major life stressors.
Table 1.
Multivariate regression analyses, including covariates, health behaviors, and major life events.
| b | C.I. (95%) | B | p | |
|---|---|---|---|---|
| Covariates | ||||
| TL at Baseline (Base pairs) |
−.24 | −.35, -.13 | −.31 | < .000 |
| Age (years) | −7.86 | −16.67, .95 | −.13 | .08 |
| Household Income | 7.20 | −16.31, 30.71 | .04 | .55 |
| Education (Completed Bachelor’s or more = 1; Bachelor’s or lower = 0) |
52.41 | −49.17, 153.98 | .07 | .31 |
| Ethnicity (Caucasian = 1; Other = 0) |
11.79 | −91.97, 115.54 | .02 | .82 |
| BMI at Baseline (Kg/m2) |
0.01 | −7.95, 7.96 | .00 | .99 |
| Antidepressant Use at Baseline (Yes = 1; No =0) |
−11.15 | −109.72, 87.42 | −.02 | .82 |
| CVD medication Use at Baseline (Yes = 1; No =0) |
0.92 | −100.91, 102.74 | .02 | .98 |
| Statins Use at Baseline (Yes = 1; No =0) |
−103.70 | −226.10, 18.71 | −.12 | .09 |
| Hormone Replacement Therapy at Baseline (Yes = 1; No =0) |
61.38 | −17.78, 140.55 | .12 | .13 |
| Cumulative Physical Activity | −10.96 | −57.03, 35.12 | −.04 | .64 |
| Cumulative Typical Diet | 33.03 | −10.44, 76.51 | .12 | .14 |
| Cumulative Sleep Quality | 6.94 | −36.09, 49.97 | .02 | .75 |
| Major Life Events | −34.65 | −62.35, −6.95 | −.18 | .01 |
Note. b = unstandardized beta; B = standardized beta
Moderating roles of each health behavior
Three separate regression equations were completed that included the covariates, major life stressors, each of three health behaviors (physical activity, sleep quality or diet), and tested whether each behavior alone moderated the relationship between major life stressors and telomere shortening. As seen in Table 2, results indicated that at one standard deviation below the mean and at the mean of each behavior separately, major life stressors significantly predicted telomere shortening. At one standard deviation above the mean of each health behavior, life stressors were unrelated to telomere shortening. However, the interaction effects for the three moderation analyses were not statistically significant (all interaction p’s > .10).
Table 2.
Simple slopes for association between life stressors and telomere shortening over one year at −1 SD below the mean, at the mean, and +1SD above the mean for each health behavior independently.
| Physical Activity | Diet | Sleep Quality | ||||
|---|---|---|---|---|---|---|
| b (SE) | p | b (SE) | p | b (SE) | p | |
| −1 SD | −48.1 (17.9) | .008 | −46.5 (18.4) | .01 | −46.2 (16.8) | .007 |
| Mean | −31.5 (14.2) | .03 | −33.5 (14.1) | .02 | −31.7 (14.2) | .03 |
| +1 SD | −14.9 (21.1) | .48 | −20.5 (19.6) | .30 | −17.2 (19.5) | .38 |
Note. Values indicate the unstandardized beta coefficient (b), standard errors (SE) and p values for the associations between life stressors and telomere shortening over the course of the year at varying levels of each behavior.
Moderating role of healthy behaviors
Results testing an interaction between major stressors and cumulative health behaviors (i.e. sum score of all three health behaviors over the year) support the hypothesis that a constellation of health behaviors during the 12 months from baseline to follow-up significantly moderated the relationship between major life stressors and telomere shortening during the same time frame (interaction B = 0.19, p = .041). For women who engaged in lower levels of health behaviors (one standard deviation below the average sample mean of cumulative health behaviors), every additional stressor was related to accelerated telomere shortening over the year by an additional mean 76.5 base pairs (95%C.I. = −123.1, −30.0 base pairs, B = -.29, p = .001) compared to women with no adverse stressors. At average levels of health behaviors, accelerated telomere shortening over the year occurred by an additional 39.6 base pairs for every major life stressor that occurred, on average (95% C.I. = −76.4, −2.7 base pairs, B = -.15, p = .04). For those women at higher levels of healthy behaviors (one standard deviation above the average sample mean of health behaviors), adverse events were unrelated to telomere shortening (unstandardized b = −2.6, 95% C.I. = −58.0, 52.7 base pairs, B = −0.01, p > .50). Figure 1 illustrates the simple slopes for major life stressors on telomere shortening at mean cumulative health behaviors, and one standard deviation above and one below the mean.
Figure 1.
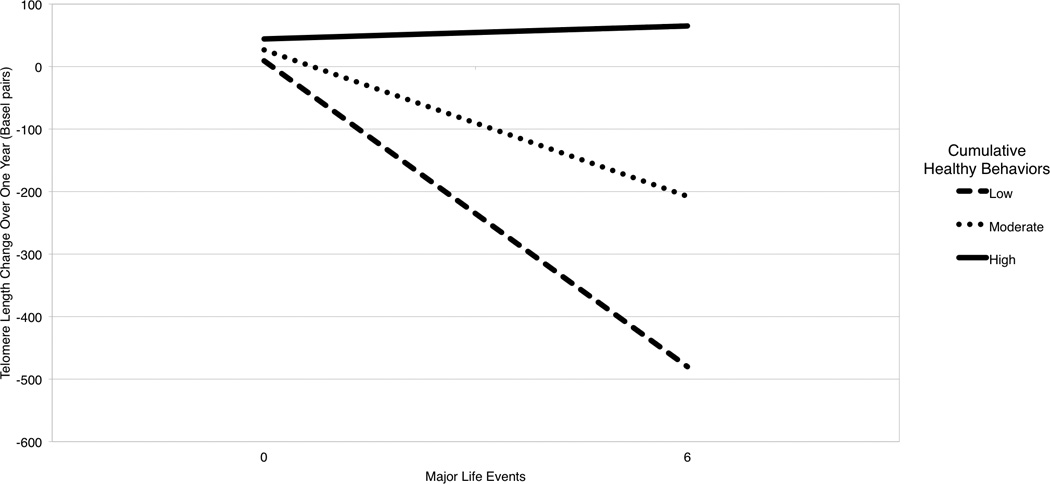
Predicted Telomere Length Change Over One Year as a Function of Major Life Stressors and Cumulative Health Behaviors
Note. Lines are predicted relationships between major life stressors and change in telomere length over the one year of study at three levels of cumulative health behaviors, low (−1 SD), moderate (mean), and high (+1 SD).
Discussion
Healthy aging is a complex interplay of genetics, lifespan stressors from the social and physical environments, and behaviors (45,46). We examined whether the interaction between life stressors from the social environment and behaviors during a one-year period shapes cell aging, indexed by telomere attrition over time. It has been an open question about whether telomere length might change in as short of a period as one year. This is the first observational study to examine short-term changes in telomere length. We found that while a majority of women remained within 5% of their original telomere length, there was still a significant amount of change, and this change was predictable based on life stressors over the previous year and modifiable lifestyle behaviors. These findings are supportive of previous models suggesting that the accumulation of varied stressful events across the lifespan promote wear and tear on physiological systems that ultimately shape cellular aging processes (3,62–64). These findings, however, are suggestive of the hopeful message that engagement in healthy behaviors during periods of high stress can perhaps attenuate immune cell aging.
Recent reviews in the literature identify strong relationships between psychological stress and physical activity (65), stress and eating (66), and stress and sleep (67,68). These three behaviors are especially important to the biological pathogenesis of depression (69). In the present study, each behavior did not independently attenuate the association of life stress on telomere attrition over time. However, the relationships between major events and telomere attrition that were evidenced at low, moderate and higher levels of each individual behavior patterned similarly to those with the cumulative health behaviors score. A composite of health behaviors has previously been evidenced to be a stronger cross-sectional predictor of telomere length than each individual behavior alone (47). It is not surprising, then, that only cumulative health behaviors were a potent moderator of the life events-telomere attrition relationship over time.
Several pathways are suggested for the protective effects of these behaviors. Physical activity promotes neurogenesis, cognitive flexibility and memory formation in both humans and other animals alike (70), perhaps shaping biological stress reactivity, and emotional and cognitive responses to stress (3,70). Previous studies have shown that fit younger and older adults have quicker biological stress recoveries than unfit adults (71–73). Physical activity also bolsters mediating immune proteins and gene expression (74,75) and more distal biological aging endpoints, such as mitochondrial health and telomerase levels (76–79), which are intimately related to telomere maintenance (20,80,81). There are suggestions of quite similar mechanistic benefits of sleep (44,82,83) and nutrition (43,84) on stress responsive systems.
Randomized controlled trials to improve these behaviors in chronically stressed individuals can help us understand the extent to which the biological damage from life stress is reversible or preventable. Ornish and colleagues (85) recently demonstrated that men with low-risk prostate cancer who adhered to recommended lifestyle changes consisting of diet, activity, stress management, and social connections had longer 5-year follow-up telomere length compared to those who did not adhere to recommendations for lifestyle change. Furthermore, across the control and intervention groups, those with greater health behaviors tended to show telomere lengthening over the five years. A next logical step is to examine lifestyle effects on cell aging in a randomized design targeting people living with high levels of chronic stress. There exists already evidence of success in combining these factors for the treatment of depression in a randomized trial setting (86). Knowledge of the impact of stressful life events across the lifespan on diseases of aging comes from an extensive literature on early life events and exposure to traumatic experiences in childhood (4) and with adults experiencing current chronic stressors, such as caregiving, unemployment, relationship conflict and bereavement (2,60,61,87,88).
Several key chromosomal genetic single nucleotide polymorphisms (SNPs) in genes regulating telomere length (i.e. rs10936599 in the TERC gene, rs2736100 in the TERT gene, rs7675998, in the NAF1 gene, etc.) place individuals at increasing risk for disease (21). Unknown, however, is the extent to which lifestyle interventions may succeed or fail in decelerating cell aging within the context of genetic polymorphisms in telomerase regulation genes. Examining the cellular benefits of a lifestyle intervention within the context of known vulnerability SNP precursors of disease and/or varied contexts of chronic stress is important to help determine for whom an exercise, sleep, and diet lifestyle intervention will most benefit.
Strengths of the study are the prospective measurement of lifestyle throughout the year, at 3 time points, and the ability to examine at short-term changes in telomere length over one year. The stability of telomere length (r = .74) suggests that telomere length tends to be stable over a short period, but is far from fixed, and many people showed changes. The current findings are perhaps limited to postmenopausal and non-smoking women. The women in the current study were also primarily Caucasian, highly educated, healthy (lower BMI, low medication use) and had lower levels of stress than national averages (89). The extent to which the effects of stress on telomere biology are reversible may depend on how long the stressor remains. For example, years of providing care significantly predicts shorter telomeres (29), thus possibly limiting the extent to which we can quickly reverse these effects with behavioral intervention. Lifespan studies that combine factors from early life and adulthood experiences together with health behaviors are needed to examine adequate doses for reversing the damage of accumulated stress across the lifespan. Finally, our behavioral measures were self-reported, allowing for reporting bias to influence our results. Future studies should utilize objective monitoring devices including accelerometers for objective activity and sleep measurement and daily food diaries utilizing computer-aided technologies to advance our understanding of the potential for healthy behaviors to directly impact aging or moderate the impact of stress on aging processes.
Currently, levels of general stress are reported at an all-time high in adult United States residents (90) and stressed adults are less likely to engage in healthy behaviors (65–68,91). It is increasingly imperative that health providers and policy makers seek to promote the engagement and maintenance of healthy behaviors to allay the destructive biological consequences of ever-increasingly stressful lives.
Acknowledgements
This study was supported by the Baumann Foundation and the Barney & Barbro Foundation. The first author is supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number R00 HL 109247. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.We gratefully acknowledgement the support of Alanie Lazaro, Aric Prather, Janet Tomiyama, and Wanda Truong.
Footnotes
Conflict of Interest
Drs. Jue Lin, Elissa Epel and Elizabeth Blackburn are co-founders of Telome Health Inc., a diagnostic company measuring telomere biology.
References
- 1.Centers for Disease Control and Prevention. The State of Aging and Health in America. Atlanta, GA: 2013. [Google Scholar]
- 2.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. J Am Med Assoc. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 4.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities building a new framework for health promotion and disease prevention. J Am Med Assoc. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BSStress, adaptation disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 6.Hänsel A, Hong S, Cámara RJA, von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35(1):115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campisi J, di Fagagna FD. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 11.Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21(6):354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calado RT, Young NS. Mechanisms of Disease: Telomere Diseases. N Engl J Med. 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, et al. Telomere Length and Risk of Incident Cancer and Cancer Mortality. JAMA J Am Med Assoc. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Gardner JP, Kimura M, Brimacombe M, Cao XJ, Srinivasan SR, et al. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis. 2009;205(2):620–625. doi: 10.1016/j.atherosclerosis.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Mainous AG, Codd V, Diaz VA, Schoepf UJ, Everett CJ, Player MS, et al. Leukocyte telomere length and coronary artery calcification. Atherosclerosis. 2010;210(1):262–267. doi: 10.1016/j.atherosclerosis.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 18.Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 19.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;475(7355) doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. doi: 10.1038/ng.2528. 427e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Gouin J-PP, Weng N-PP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood Adversity Heightens the Impact of Later-Life Caregiving Stress on Telomere Length and Inflammation. Psychosom Med. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JYY, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2012;17(7):719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68(6):e21–e22. doi: 10.1016/j.biopsych.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damjanovic AK, Yang YH, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood Adversity Heightens the Impact of Later-Life Caregiving Stress on Telomere Length and Inflammation. Psychosom Med. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, et al. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 2012 Feb 02; doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puterman E, Lin J, Blackburn EH, O’Donovan A, Adler NE, Epel ES. The Power of exercise: Buffering the effect of chronic stress on telomere length. PLoS One. 2010;5(5):e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere Shortening in Formerly Abused and Never Abused Women. Biol Res Nurs. 2011 doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel ES, Lin J, et al. Depression and Leukocyte Telomere Length in Patients With Coronary Heart Disease: Data From The Heart and Soul Study. Psychosom Med. 2011;73(7):541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BWJH. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. doi: 10.1038/mp.2013.151. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su YL, et al. Leukocyte Telomere Length in Major Depression: Correlations with Chronicity, Inflammation and Oxidative Stress - Preliminary Findings. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prather AA, Puterman E, Lin J, O’Donovan A, Krauss J, Tomiyama AJ, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011:721390. doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krauss J, Farzaneh-Far R, Puterman E, Na B, Lin J, Epel ES, et al. Physical fitness and telomere length in patients with coronary heart disease: Findings from the Heart and Soul Study. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0026983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 39.Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22(10):895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Shiels PG, McGlynn LM, MacIntyre A, Johnson PCD, Batty GD, Burns H, et al. Accelerated Telomere Attrition Is Associated with Relative Household Income, Diet and Inflammation in the pSoBid Cohort. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo S-S, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31(2):197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Schuit AJ, van Loon AJM, Tijhuis M, Ocké M. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002;35(3):219–224. doi: 10.1006/pmed.2002.1064. [DOI] [PubMed] [Google Scholar]
- 46.Poortinga W. The prevalence and clustering of four major lifestyle risk factors in an English adult population. Prev Med. 2007;44(2):124–128. doi: 10.1016/j.ypmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, et al. Healthy lifestyle and leukocyte telomere length in U.S. women. 2012:e38374. doi: 10.1371/journal.pone.0038374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puterman E, Epel ES. An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2012;6(11):807–825. doi: 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puterman E, Epel ES, Lin J, Blackburn EH, Gross JJ, Whooley MA, et al. Multisystem resiliency moderates the major depression-Telomere length association: Findings from the Heart and Soul Study. Brain Behav Immun. 2013;33:65–73. doi: 10.1016/j.bbi.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39(20):e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farzaneh-Far R, Lin J, Epel ES, Lapham K, Blackburn EH, Whooley MA. Telomere Length Trajectory and Its Determinants in Persons with Coronary Artery Disease: Longitudinal Findings from the Heart and Soul Study. PLoS One Public Library of Science. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor-Piliae RE, Norton LC, Haskell WL, Mahbouda MH, Fair JM, Iribarren C, et al. Validation of a new brief physical activity survey among men and women aged 60–69 years. Am J Epidemiol. 2006;164(6):598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 55.Kiernan M, Schoffman DE, Lee K, Brown SD, Fair JM, Perri MG, et al. The Stanford Leisure-Time Activity Categorical Item (L-Cat): a single categorical item sensitive to physical activity changes in overweight/obese women. Int J Obes. 2013 doi: 10.1038/ijo.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor-Piliae RE, Fair JM, Haskell WL, Varady AN, Iribarren C, Hlatky MA, et al. Validation of the Stanford Brief Activity Survey: examining psychological factors and physical activity levels in older adults. J Phys Act Health. 2010;7(1):87–94. doi: 10.1123/jpah.7.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J, Cohen P, West SG, Aiken LS, Mahwah USNJ. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- 60.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109(Suppl Supplement_2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGinnis JM, Williams-Russo P, Knickman JR. The Case For More Active Policy Attention To Health Promotion. Health Aff. 2002;21(2):78–93. doi: 10.1377/hlthaff.21.2.78. [DOI] [PubMed] [Google Scholar]
- 62.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 63.Seeman TE, Gruenewald T, Karlamangla A, Sidney S, Liu KA, McEwen B, et al. Modeling Multisystem Biological Risk in Young Adults: The Coronary Artery Risk Development in Young Adults Study. Am J Hum Biol. 2010;22(4):463–472. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports Med. 2013 doi: 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Kahn M, Sheppes G, Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol. 2013;89(2):218–228. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010;14(4):219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J Affect Disord. 2013;148(1):12–27. doi: 10.1016/j.jad.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 70.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 71.Traustadóttir T, Bosch PR, Cantu T, Matt KS. Hypothalamic-Pituitary-Adrenal Axis Response and Recovery from High-Intensity Exercise in Women: Effects of Aging and Fitness. J Clin Endocrinol Metab. 2004 Jul 1;89(7):3248–3254. doi: 10.1210/jc.2003-031713. [DOI] [PubMed] [Google Scholar]
- 72.Rimmele U, Zellweger BC, Marti B, Seiler R, Mohiyeddini C, Ehlert U, et al. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 2007;32(6):627–635. doi: 10.1016/j.psyneuen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34(2):190–198. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 74.Handschin C, Spiegelman BM. Nature. 7203. Vol. 454. Nature Publishing Group; 2008. The role of exercise and PGC1alpha in inflammation and chronic disease; pp. 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. Elsevier. 2012;15(3):405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol - Regul Integr Comp Physiol. 2004;286(3):R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 77.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci. 2011;108(10):4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, et al. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 79.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52(6):470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 80.Sahin E, Depinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13(6):397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470(7334):359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Andreazza AC, Andersen ML, Alvarenga TA, de-Oliveira MR, Armani F, Ruiz FS, et al. Impairment of the mitochondrial electron transport chain due to sleep deprivation in mice. J Psychiatr Res. 2010;44(12):775–780. doi: 10.1016/j.jpsychires.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 84.Kiecolt-Glaser JK. Stress, Food, and Inflammation: Psychoneuroimmunology and Nutrition at the Cutting Edge. Psychosom Med. 2010;72(4):365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 86.García-Toro M, Ibarra O, Gili M, Serrano MJ, Oliván B, Vicens E, et al. Four hygienic-dietary recommendations as add-on treatment in depression: a randomized-controlled trial. J Affect Disord. 2012;140(2):200–203. doi: 10.1016/j.jad.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 87.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 88.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 89.Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, Mendes WB. Wandering minds and aging cells. Clin Psychol Sci. 2012;1(1):75–83. [Google Scholar]
- 90.Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. J Appl Soc Psychol. 2012;42(6):1320–1334. [Google Scholar]
- 91.American Psychological Association. Stress in America: Our Health at Risk. 2012:1–78. [Google Scholar]


