Abstract
Objective
This study investigated whether arginase contributes to endothelial dysfunction and hypertension in obese rats.
Design and Methods
Endothelial function and arginase expression were examined in skeletal muscle arterioles from lean and obese Zucker rats (ZR). Arginase activity, arginine bioavailability, and blood pressure were measured in lean and obese animals.
Results
Arginase activity and expression was increased while global arginine bioavailability decreased in obese ZR. Acetylcholine or luminal flow caused dilation of isolated skeletal muscle arterioles but this was reduced or absent in vessels from obese ZR. Treatment of arterioles with a nitric oxide synthase inhibitor blocked dilation in lean arterioles and eliminated differences among lean and obese vessels. In contrast, arginase inhibitors or L-arginine enhanced vasodilation in obese ZR and abolished differences between lean and obese animals, while D-arginine had no effect. Finally, mean arterial blood pressure was significantly increased in obese ZR. However, administration of L-arginine or arginase inhibitors lowered blood pressure in obese, but not lean animals, and this was associated with an improvement in systemic arginine bioavailability.
Conclusions
Arginase promotes endothelial dysfunction and hypertension in obesity by reducing arginine bioavailability. Therapeutic approaches targeting arginase represent a promising approach in treating obesity-related vascular disease.
Keywords: obesity, arginase, arginine, hypertension, endothelial dysfunction
Introduction
Obesity is a major public health problem affecting more than 30% of the adult population in the United States.1 Obesity is the principal causative factor in the occurrence of insulin resistance and type 2 diabetes, and is an independent risk factor for the development of cardiovascular disease, including hypertension.2 It is estimated that at least 75% of the incidence of hypertension is related directly to obesity, and that the combination of obesity and hypertension is a major contributor to cardiovascular morbidity and mortality.3 While a number of pathogenic factors have been implicated in obesity-associated hypertension, endothelial dysfunction characterized by decreased bioavailability of nitric oxide (NO) and impaired endothelium-dependent vasodilation is a key component that links obesity to the rise in total peripheral resistance noted in obese hypertensive patients.4 Endothelial dysfunction, including blunted NO-dependent vasodilator responses, has been documented in obese subjects and animals.5 In this respect, the obese Zucker rat (ZR) is a well-established genetic model of obesity that has been extensively used to characterize vascular abnormalities associated with excessive weight gain.6 Due to a non-functional leptin receptor and consequent hyperphagia, homozygous (fa/fa) ZR develop characteristic features associated with human obesity, including hyperlipidemia, insulin resistance, and hypertension.6,7 Moreover, obese ZR exhibit pronounced impairment in NO-dependent arteriolar vasodilation that encompasses multiple vascular beds, including skeletal muscle.5,8,9
Although many factors can trigger endothelial dysfunction, recent work has identified arginase as a potent inducer of endothelial malfunction. Arginase is the central enzyme of the urea cycle that metabolizes arginine to ornithine and urea. Two distinct isoforms of arginase, arginase I and II, exist that display different tissue distribution and subcellular localization but similar enzymatic properties.10,11 Significantly, arginase can provoke endothelial dysfunction by competing with endothelial NO synthase (eNOS) for substrate, arginine, resulting in arginine deficiency and impaired NO synthesis.10–12 Interestingly, studies from our laboratory and others found that arginase contributes to endothelial dysfunction in various pathological states, including diabetes, atherosclerosis, aging, hemorrhagic shock, and hypertension.see 10–12
Despite intensive investigations, the mechanisms by which obesity promotes hypertension are not completely known. Moreover, while the importance of endothelial dysfunction in contributing to high blood pressure in obesity is well appreciated, no effective therapeutic strategies are currently available to prevent abnormalities of resistance arteries in obese individuals. Given the recent emergence of arginase as a key driver of arginine catabolism, the present study investigated whether arginase contributes to systemic arginine bioavailability, endothelial dysfunction, and hypertension in obese ZR.
Methods
Materials
Arginine, heparin, acetylcholine, and Nω-nitro-L-arginine methyl ester (L-NAME) were from Sigma (St. Louis, MO); Nω-hydroxy-nor-L-arginine (OHNA) and S-(2-boronoethyl)-L-cysteine (BEC) were purchased from EMD Biosciences (San Diego, CA); L-[guanido-14C]arginine (52 mCi/mmol) was from Amersham (Arlington Heights, IL). All other reagents were from Fisher Scientific (Houston, TX).
Animals
Male obese (fa/fa) and lean (Fa/?) ZR were obtained from Harlan (Indianapolis, IN) and used for experiments between 12–14 weeks of age. Animals were housed under controlled temperature and humidity, with ad libitum access to tap water and standard rodent chow (Harlan Teklad, Madison, WI). All experimental procedures were approved by the institutional animal care and use committee and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Blood Chemistry and Tissue Collection
Rats were weighed, anesthetized with isoflurane, and femoral arterial catheters implanted for blood collection and determination of blood glucose (Accu-Check Compact, Roche Diagnostics, Indianapolis, IN) and lipid profile (Cardio-Check PA analyzer, QAS, Orlando, FL). Additional blood was collected in tubes containing EDTA and plasma obtained by centrifugation. Plasma levels of specific proteins were determined using commercial ELISA kits for insulin (Cayman Chemical, Ann Arbor, MI), tumor necrosis factor-α (TNFα) (Thermo Scientific, Waltham, MA), and oxidized low-density lipoprotein (oxLDL) (Mercodia, Winston Salem, NC). Circulating levels of amino acids were quantified by ion exchange chromatography (Molecular Genetics Laboratory at Baylor College of Medicine, Houston, TX). Global arginine bioavailability, which is a more sensitive indicator of disturbances in arginine metabolism than levels of individual amino acids, was calculated as the ratio of plasma arginine to the sum of plasma ornithine plus citrulline.13,14 Following blood collection, animals were heparinized (1000U/kg, iv) and the thoracic aorta and gracilis anticus muscles removed.
Arginase Assay
Vascular arginase activity was determined by measuring the formation of [14C]urea from L-[guanido-14C]arginine, as we previously described.15 For plasma arginase measurement, plasma samples were depleted of urea using Centricon YM10 filters (Amicon Inc, Beverly, MA) and subsequent urea formation from exogenously applied arginine determined by absorption spectroscopy, using a commercially available kit (QuantiChrom™ Arginase Assay Kit, BioAssay Systems, Hayward, CA).
Arginase and eNOS expression
Arginase expression was monitored by quantitative real-time PCR. Total RNA was extracted using TRIzol reagent and reverse transcribed to cDNA using iScript cDNA synthesis kits (Bio-Rad, Hercules, CA). Quantitative real-time PCR was carried out using SYBR Green Supermix (Bio-Rad, Hercules, CA) in a SYBR Green Cylcer iQ™ 5 RT-PCR detection system as previously described.15,16 Transcript levels were quantified using the ΔΔCT method using 18 S rRNA as a control, and presented as fold increase over tissues from non-obese animals.
Arteriolar Endothelial Function
First-order gracilis arterioles were isolated from the hindlimb of rats and cannulated at both ends with glass micropipets in a vessel chamber (Living Systems Instrumentation, Burlington, VT). The chamber was continuously perfused with Krebs buffer (in mM; 118.5 NaCl, 4.7 KCl, 1.4 CaCl2, 1.2 KH2PO4, 1.1 MgSO4, 25.0 NaHCO3, and 11.1 dextrose) gassed with 14% O2 and 5% CO2, balanced with N2, in a non-circulating system. For internal diameter measurements, the vessel chamber was mounted on an inverted microscope fitted with a video camera that was connected to a computer equipped with video dimensioning software (ImagePro Express, Media Cybernetics).
For acetylcholine experiments, the proximal micropipette was connected to a pressure servo-controller and the distal micropipette to a closed stopcock to achieve and maintain 80 mmHg constant luminal pressure with no flow. Following a one hour stabilization period, drugs were added to the superfusion buffer 20 minutes prior to the administration of acetylcholine. Increasing concentrations of acetylcholine were added to the superfusion buffer and internal diameter recorded for each concentration of acetylcholine. For flow experiments, both the proximal and distal micropipettes were connected to pressure servo controllers and an inline microflowmeter (Living Systems Instrumentation). Drugs were included in the luminal buffer. Proximal and distal pressures were adjusted equally in opposite directions to maintain midline pressure at 80 mmHg and establish graded levels of luminal flow.
Blood Pressure Measurements in Awake Animals
Animals were anesthetized with isoflurane and fitted with femoral arterial catheters.9 Following a three-day recovery interval, inline blood pressure was measured daily using a pressure transducer coupled to a polygraph system (Biopac Systems, Santa Clara, CA). Animals received a continuous infusion of the arginase inhibitor BEC (55.6μg/hour, ip) or isotonic saline via osmotic pumps (ALZA Corporation, Palo Alto, CA) for 6 days. Alternatively, other animals were treated with OHNA (50 mg/kg, ip) or received L-arginine (10 g/L) in the drinking water for 6 days.
Data Analysis
Results are expressed as the means ± SEM. Statistical differences between groups were evaluated with a Student’s two-tailed t-test or by ANOVA with the Tukey post hoc test when more than two treatment regimens were compared. A value of P < 0.05 was considered statistically significant.
Results
Baseline body weight and metabolic parameters of lean and obese ZR are summarized in Table 1. Body weight and circulating levels of cholesterol, triglycerides, glucose, insulin, TNFα, and oxLDL were significantly increased in obese ZR relative to lean animals. Vascular arginase activity was also elevated in obese ZR and this was paralleled by a significant increase in the expression of both arginase I and II mRNA in gracilis muscle arterioles without any change in eNOS expression (Figures 1A and B). A greater than three-fold increase in plasma arginase activity was also detected in obese ZR (Figure 1C).
Table 1.
Characteristic features of lean and obese Zucker rats.
| Lean ZR | Obese ZR | |
|---|---|---|
| Body weight (g) | 344±8 | 522±7* |
| Glucose (mg/dL) | 131±7 | 159±10* |
| Total cholesterol | 69±4 | 96±7* |
| Triglycerides (mg/dL) | 33±5 | 467±54* |
| Insulin (ng/ml) | 1.9±0.3 | 22.8±4.5* |
| TNFα (pg/mL) | 88±7 | 177±21* |
| OxLDL (U/L) | 3.0±0.3 | 7.9±0.5* |
Values are expressed as the mean±SEM (n=6–12). ZR, Zucker rats; TNFα, tumor necrosis factor-α; OxLDL, oxidized low-density lipoprotein.
Statistically significant effect of obesity.
Figure 1.
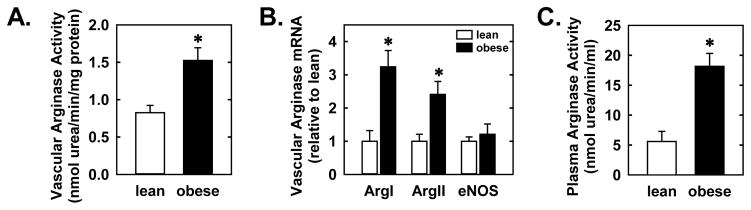
Obesity stimulates vascular arginase activity and expression, and plasma arginase activity. Obesity increases arginase activity in the aorta (A), arginase (Arg) I and II, but not endothelial nitric oxide synthase (eNOS), mRNA expression in gracilis muscle arterioles (B), and arginase activity in the plasma (C). Results are means ± SEM (n=4–5). *Statistically significant effect of obesity.
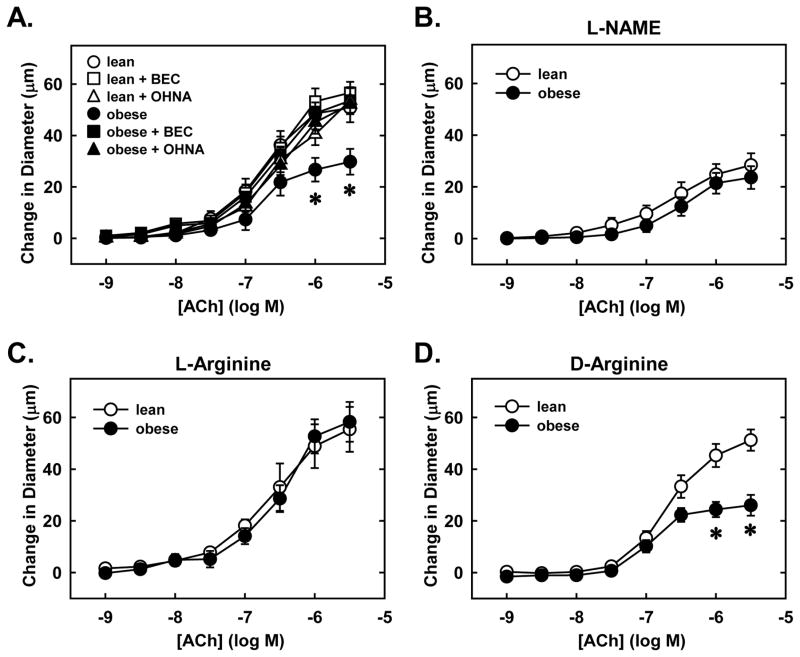
Endothelial function was severely compromised in obese animals. Administration of the endothelium-dependent vasodilator acetylcholine stimulated a concentration-dependent increase in the diameter of skeletal muscle arterioles isolated from lean ZR (Figure 2A). Acetylcholine also induced a dose-dependent dilation in arterioles from obese ZR but the increase in vessel wall diameter was significantly attenuated compared to arterioles isolated from lean animals. Inhibition of NOS with L-NAME markedly decreased acetylcholine-mediated vasodilation in lean animals while it minimally altered the response in obese animals (Figure 2B). Furthermore, L-NAME eliminated the difference in acetylcholine-mediated dilation between lean and obese arterioles. Since arginase expression is elevated in gracilis muscle arterioles of obese animals, we determined whether arginase was responsible for impairing endothelial function in these animals. Pretreatment of blood vessels with the arginase inhibitors, BEC or OHNA, enhanced the response of obese arterioles to acetylcholine and abolished the difference between obese and lean vessels (Figure 2A). Similarly, addition of the arginase and eNOS substrate, L-arginine, to the superfusion buffer abrogated differences in acetylcholine-mediated vasodilation between lean and obese rats (Figure 2C). In contrast, D-arginine, which is not a substrate for either enzyme, failed to normalize acetylcholine-mediated responses between the two groups of animals (Figure 2D).
Figure 2.
Obesity-induced impairment in acetylcholine (Ach)-mediated vasodilation is corrected by arginase inhibition or L-arginine administration. Ach-mediated increases in luminal diameter of gracilis muscle arterioles are reduced in obese relative to lean Zucker rats (ZR) and this is reversed by S-(2-Boronoethyl)-L-cysteine (BEC; 100μM) or Nω-hydroxy-nor-L-arginine (OHNA; 100μM) (A). Nω-nitro-L-arginine methyl ester (L-NAME; 1 mM) attenuates Ach-mediated vasodilation (B). L-Arginine (1 mM) (C), but not D-arginine (1 mM) (D) restores Ach-mediated vasodilation in obese ZR. Results are means ± SEM (n=4–6). *Statistically significant effect of obesity.
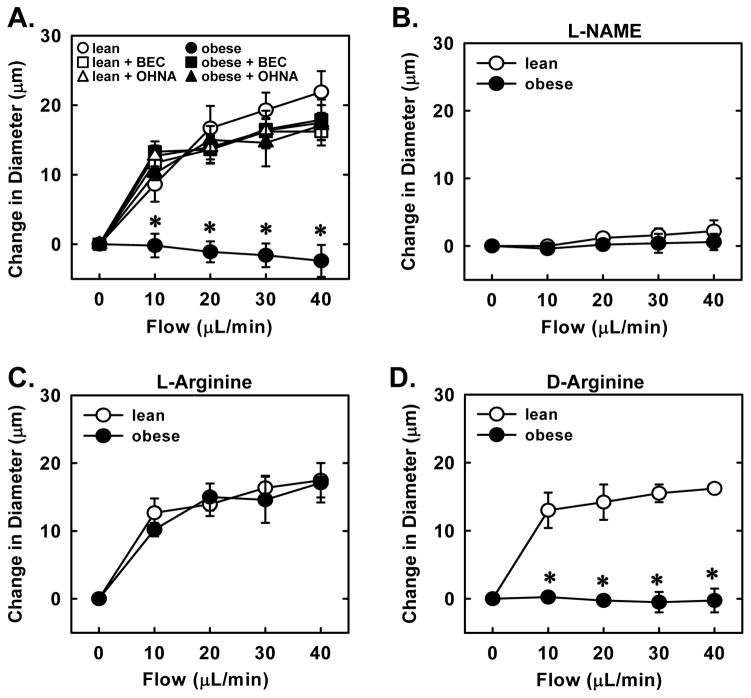
Endothelial function was also examined in response to a receptor-independent stimulus, fluid flow. Step-wise increases in luminal flow resulted in dilation of arterioles isolated from lean ZR but not in arterioles from obese ZR (Figure 3A). Pretreatment of vessels with L-NAME prevented flow-mediated vasodilation in arterioles isolated from lean animals demonstrating that the dilator response to flow is strictly dependent on NOS activity (Figure 3B). In addition, pretreatment of arterioles with the arginase inhibitors, BEC or OHNA, restored flow-induced dilation in obese arterioles and abolished the difference between lean and obese vessels (Figure 3A). Furthermore, acute in vitro pretreatment of blood vessels with L-arginine reestablished flow-induced dilation in obese arterioles and nullified the difference in vasodilation between the two groups of animals (Figure 3C). However, D-arginine failed to restore flow-induced dilation in obese arterioles (Figure 3D).
Figure 3.
Obesity-induced impairment in flow-mediated vasodilation is corrected by arginase inhibition or L-arginine administration. Flow-mediated increases in luminal diameter of gracilis muscle arterioles are reduced in obese relative to lean Zucker rats (ZR) and this is reversed by S-(2-boronoethyl)-L-cysteine (BEC;100μM) or Nω-hydroxy-nor-L-arginine (OHNA;100μM) (A). Nω-nitro-L-arginine methyl ester (L-NAME; 1 mM) blocks flow-mediated vasodilation (B). L-arginine (1 mM) (C), but not D-arginine (1 mM) (D) restores flow-mediated vasodilation in obese ZR. Results are means ± SEM (n=4–6). *Statistically significant effect of obesity.
Since plasma arginase activity was elevated in obese ZR, we examined whether arginine bioavailability was altered in these animals. Indeed, there was a striking 30% decline in plasma arginine concentration and a corresponding rise in the arginase product, ornithine (Figure 4A). Significant increases in citrulline, the branched chain amino acids, (leucine, isoleucine, and valine), and the aromatic amino acids phenylalanine and tyrosine were also detected in the plasma of obese rats. However, the plasma concentration of other essential amino acids (histidine, lysine, methionine, and threonine) was unchanged in obese animals. Furthermore, global arginine bioavailability was reduced by approximately 50% in obese ZR (Figure 4B). We attempted to normalize systemic arginine bioavailability in obese ZR by administering L-arginine in the drinking water. Following 6 days of L-arginine supplementation, global arginine bioavailability was increased in obese and lean ZR but levels in obese rats remained significantly lower than that observed in lean animals (Figures 4B). Similarly, chronic delivery of the arginase inhibitor BEC for 6 days elevated global arginine bioavailability in both obese and lean animals; however, bioavailability remained significantly lower in obese rats (Figure 4B).
Figure 4.
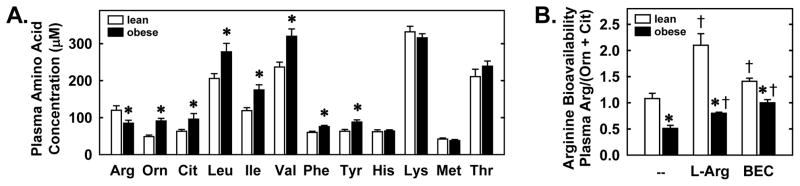
Obesity inhibits plasma arginine concentration and global arginine bioavailability. Plasma amino acid concentrations in lean and obese Zukcer rats (ZR) (A). Global arginine bioavailability is improved by dietary L-arginine supplementation (L-Arg; 1% in drinking water for 6 days) or arginase inhibition (intraperitoneal administration of S-(2-boronoethyl)-L-cysteine (BEC; 55.6μg/h for 6 days) (B). Results ± SEM (n=5–8). *Statistically significant effect of obesity. †Statistically significant effect of L-arginine or BEC. Arg, arginine; Orn, ornithine; Cit, citrulline; Leu, leucine, Ile, isoleucine; Val, valine; Phe, phenylalanine; Tyr, tyrosine; His, histidine; Lys, lysine; Met, methionine; Thr, threonine.
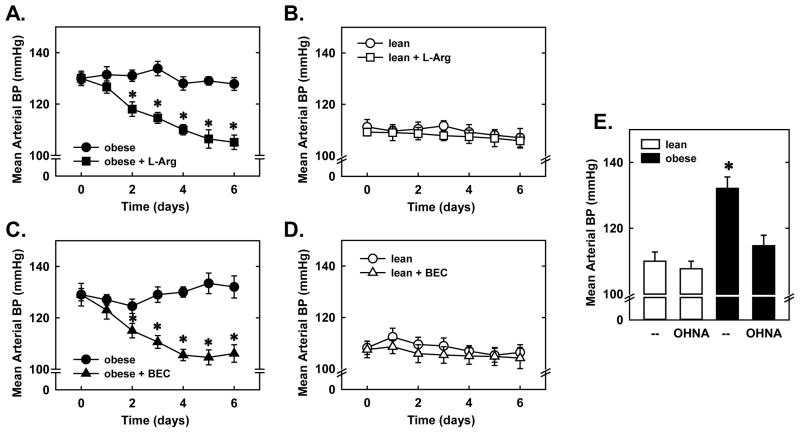
Notably, administration of L-arginine or BEC normalized blood pressure in obese animals. Mean arterial blood pressure was significantly elevated in obese ZR and L-arginine or BEC evoked a significant drop in blood pressure after two days of treatment and this decrease in blood pressure was sustained for 6 days (Figures 5A and C). Likewise, treatment of obese ZR with OHNA for 6 days reduced blood pressure in these animals (Figure 5E). In contrast, L-arginine, BEC, or OHNA had no effect on arterial blood pressure in normotensive lean ZR (Figures 5B, D and E). Furthermore, treatment of obese or lean ZR with L-arginine or BEC had no significant effect on animal weight and circulating levels of cholesterol, glucose, triglyceride, and insulin (Table 2).
Figure 5.
Obesity-induced hypertension is reversed by arginase inhibition or L-arginine administration. Mean arterial blood pressure (BP) is reduced in obese, but not lean, Zucker rats (ZR) by dietary L-arginine supplementation (L-Arg; 1% in drinking water for 6 days) (A and B) or arginase inhibition (intraperitoneal administration of S-(2-boronoethyl)-L-cysteine (BEC;55.6μg/h for 6 days) (C and D). Nω-hydroxy-nor-L-arginine (OHNA;50 mg/kg, ip, daily for 6 days) reduces mean arterial BP in obese, but not lean, ZR (E). Results are means ± SEM (n=5–6). *Statistically significant effect of obesity.
Table 2.
Metabolic parameters in obese and lean Zucker rats (ZR) following administration of L-arginine or S-(2-boronoethyl)-L-cysteine (BEC).
| H2O (drinking water) | L-Arginine (drinking water) | Saline (ip) | BEC (ip) | |
|---|---|---|---|---|
| Lean ZR | ||||
| Body weight (g) | 368±11 | 355±10 | 372±13 | 366±16 |
| Glucose (mg/dL) | 124±8 | 118±7 | 120±6 | 116±12 |
| Total cholesterol (mg/dL) | 75±7 | 78±5 | 72±6 | 71±9 |
| Triglycerides (mg/dL) | 29±6 | 33±7 | 34±8 | 32±5 |
| Insulin (ng/ml) | 1.5±0.5 | 2.2±0.4 | 2.4±0.8 | 1.8±0.6 |
| Obese ZR | ||||
| Body weight (g) | 548±7* | 535±16* | 552±10* | 544±13* |
| Glucose (mg/dL) | 149±6* | 159±9* | 153±12* | 156±7* |
| Total cholesterol (mg/dL) | 95±7* | 102±8* | 98±7* | 90±10* |
| Triglycerides (mg/dL) | 455±21* | 488±35* | 495±33* | 436±56* |
| Insulin (ng/ml) | 19.2±4.1* | 22.3±5.4* | 18.4±4.4* | 14.7±5.9* |
Dietary L-arginine (1% in drinking water for 6 days) or intraperitoneal BEC administration (55.6μg/h for 6 days). Values are expressed as the mean±SEM (n=5–8).
Statistically significant effect of obesity.
Discussion
The present study demonstrates that arginase plays a fundamental role in promoting endothelial dysfunction and hypertension in obesity. We found that arginase activity is increased in blood vessels and plasma of obese ZR and this is associated with a significant decline in plasma arginine and global arginine bioavailability. We also discovered that skeletal muscle arteriolar endothelial function is compromised in obese ZR and that acute pretreatment of isolated blood vessels with L-arginine or arginase inhibitors restore endothelium-dependent vasodilator responses in obese animals. Moreover, we demonstrated that chronic administration of L-arginine or an arginase inhibitor improves systemic arginine bioavailability and normalizes blood pressure in hypertensive obese ZR. These findings indicate that arginase contributes to endothelial dysfunction and hypertension in obese ZR by restricting arginine availability.
The obese ZR is a well established genetic model of obesity that exhibits many of the metabolic abnormalities found in overweight humans. We found that obese ZR have elevated levels of circulating insulin, cholesterol, triglycerides, and glucose. Furthermore, we show that arginase activity is elevated in obese ZR. A significant rise in arginase activity is observed in blood vessels from obese ZR and this is associated with increases in vascular arginase I and II expression independent of any alteration in eNOS expression, indicating that obesity can differentially affect the expression of L-arginine metabolizing enzymes. The underlying mechanism responsible for the induction of arginase in the vessel wall of obese animals is not known. While hyperglycemia can induce arginase expression, the modest elevation in blood glucose observed in obese ZR is unlikely to elevate arginase activity.17 Instead, increases in circulating TNFα and oxLDL that are detected in obese rats may contribute to the induction of arginase activity since both factors are known to stimulate arginase expression.12 In addition, elevations in plasma insulin may contribute to the rise in vascular arginase activity in obese ZR since insulin is able to stimulate arginase expression in endothelial cells.18
We also found that plasma arginase activity, which arises from arginase I,19 is markedly elevated in obese ZR. Since arginase I is most abundantly expressed in the liver and because hepatic damage is present in obese ZR,20 the release of arginase by hepatic cells may contribute to the increase in plasma arginase in obese rodents. Consistent with this notion, serum arginase I is a highly sensitive marker for liver injury.21 Furthermore, as arginase I is induced in monocytes of overweight adult subjects,22 these cells may also contribute to the rise in plasma arginase activity. Our finding that plasma arginase activity is up-regulated in a genetic animal model of obesity is in agreement with studies in mice showing that a high fat-high cholesterol diet increases plasma arginase and with recent clinical reports demonstrating that circulating arginase I is elevated in obese subjects.22–24 Interestingly, serum arginase I correlates with body mass index and other phenotypic biomarkers of obesity, and may serve as an early predictor for the development of diabetes mellitus.23,25
We also observed marked disturbances in the concentration of amino acids in the plasma of obese ZR. Most striking, a prominent decrease in plasma arginine and global arginine bioavailability is detected in genetically obese rats. A decrease in arginine bioavailability has also been noted in diet-induced obese mice,26,27 suggesting the presence of a generalized arginine deficiency state in obesity. The finding that systemic arginine bioavailability is diminished in obesity may be of clinical significance since reductions in arginine availability are associated with increases in cardiovascular disease and mortality.13,14 We also show that the decrease in plasma arginine in obese rats is mediated by increases in arginase activity since it is accompanied by elevations in the arginase product ornithine and improved following arginase inhibition. Consistent with previous reports in obese humans and rodents,26,28–30 an increase in the concentration of branched chain amino acids is observed in the plasma of obese ZR, probably due to reductions in the activity of enzymes involved in branched chain amino acid metabolism.27 In addition, the rise in circulating citrulline in obese animals may reflect a larger flux of arginine through the hepatic urea cycle, increased intestinal synthesis, and/or reduced renal metabolism.26 The rise in plasma phenylalanine and tyrosine noted in our study is consistent with previous reports in obese humans and may reflect alterations in liver function.29,31
We also demonstrate that arginase plays an integral role in promoting endothelial dysfunction in obesity. As with previous reports,8,9 we found that obese ZR exhibit pronounced endothelial dysfunction. Gracilis muscle arterioles isolated from obese animals display attenuated endothelium-dependent dilator responses to acetylcholine and fail to dilate in response to flow. Interestingly, the endothelial dysfunction observed in obese vessels is likely due to reduced production of NO since the NOS inhibitor L-NAME selectively impairs the vasodilatory response of lean vessels and is able to eliminate differences in both acetylcholine- and flow-mediated vasodilation among lean and obese animals. Significantly, acute pretreatment of isolated arterioles with arginase inhibitors, BEC or OHNA, restores flow-mediated dilation in obese arterioles and abolishes differences in endothelial responses to acetylcholine and flow between obese and lean vessels, suggesting that elevated vascular arginase activity contributes to endothelial dysfunction in obese arterioles by restricting NO synthesis. Consistent with a role for arginase in depleting substrate for eNOS, we found that acute exogenous administration of L-arginine to blood vessels mimics the action of BEC or OHNA and normalizes acetylcholine- and flow-induced dilation in arterioles from obese animals. In contrast, the inactive isomer, D-arginine, fails to restore arteriolar dilation in obese vessels. Our findings in genetically obese rats are consistent with a previous study showing that L-arginine administration improves endothelium-dependent relaxation in dietary-induced obese hamsters,32 further implicating an arginase-mediated functional arginine insufficiency in obesity.
We also show for the first time that arginase contributes to the development of hypertension in obesity. Mean arterial blood pressure is elevated in obese ZR but chronic administration of BEC or OHNA lowers blood pressure in these animals without affecting blood pressure in lean normotensive rats, suggesting that increases in arginase activity contributes to the development of hypertension in obesity. Interestingly, arginase has been implicated in mediating hypertension in diabetic and fructose-fed insulin-resistant rodents, suggesting a critical role for this enzyme in promoting metabolic stress-induced hypertension.33,34 In addition, we found that chronic administration of L-arginine normalizes blood pressure in obese ZR and this is associated with an increase in arginine bioavailability. In contrast, L-arginine supplementation fails to lower blood pressure in lean ZR despite a significant increase in global L-arginine bioavailability. The selective blood pressure lowering effect in response to L-arginine administration or arginase inhibition is consistent with the notion that eNOS-mediated NO production is substrate-limited by arginase in obese but not lean ZR. Limitations in substrate availability in obesity may also contribute to the preferential vasodepressor action of acute L-arginine infusions noted in obese hypertensive relative to lean normotensive subjects.35
Restoration of arginine bioavailability following arginase inhibition may improve vascular function in obesity via multiple mechanisms. Since arginine is required for functional coupling of eNOS, increases in arginine bioavailability may protect against the uncoupling of the enzyme and better defend against the endogenous eNOS inhibitor, asymmetric dimethylarginine, which is increased in obesity.36 Furthermore, by augmenting NO synthesis, elevations in arginine may counteract the enhanced release of vasoconstrictors and diminished production of vasodilators seen in obesity. The NO-mediated vasodilatory action of arginine may be further amplified by its ability to suppress circulating levels of angiotensin II via the inhibition of angiotensin-converting enzyme.37 Moreover, as arginine can directly scavenge and inhibit superoxide formation, rises in arginine concentration may limit the degree of oxidative stress in obesity.37
Finally, the antihypertensive effect observed with BEC, OHNA, or L-arginine in obese ZR is not secondary to improvements in body weight or circulating levels of glucose, cholesterol, triglycerides, or insulin. Inability of arginase inhibition to modify blood lipids is consistent with previous work in atherogenic animals.38 The lack of improvement in the metabolic profile of obese rats may also arise from the short duration of treatment. Indeed, longer duration of L-arginine supplementation has been demonstrated to improve body weight and insulin sensitivity in obese rodents and humans 39,40 while sustained arginase inhibition for 6 weeks ameliorates insulin-resistance in fructose-fed rats.34
In conclusion, the present study identifies arginase as a critical mediator of skeletal muscle arteriolar endothelial dysfunction and hypertension in obesity. We found that vascular and plasma arginase activity is increased in obese ZR and this is associated with impairments in systemic arginine bioavailability and endothelial function. Importantly, pretreatment of blood vessels with L-arginine or arginase inhibitors restores endothelium-mediated vasodilation in arterioles from obese animals. Moreover, we discovered that chronic administration of L-arginine or an arginase inhibitor improves global arginine bioavailability and normalizes blood pressure in obese ZR. These results provide novel insight regarding the role of arginase in obesity, and establish arginase as a potential therapeutic target in treating obesity-related alterations in arginine bioavailability and vascular disease.
What is already known about this subject?
Obesity is an independent risk factor for the development of cardiovascular disease, including hypertension.
Obesity is associated with impaired endothelial cell function.
Arginase can trigger endothelial cell dysfunction and hypertension by limiting arginine availability.
What does this study add?
This study found that arginase activity is increased in blood vessels and plasma of obese animals and this causes a significant decline in arginine bioavailability.
This study demonstrated that correction of the arginine deficit via the use of arginase inhibitors or dietary supplementation of arginine restores endothelial function and blood pressure in obesity.
This study identifies arginase as a promising therapeutic target in treating obesity-related hypertension.
Acknowledgments
FKJ, RAJ, and WD designed the study. FKJ, KJP, XL, ARS, MAA, and RAJ carried out experiments. FKJ, KJP, and WD analyzed the data and interpreted the results. FKJ, RAJ, and WD wrote the manuscript. The authors have no competing interests and acknowledge the support of grants from the National Institutes of Health National Heart, Lung, and Blood Institute (Grants R01-HL74966 and R01-HL59976), and the American Heart Association MidWest (855715G) and South Central (BGIA 0865241B) Affiliates.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Heart Association. Overweight and obesity statistics-2009 Update. 2009. [Google Scholar]
- 4.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment- a position paper of the Obesity Society and the American Society of Hypertension. Obesity. 2013;21:8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]
- 5.Stepp DW. Impact of obesity and insulin resistance on vasomotor tone: nitric oxide and beyond. Clin Exp Pharmacol Physiol. 2006;33:407–414. doi: 10.1111/j.1440-1681.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 6.Zucker LM. Hereditary obesity in the rat associated with hyperlipidemia. Ann NY Acad Sci. 1965;131:447–458. doi: 10.1111/j.1749-6632.1965.tb34810.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, O’Donnel MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension. 1992;19:110–115. doi: 10.1161/01.hyp.19.1_suppl.i110. [DOI] [PubMed] [Google Scholar]
- 8.Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2001;281:H1304–H1311. doi: 10.1152/ajpheart.2001.281.3.H1304. [DOI] [PubMed] [Google Scholar]
- 9.Johnson FK, Johnson RA, Durante W, Jackson KE, Stevenson BK, Peyton KJ. Metabolic syndrome increases endogenous carbon monoxide production to promote endothelial dysfunction in obese Zucker rats. Am J Physiol Reg Integr Comp Physiol. 2006;290:R601–R608. doi: 10.1152/ajpregu.00308.2005. [DOI] [PubMed] [Google Scholar]
- 10.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 12.Durante W. Role of arginase in vessel wall remodeling. Front Immunol. 2013;4:111. doi: 10.3389/fimmu.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–2067. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson FK, Johnson RA, Peyton KJ, Shebib AR, Durante W. Arginase promotes skeletal muscle arteriolar endothelial dysfunction in diabetic rats. Front Immunol. 2013;4:119. doi: 10.3389/fimmu.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulton D, Marris MB, Kemp BE, Venema RC, Marrero MB, Stepp DW. Insulin resistance does not diminish eNOS expression, phosphorylation, or binding to HSP-90. Am J Physiol Heart Circ Physiol. 2004;287:H2384–2393. doi: 10.1152/ajpheart.00280.2004. [DOI] [PubMed] [Google Scholar]
- 17.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giri H, Muthuramu I, Dhar M, Rathnakumar K, Ram U, Dixit M. Protein tyrosine phosphatase SHP2 mediates chronic insulin-induced endothelial inflammation. Arterioscler Thromb Vasc Biol. 2012;32:1943–1950. doi: 10.1161/ATVBAHA.111.239251. [DOI] [PubMed] [Google Scholar]
- 19.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137(Suppl 2):1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 20.Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation. 2001;72:1625–1631. doi: 10.1097/00007890-200111270-00008. [DOI] [PubMed] [Google Scholar]
- 21.Ikemoto M, Tsunekawa S, Toda Y, Totani M. Liver-type arginase is a highly sensitive marker for hepatocellular damage in rats. Clin Chem. 2001;47:946–948. [PubMed] [Google Scholar]
- 22.Erdely A, Kepka-Lenhart D, Salmen-Muniz R, Chapman R, Hulderman T, Kashen M, Simeonova PP, Morris JM., Jr Arginase activities and global arginine bioavailability in wild-type and ApoE-deficient mice: responses to high fat and high cholesterol diets. PLoS One. 2010;5:e15253. doi: 10.1371/journal.pone.0015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim OY, Lee SM, Chung JH, Do HJ, Moon J, Shin MJ. Arginase I and the very low-density lipoprotein receptor are associated with phenotypic biomarkers for obesity. Nutrition. 2012;28:635–639. doi: 10.1016/j.nut.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Jung C, Figulla HR, Lichtenauer M, Franz M, Pernow J. Increased levels of circulating arginase I in overweight compared to normal weight adolescents. Pediatr Diabetes. 2014;15:51–56. doi: 10.1111/pedi.12054. [DOI] [PubMed] [Google Scholar]
- 25.Ogino K, Takahashi N, Takigawa T, Obase Y, Wang D-H. Association of serum arginase I with oxidative stress in a healthy population. Free Radic Res. 2011;42:147–155. doi: 10.3109/10715762.2010.520318. [DOI] [PubMed] [Google Scholar]
- 26.Sailer M, Dahlhoff C, Giesbertz P, Eidens MK, de Wit N, Rubio-Aliaga I, et al. Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS One. 2013;8:e63950. doi: 10.1371/journal.pone.0063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.She P, Van Horne C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT. Amino acid metabolism in the Zucker diabetic fatty rat: effect of insulin resistance and type 2 diabetes. Can J Physiol Pharmacol. 2004;82:506–514. doi: 10.1139/y04-067. [DOI] [PubMed] [Google Scholar]
- 29.Caballero B, Finer N, Wurtman RJ. Plasma amino acids and insulin levels in obesity: response to carbohydrate intake and dietary supplements. Metabolism. 1988;37:672–676. doi: 10.1016/0026-0495(88)90089-3. [DOI] [PubMed] [Google Scholar]
- 30.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched chain amino acid related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felig P, Marliss E, Cahill GF. Plasma amino acid levels and insulin secretion secretion in humans. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 32.Popov D, Costache G, Georgescu A, Enache M. Beneficial effects of L-arginine supplementation in experimental-hyperglycemia in the hamster. Cell Tissue Res. 2002;308:109–120. doi: 10.1007/s00441-001-0509-4. [DOI] [PubMed] [Google Scholar]
- 33.El Bassossy H, El-Fawal R, Fahmy A. Arginase inhibition alleviates hypertension associated with diabetes: effect on endothelial dependent relaxation and NO production. Vascul Pharmacol. 2012;57:194–200. doi: 10.1016/j.vph.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 34.El-Basossy HM, El-Fawal R, Fahmy A, Watson ML. Arginase inhibition alleviates hypertension in the metabolic syndrome. Br J Pharmacol. 2013;169:693–703. doi: 10.1111/bph.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castejon AM, Hoffmann IS, Jimenez E, Cubeddu RJ, Baldonedo RM, Cubeddu LX. Differential blood pressure effects of oral glucose and intravenous L-arginine in healthy lean normotensive and obese hypertensive subjects. J Hum Hypertens. 2002;16:S133–S136. doi: 10.1038/sj.jhh.1001359. [DOI] [PubMed] [Google Scholar]
- 36.Marliss EB, Chevalier S, Gougeon R, Morais JA, Lamarche M, Adegoke OA, Wu G. Elevations in plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006;49:351–359. doi: 10.1007/s00125-005-0066-6. [DOI] [PubMed] [Google Scholar]
- 37.Wu G, Meininger CJ. Arginine nutrition and cardiovascular function. J Nutr. 2000;130:2626–2629. doi: 10.1093/jn/130.11.2626. [DOI] [PubMed] [Google Scholar]
- 38.Rhoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 39.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, et al. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–721. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 40.Bogdanski P, Suliburska J, Grabanska K, Musialik K, Cielslewicz A, Skoluda A, et al. Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur Rev Med Pharmacol Sci. 2012;16:816–823. [PubMed] [Google Scholar]