Abstract
Magnetic sorting using magnetic beads has become a routine methodology for the separation of key cell populations from biological suspensions. Due to the inherent ability of magnets to provide forces at a distance, magnetic cell manipulation is now a standardized process step in numerous processes in tissue engineering, medicine, and in fundamental biological research. Herein we review the current status of magnetic particles to enable isolation and separation of cells, with a strong focus on the fundamental governing physical phenomena, properties and syntheses of magnetic particles and on current applications of magnet-based cell separation in laboratory and clinical settings. We highlight the contribution of cell separation to biomedical research and medicine and detail modern cell separation methods (both magnetic and non-magnetic). In addition to a review of the current state-of-the-art in magnet-based cell sorting, we discuss current challenges and available opportunities for further research, development and commercialization of magnetic particle-based cell separation systems.
Keywords: biomedical, magnetic, cells, separation
1. Introduction: Cell Separation Context and Motivation
The separation and sorting of biological cells is critical to a variety of biomedical applications including diagnostics, therapeutics, and fundamental cell biology. As samples of interest are often heterogeneous populations of cells that are in culture or that comprise a tissue, techniques to isolate specific cells are essential for understanding how cells function and respond to various stimuli. Blood, for example, is an extremely information-rich and easily accessible tissue that is a complex blend of cells; accurate analysis of blood character and condition requires isolation of a few desired cells. Effective cell sorting to support numerous biomedical pursuits relies upon optimal matching between the target cell attributes, desired outcomes, and the parameters of the sorting technique. Numerous cell isolation and sorting techniques have been developed for benchtop and clinical settings that are based on either physical properties of the cell, such as density or size, or on cell affinity that describes electric, magnetic or adhesive properties specific to each cell type. Standard techniques for the separation of cells include processing steps of filtration, centrifugation and sedimentation, which are carried out either in a batch or in a continuous manner and can be easily translated to large-scale operation. However, in situations where cell size or density differences are not significant, effective cell separation is impeded in these techniques and other methods must be employed, including fluorescence activated cell sorting (FACS) and magnetic activated cell sorting (MACS). In this context, magnetic particles —nanoparticles (mean diameter 10 – 100 nm), sub-micron particles (0.1 – 1 microns), and microparticles (mean diameter 1 – 50 microns) — have been an important component of cell separation techniques in both biomedical research and in clinical medicine for the past four decades (Borlido et al., 2013). The ability to utilize magnetic forces to easily manipulate and control magnetic particles and magnetic entities without wires or contacts has been recognized to have great potential for biomedical use; as such, magnetic particles have been widely utilized for the isolation of key cell population for a variety of applications including clinical diagnostics and regenerative medicine as well as facilitate fundamental understanding of biological phenomena.
This paper reviews the current status of magnetic particle attributes relevant to the field of cell separation, with a general focus on the governing physical and fluid dynamic properties of magnetic particles and on current applications of magnet-based cell separation. Aspects such as synthesis of magnetic particles used in cell isolation, platform design considerations and future prospects for magnetic-enabled cell separation methods are reviewed. Introductory material (Section 1) presents highlights of the contribution of cell separation to biomedical research and medicine and is followed by an overview of cell separation methods (Section 2). Presentation of relevant theory and phenomena of magnetism underlying the action of magnetic particle-based cell separation is provided in Section 3. Sections 4 and 5 describes examples of magnetic cell-separation systems, including consideration of magnetic particle and non-magnetic cell separation techniques, while Section 6 discusses challenges and opportunities for further research and commercialization of magnetic particle-based cell separation systems.
1.1 Cell Separation: Enabling Modern Biology and Biomedicine
The use of pure, sorted cells helps to reduce variations among experiments and thus expedites scientific discovery. Understanding cell behavior often requires isolation of cell subpopulations to reduce heterogeneity in the studied sample: cell populations of interest can include stem cells, circulating tumor cells (CTCs), cancer stem cells, and white blood cell subpopulations. The enrichment of a target cell population, and subsequent cultivation of desired cells from a defined cell population, is an important first step in the fields of molecular genetics (Szaniszlo et al., 2004) and proteomics (Altelaar and Heck, 2012, Gomase et al., 2008, Matt et al., 2008), as well as in a number of fundamental biological assays. Other important applications that rely upon cell sorting are enrichment of malaria-infected cells for diagnostics, blood cleansing (the removal of bacteria from blood before returning the blood to its donor), and filtering out CTCs to prevent the spread of cancer. In this Introductory section, selected examples that illustrate the importance of cell separation in the fields of biomedical research and medicine are described. These examples are excerpted from the areas of fundamental biological research, tissue engineering and regenerative medicine, personalized medicine and diagnostics/therapeutic health monitoring.
As this review covers both magnetism and cell separation, it is important to define several terms relevant to cell separation to assist in the overall understanding and comprehension. In the field of biologics enrichment or isolation, the target population of cells is referred to as the “specific” cell of interest and those cells that are isolated, but are not desired, are termed “non-specific”. In the past decade, cell isolation has played a large role in the separation of stem cells and progenitor cells. To avoid confusion, a progenitor cell is more specific than a stem cell and has properties similar to its terminal cell type. The most important difference between stem cells and progenitor cells is that stem cells can replicate indefinitely and progenitor cells have a limited lifetime. Moreover, many applications in cell separation are closed linked to the fields of diagnostic and therapeutic monitoring; in these situations the numbers of cells that can be isolated and counted are directly related to the accuracy of the disease prognosis. The number of specific cells in a sample serves as a “biomarker” – the indication of disease presence or a change in disease severity. In the targeting process, the cell population of interest, or “phenotype”, can be characterized based on the expression of biomolecules known as antibodies that are present on the surface of the cell. These antibody ligands on the cells, or the immunophenotype of the cells, can be used to attach an additional molecule or magnetic beads onto the cells. The details on this “affinity” methodology are described Section 2.1.3. These unique binding events result in an external, or exogenous, “labeling” or “tagging” of the cell.
Fundamental Biological Research
The capability to probe distinct characteristics of a select cell implies the need for a pure, homogeneous, population of the desired cell population within the research system. A wide variety of subfields ranging from genomics and proteomics to synthetic biology and organ-level research require precise control over the cells under investigation (i.e., the target cells) without the biological interference of non-target cells. Thus the ability to isolate and enrich a population of cells extracted from their complex native environment is a necessary pre-processing step to yield meaningful and impactful results.
Successful sorting of key cells from their biological milieu has resulted in numerous discoveries that have lead to, or soon will lead to, important advances in medicine. As one example, the production of induced pluripotent stem cells, first discovered in 2006 in mice (Takahashi and Yamanaka, 2006) and 2007 in humans (Yu et al., 2007, Takahashi et al., 2007) derived from fibroblasts has provided a less politically and religiously polarizing research alternative to the use of embryonic stem cells. Although this initial work utilized a culture-based cell separation method, it is clear that cell enrichment played a key role in this research. Since these initial discoveries, higher-throughput sorting methods have been adopted in the field of stem cell research (Meng et al., 2011, Singh et al., 2013, Giorgetti et al., 2010, Vickers et al., 2012). Another example of an advance enabled by cell separation is the isolation of HIV-infected white blood cells from patients (Douek et al., 2002, Pitcher et al., 1999, Brenchley et al., 2004) for individual testing; these developments have provided essential insight into the pathology of HIV, leading to better treatment and management of the disease.
Cell Sorting for Applications in Tissue Engineering and Regenerative Medicine
Over the last decade, the innate regenerative capacity of stem and progenitor cells resident in blood and tissue has been the basis of several promising tissue-based therapeutic strategies. All of these strategies require isolation of the stem or progenitor cells from their native environments in blood or tissue in viable condition and in sufficient quantity. Across most organ systems, the abundance of these cells is generally quite low (≤1% of the total cell population in a given sample), posing a technical challenge at the outset. These overall low cell concentration levels in these cases preclude the use of bulk cell separation techniques, such as density gradient centrifugation-based techniques (e.g. RosetteSep (Naume et al., 2004)) and macroscale cell affinity chromatography methods (Hertz et al., 1985). Antibody-mediated techniques, such as labeling cells with fluorescent or magnetic particle tags, have better sensitivity and selectively as compared to bulk separation methods and are currently the most widely-used methods in both foundational and early-stage clinical studies of stem/progenitor cell-mediated regeneration from tissue sources.
The majority of cell separations currently performed for clinical cell therapy and regenerative medicine use cells isolated from tissues such as bone marrow and blood. These separations isolate the non-red cell population from blood, including the stem cell fraction, and can be used to repopulate the blood (hematopoietic) system of a patient suffering from, for example, chronic myeloid leukemia, following immune-comprising therapies. At the present time, the largest challenge for clinical cell separation is to achieve a robust isolation of rare cell populations with multiple surface markers from a large initial pool of cells. Currently, cell-separation technologies based on centrifugation allow for the isolation of cells from a large initial cell sample, and technologies that employ magnetic particles can isolate specific populations of cells; however, these technologies identify cells with only single biomarkers so that cells of interest that possess two or more biomarkers cannot be specifically isolated. The topic of multiple marker separation is a very active area of research and this topic will be addressed in Section 6.2.
Personalized Medicine
Personalized medicine is a relevantly young but rapidly advancing field of healthcare that is informed by each person’s unique clinical, genetic, genomic, and environmental information (Hamburg and Collins, 2010). Because these factors are different for every person, it is a tenant that the nature of diseases—including their onset, their course, and how they might respond to drugs or other interventions—is as individual as the people who experience them. Personalized medicine seeks to make the treatment as individualized as the disease, and accurate and rapid cell sorting is indispensible to this vision. Completion of the Human Genome Project in 2003 (Collins et al., 2003) provided crucial insight into the biological mechanisms underlying countless medical conditions, allowing scientists and physicians to advance the field of personalized medicine at a remarkable pace. While not yet an established part of clinical practice, a number of top-tier medical institutions now have personalized medicine programs, and many are actively conducting both basic research and clinical studies in genomic medicine, proteomics, and drug development (Hamburg and Collins, 2010).
It is routine in oncology and hematology to characterize the morphology and type of cancer cells for diagnostic and therapeutic purposes. Unraveling the detailed molecular characteristics of cancer cells from clinical samples will thus play a paramount role for the progress of cancer research, diagnosis and treatment. Regarding clinical sampling, the current trend towards minimally-invasive diagnostic procedures follows several different tracks including the identification and molecular typing of CTCs in peripheral blood (Pachmann et al., 2008) (i.e., that blood found within the circulating pool of blood and not sequestered within the lymphatic system, spleen, liver, or bone marrow). Such investigations are currently performed using the commercial magnetic cell separation platform, the CELLSEARCH® system (Allard et al., 2004, Budd et al., 2006, Cohen et al., 2008, Cristofanilli et al., 2007, Hayes et al., 2006, Moreno et al., 2005, Pantel et al., 2008). The deconvolution of profiling data to extract the relevant biology of cancer cells from the mixture of white blood cells (WBCs or leukocytes) is challenging, and in most cases impractical (Calvano et al., 2005, Smirnov et al., 2005). Efficient enrichment of these cells of interest is critical prior to characterization; otherwise, plentiful leukocyte cell contamination would overwhelm any subsequent molecular analyses of rare cells. Although most of the work in personalized medicine has focused on cancer cells, numerous other cells of interest can be used for personalized medicine including fetal material blood cells for prenatal diagnostics (Wachtel et al., 2001), endothelial progenitor cells for cardiovascular risk assessment (Werner et al., 2005), hematopoietic stem cells for hematology diagnostics (Solovey et al., 1997, van Beem et al., 2009), and other stem cells in various diseases and conditions (Prasongchean and Ferretti, 2012, Chun et al., 2011).
Diagnostics and Therapeutic Monitoring
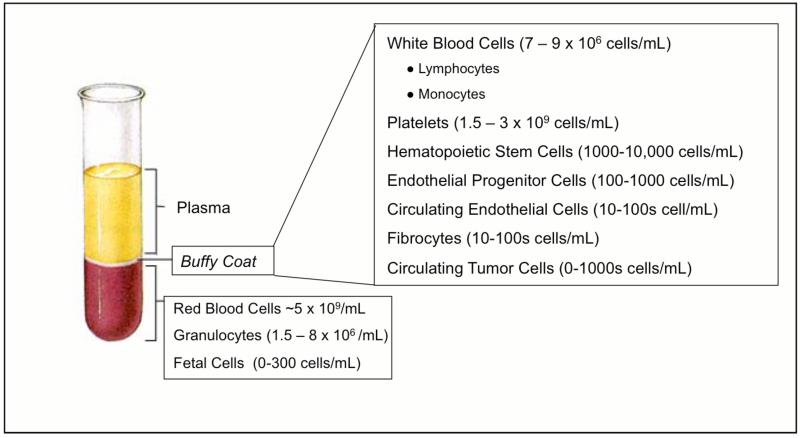
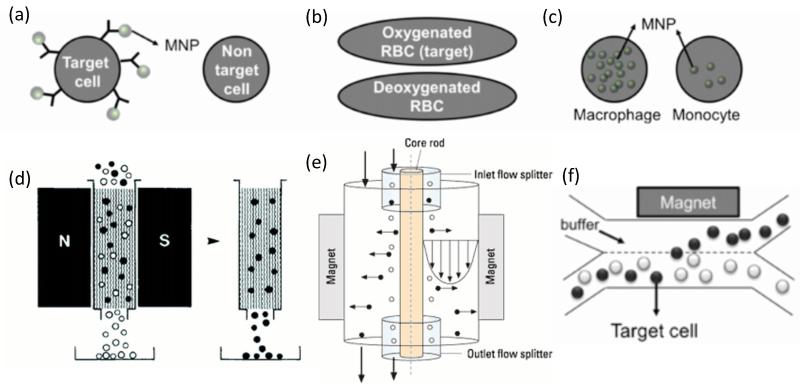
Cell separation technologies have enabled high-precision tests for the diagnosis of cancer. Many current diagnostic tests depend on individual aspects of fractionated blood components: plasma, red blood cells, white blood cells, and platelets. Clean, cell-free plasma is necessary for early cancer detection via blood-borne cancer biomarkers (Li et al., 2002, Villanueva et al., 2006, Bunn, 1997). Leukocytes are required for several hematological tests as well as for DNA sequencing. Toner and Irimia (Toner and Irimia, 2005) presented a thorough review of blood-on-a-chip technology which describes the challenges of handling blood and the information that can be gleaned from the various components of blood. A number of rare cells (defined as comprising less than 1% of the total cell number) useful for disease diagnosis may also be found in healthy blood (Bhagat et al., 2010, Miltenyi et al., 1990) (Figure 1). For example, rare cells such as circulating tumor cells (CTCs) may be useful for adapting therapies to the characteristics of a patient populations (Cristofanilli et al., 2004). Fetal cells are also present in limited quantities in the maternal circulation or cord blood and may be used in noninvasive prenatal diagnostics (Krabchi et al., 2001). Furthermore, the measurement of immunologically-defined mature circulating endothelial cells (CECs) in the peripheral blood is gaining ground as an important and novel technique for assessment of cardiovascular and endothelial injury (Boos et al., 2006). In addition to CECs there is recent evidence of another rare endothelial cell in the blood that can give further indication of cardiovascular disease status called an endothelial progenitor cell (EPC) (Hill et al., 2003, Hristov and Weber, 2008, Werner et al., 2005). In addition to their value in diagnostics, EPCs have shown significant promise as easy cell source for engineered vascular grafts (Masuda et al., 2000, Melero-Martin et al., 2007, Roncalli et al., 2008) and heart valves (Sales et al., 2007a, Sales et al., 2007b).
Figure 1.
Blood is a rich source of cells for tissue engineering, diagnostics, and fundamental biology, containing several rare populations.
Beyond diagnostics, blood components derived from cell sorting are used in therapeutics. Purified platelets are often transfused during surgery (Sethu et al., 2006), and stem and progenitor cells derived from tissue niches may be found in many clinical samples. These cells, once isolated, can be reintroduced into the body to assist the natural cell repair mechanisms. Enzyme-digested adipose (fatty) tissue can yield personalized donor cells that may be later used for tissue engineering and disease treatment (Tandon et al., 2013); a more accessible source of valuable stem cell populations is the amniotic fluid. Many cell populations can be used to diagnose disease and correlation disease status with therapeutic success. The body has a natural tendency to tune the concentration of particular cells as mechanism to repair functions within the organs. Furthermore, the presence of particular mutations in the native cells within organs, blood, or bone marrow can be indicative of a disease state.
1.2 Societal Interest and Motivation for Cell Separation
In addition to enabling a myriad of biomedical procedures and diagnostic techniques that improve the quality of life and open up many new avenues of fundamental inquiry, advances in cell sorting helps to fuel the global economic engine through public and private investment in medical research. Four specific health diagnosis/monitoring examples of heightened societal interest that greatly benefit from cell isolation techniques are provided here pertinent to cancer, cardiovascular disease, prenatal diagnostics and malaria. Completing this picture, a brief overview of the economic impact and implications of biomedical research, critically supported by cell separation technologies, is also provided.
Cancer
Although much progress has been made in the diagnosis and treatment of malignancy, cancer is still one of the most common causes of death worldwide (Society, 2012). There were an estimated 14.1 million cancer cases around the world in 2012, of these 7.4 million cases were in men and 6.7 million in women. This number is expected to increase to 24 million by 2035 (Society, 2012). Most patients with cancer have symptoms and distant metastases when diagnosed, which makes it more difficult to successfully treat the disease. Therefore, accurate prognosis and diagnosis at early stages of the disease are the most critical issues in cancers (Rusling et al., 2010). Recently, significant efforts have been devoted to identifying informative cancer biomarkers that can contribute to the establishment of cancer diagnosis. The biomarkers encompass mutated DNAs and RNAs (Rusling et al., 2010), secreted proteins (Rusling et al., 2010), and tumor cells (both circulating tumor cells, tumor stem cells) (Reya et al., 2001, Paterlini-Brechot and Benali, 2007, Huntly and Gilliland, 2005). The major cause of cancer-associated mortality is tumor metastasis, occurring when tumor cells invade the surrounding tissue of the primary tumor and enter into the blood and lymphatic systems, travelling to distant tissues where they adapt to new microenvironments, and eventually seed, proliferate, and colonize. Because cell dissemination mostly occurs through the blood, circulating tumor cells (CTCs) that have been shed into the vasculature and may be on their way to potential metastatic sites are of obvious interest (Chaffer and Weinberg, 2011). The presence of CTCs in cancer patients was first detected in 1869 (Gupta and Massagué, 1869). Numerous studies in the past decade have shown that CTCs may be used as a marker to predict disease progression and survival in metastatic (Cohen et al., 2008, Cristofanilli et al., 2007, Cristofanilli et al., 2004, Maheswaran and Haber, 2010, Moreno et al., 2005, Paterlini-Brechot and Benali, 2007, Stott et al., 2010b, Nagrath et al., 2007) and possibly even in early-stage cancer patients (Rhim et al., 2012). High CTC numbers correlate with aggressive disease, increased metastasis, and decreased time to relapse (Chaffer and Weinberg, 2011). Because blood collection is simple and minimally invasive, identification and quantification of CTCs could be used as a real-time marker for disease progression and survival. CTCs also have the potential to guide therapeutic management, indicate therapy effectiveness or necessity, even in the absence of detectable metastases, and offer insights into mechanisms of drug resistance. All of these attributes of CTC’s make their separation an important priority in biomedicine, with a number of cell separation platforms poised to contribute to the next generation in metastatic cancer diagnostics and oncological therapeutic monitoring (Cristofanilli et al., 2007, Cristofanilli et al., 2004, Ozkumur et al., 2013, Karabacak et al., 2014). Non-metastatic cell populations have also been successfully separated and enumerated for diagnostics of acute and chronic leukemia (Vickers et al., 2011).
Cardiovascular Disease
In the past decade, there has been growing interest in endothelial progenitor (EPCs) and mature circulating endothelial cells (CEC) in the peripheral blood, as it has been shown that both EPC and CEC numbers are positively correlated with cardiovascular disease risk (Blann et al., 2005, Boos et al., 2006, Bull et al., 2003, Burger and Touyz, 2012, Damani et al., 2012, Goon et al., 2006, Kraan et al., 2012, Diller et al., 2010, Dzau et al., 2005, Hristov and Weber, 2008, Mead et al., 2007, Urbich and Dimmeler, 2004, Yoder, 2012). Currently there are two main approaches to the separation of endothelial cells populations from blood (1) cell sorting using cell surface markers, via MACS (Plouffe et al., 2012, Damani et al., 2012), affinity-chromatography (Plouffe et al., 2009b, Hansmann et al., 2011, Hatch et al., 2011, Hatch et al., 2012), and FACS (Van Craenenbroeck et al., 2008, Kraan et al., 2012); (2) in vitro cell culture of the blood mononuclear cell fraction (Masuda and Asahara, 2013). The measurement of EPCs as cardiovascular biomarkers in large clinical trials requires simple, rapid, and reproducible cell separation methods, with techniques such as flow cytometry widely applied.
Prenatal Diagnostics
To date fetal cells separated from maternal blood have so far identified the sex of the fetus (Bianchi et al., 1992) and various genetic disorders (including human leukocyte antigen and Rh blood types (Geifman-Holtzman et al., 1996); trisomy 13, 18 and 21 (Ganshirt-Ahlert et al., 1993, Oosterwijk et al., 1998); triploidy (de Graaf et al., 1999) and sickle cell anemia and thalassemia (Cheung et al., 1996)). Thus, fetal cell separation might one day be used for screening of common genetic conditions and, ultimately, for prenatal diagnosis. Individual fetal red blood cells precursors have been cultured after separation in some laboratories. Culturing and genotyping of separated fetal cells might enable diagnosis of a spectrum of chromosomal and genetic disorders. As current separation techniques do not fully achieve the purities needed for precise prenatal care, further development of fetal cell separation technology will be required before regular clinical application of these methodologies is adopted (Wachtel et al., 2001, Hemberger, 2012, Kavanagh et al., 2010, Torricelli and Pescucci, 2001).
Malaria
Malaria infection is a serious public health problem in developing countries with up to 300-500 million clinical cases and more than 1 million deaths each year (Heidelberger et al., 1946). Upon infection, malaria parasites invade liver cells and produce thousands of spores, which can then invade red blood cells (RBCs) and rapidly spread (Cowman and Crabb, 2006). Currently, the Giemsa staining method is the standard technique for diagnosis, but the procedure for to conduct this method is complex, and well-trained personnel are required for reliable evaluation. It is also difficult to achieve high detection accuracy at low infection rates (< 100 parasites/μL) through the use of staining procedures (Makler et al., 1998). It is an interesting and useful fact that healthy RBCs are magnetic, by virtue of their significant iron content, and become distinguishably more magnetic when infected with the malarial parasite (Nam et al., 2013). This attribute distinguishes infected RBCs from the surrounding cell populations and allows them to be magnetically manipulated and separated in a label-free manner, without the need to incorporate magnetic particles into the blood sample. In this manner, the target cells of interest may be concentrated to allow for early infection diagnosis and more accurate prognostication (Bhakdi et al., 2010, Kim et al., 2012, Miao and Cui, 2011, Moore et al., 2006, Nam et al., 2013, Ribaut et al., 2008).
1.3 Laboratory and Clinical Research Support, Impact
There is no doubt that biomedical research investment and associated spillover effects play an extremely substantial role in the global economy. Reports tend to focus on the economic burden of select categories of health challenges (obesity, lung cancer, etc.), with very few wholistic assessments. A 2008 report commissioned by the Wellcome Trust, the UK Medical Research Council and the UK Academy of Medical Sciences critically examined the economic benefits of public and charitably funded medical research in the UK (Group et al., 2008). While the elements of the study are numerous and complex, the conclusions, based in large part on a comprehensive study of cardiovascular disease, suggest that the proportion of UK health care benefit attributable to UK research lies in the range from 10% to 25% with a central estimate of 17%. Expanding the view beyond the UK to assess the economic impact of cancer within the European Union, it is reported that cancer incurred costs of €126 billion in 2009, yielding an equivalent healthcare cost of €102 per citizen. In the U.S., the National Cancer Institute (2012) reports 2010 U.S. direct costs for cancer care as $124.57 B. This amount is projected to steadily increase due to anticipated increases in occurrence in the U.S. population as well as to as new, more advanced techniques for diagnosis and treatment that will be adopted as standards of care. Innovation for future biomedical technology development, including that underlying cell sorting techniques, will be actively pursued in the private sector, as discussed in the next section.
1.4 Investments by Large Corporations and Start-Up Companies
According to BCC Research, the microspheres market, spanning all applications, is projected to be worth $3.5 billion by 2015. The market for cell separation technologies overall is projected to reach the $1.4 billion level by 2015. The market for MACS beads, which is a subset of both of these market areas, is projected to be at the $380 million level by 2015 (Research, 2011). MACS is by now a well-established platform in both basic research as well as clinical medicine as a major attraction is its scalability and very low capital costs relative to FACS. There are numerous companies that exclusively produce magnetic particles for a variety of applications including cell separation; several larger corporations can be found in the magnetically-enabled cell separation space. Some of the largest global providers of magnetic beads include BD Sciences, Bang Laboratories, Thermo Fisher Scientific (formally Life Technologies), Micromod Partikeltechnologie GmbH, Miltenyi Biotec GmbH, and Millipore. The largest companies in the MACS space are Miltenyi Biotec and Thermo Fisher Scientific, as they provided a fully automated separation platform (MultiMACS™ Cell24 Separator and autoMACS® Pro Separator, and the KingFisher™ Flex Magnetic Particle Processors, respectively). The RoboSep® platform by StemCell Technologies provides a smaller automated platform for separation of cells up to 8.5 mL of sample with 4 simultaneous separations. For small-scale separation the BD™ IMag Cell Separation System has been shown to effectively separate, either through positive or negative approaches, a high purity fraction of target cells. Currently R&D on improving bead technologies is very active within large companies not only on the particle side but also in the design of automated instrumentation (e.g. Miltenyi AutoMACS) or of user-friendly kits (Stem Cell Technologies). The key drivers of technological development appear to be (i) improving bead performance in terms of recovery and specificity, (ii) the ability to custom-design beads for kits sold by the large company itself or for kits that these companies manufacture for other vendors, (iii) providing improved automated platforms to promote user adoption. A comprehensive table of bead manufacturers and their current diversity in capabilities and functionalities is presented by Borlido et al. (Borlido et al., 2013) and Safarik and Safarikova (Safarik and Safarikova, 1999). In addition to the variety of chemically coated microbeads, many vendors also sell beads towards specific cells of interest.
Within the magnet-based cell separation market space there are also several start-up companies that are either developing new cell separation devices or new magnetic beads for cell separation. Many small companies are designing platforms specifically for cancer diagnostics via circulating tumor cell separation and enumeration, including Cynvenio Biosystems (US), BioCep (Israel), and Aviva Bioscience (China). Sepmag (Spain) is one of the leading providers of commercial permanent magnet separators, with capacities of separating 1μL to 50 L. Several novel magnetic beads are also currently in development, such as QuickGel™ beads from Quad Technologies (US), new “big beads” from CellCap Technologies Ltd (UK), and metallic beads from TurboBeads Llc (Switzerland). Many of the innovative efforts attempt to challenge the current paradigm of iron oxide beads (50 nm – 10 μm in diameter) that remain attached to the cell surface. QuickGel™ beads are synthesized from a patented hydrogel technology that allows for facile release of the beads from the cell surface. CellCap beads possess diameters in excess of 50 μm thus their operation relies on gravitational forces combined with magnetic forces to separate labeled cells from suspension. TurboBeads® possess a magnetic metal core and a graphene shell of monolayer thickness; they thus present a high moment and stable labeling potential. Overall, magnetic cell separation is a growing industry and shows much promise for continued future innovations.
2. Overview of Cell Separation Methods
As introduced in previous sections, both clinical laboratory and basic biology research applications contain significant challenges in the isolation and study of target cells of interest due to the abundance of non-target cells present in the surrounding normal tissue, such as blood cells. It is thus necessary to reduce the molecular “noise” from normal cells by enriching these target cells through application of a precise cell separation method that specifically isolates the cells of interest from the dense heterogeneous cell environment.
Assessment of the efficacy of any cell separation technique involves three paramount considerations: purity, recovery and viability (Sharp, 1988). The consideration of “purity” relates to the enrichment of specific cells of interest that are derived from a heterogeneous cell population using known factors, such as cell surface phenotype, associated with the target cells. The percentage of target cells compared to isolated non-target cells within a sample can be calculated from this separated fraction, simply represented as the number of target cells divided by the total number of cells separated. “Recovery” describes the efficiency of cell separation and is quantified by the percentage (by number) of cells that are obtained post-sorting as compared to the number of total cells or target cells in the original suspension. There are two measurements that can quantify cell recovery: the number of separated cells versus the total cell count and the number of separated cells versus the target cells in the original cell suspension; the latter measurement is generally more informative. The former measurement yields information on the percentage of cells isolated from the total number of cells, providing guidance on the cell separation efficiency when working with a cell suspension of a well-defined composition. However, the value of this quantity is limited, especially for original cell suspensions with a variable cell content due to, for example, a disease state. To determine the true cell separation efficiency, the number of recovered cells must be compared to the number of target cells in the original suspension. It is therefore important to quantify the number of cells obtained following separation as well as those in the original cell suspension. Finally, the consideration of “viability” refers to ’cells that are not dead’ at its most basic level. This descriptor is clearly important, as a separation process that does not yield live cells is of little value when the downstream application is a live cell assay or cell culture for clinical applications. However, attainment of a living population of cells does not of itself necessarily meet the requirements of some downstream applications; for example, dormant cells are also live but do not possess the capability to proliferate or differentiate. Therefore, viability and function are both essential metrics of cell separation efficacy.
2.1 Current Methodologies of Cell Separation
Conventional cell separations are often achieved on the basis of the differences in cell physical properties, such as density and size, or by exploiting more specific biochemical properties, such as surface antigen expression (Radisic et al., 2006, Pratt et al., 2011, Bhagat et al., 2010, Recktenwald and Radbruch, 1997). Rather than providing a comprehensive review of all cell separation techniques, representative examples of different methods are briefly described here to illustrate each isolation technique. These techniques are divided into three categories: culture-based cell separation, separation based on physical properties and biochemical affinity-based cell separation. The last class of cell separation technologies includes the important techniques of fluorescent-activated cell sorting (FACS) and magnet-activated cell sorting (MACS). All of these techniques are summarized in Table 1, which provides a general overview of the specific cell characteristics that are used to achieve isolation, in addition to describing the advantages and disadvantages inherent in each technique. There is no perfect cell isolation technique, and development of such a platform would be a quixotic approach, thus the choice of separation platform is dependent on application and need.
Table 1.
Descriptions and Comparisons Among Different Cell Separation Techniques
| Technique | Key parameters | Advantages | Disadvantages |
|---|---|---|---|
| Culture-based Separation | Cellular adhesion profile | Label-free | Adhesion and growth property differences generally comparable across cell types Very low throughput (> 3-5 days) |
| Centrifugation | Cell size Cell cytoplasm density |
Label-free Standardized Equipment High throughput (>1011 cells/hr) |
Physical and biological differences can be too subtle Cell perturbations due to physical forces |
| Acoustophoresis | Contract Factor (cell density and compressibility) |
Label-free Gentle on cells |
Most cells have contrast factors of the same sign |
| Dielectrophoresis | Cell dielectric properties Cell size Electric field parameters |
Label-free Easy incorporation into devices |
Biological basis underexplored Potential differences can be too subtle Low to medium throughput (107-108 cells/hr) |
| Mechanical/hydrodynamic | Cell size Cell shape Cell deformability |
No exogenous labeling High throughput (>1010 cells/hr) |
Problems with clogging Physical and biological differences can be too subtle Damage to cells |
| Cell-affinity Chromatography | Cell surface marker expression Antibody/ligand binding kinetics Cell interaction with surface |
Highly specific | Requires cell-specific marker Dependent on antibody-ligand specificity Lack of a standard detachment method |
|
Fluorescence-activated Cell
Sorting (FACS) |
Optical signal intensity or morphological features |
Gives spatially specific information Identifies complex/subtle phenotypes |
Often requires exogenous labeling Trade-off between speed and resolution Low throughput 107 cells/hr |
|
Magnet-activated Cell Sorting
(MACS) |
Magnetic field strength Cell surface marker expression Magnetic label binding kinetics |
Can be highly specific Easy incorporation into devices |
Often requires exogenous labeling Medium throughput 109 cells/hr |
Cell separation approaches can be categorized into either positive selection or negative selection. In positive selection approaches, the cells of interest are collected as the target cell population. This mode of selection continues to remain the most prevalent technique for cell separation, as cells can be selectively targeted via ligand affinities and excellent purities of collection (> 99%) are easily achievable. Positive selection techniques have only recently shown promise as methodologies for rare cell isolation; due to low recoveries, albeit high purities, of positive selection, the efficiency of separation using this method has not yet met the high requirements necessary for laboratory and/or clinical settings. On the other hand, negative selection techniques isolate the non-specific cells from heterogeneous suspensions, leaving behind the target cells in suspension. A major shortcoming of negative selection techniques is the unintended collection of non-target cells in the effluent stream. The significance advantage of negative selection techniques is the ability to (i) separate cells without deleterious labeling or stresses and (ii) selective separation of cell with no known markers. Although a large percentage (> 95%) of the non-target cells can be removed from a given sample, there remains a small population of undesired cells in the target cell suspension that results in low purities of collection and may adversely influence post-separation applications.
2.1.1: Culture-based Cell Separation Techniques
One of the simplest ways to separate target cells from a heterogeneous cell population is to harness the unique differential adhesion profile of different cells in the heterogeneous suspension (Lavasani et al., 2013, Brown et al., 2008, Laugwitz et al., 2005). Cells can be placed in culture with a growth medium, either on native polystyrene or coated with cell adhesion biomolecules, and over a given period of time specific cell populations will adhere to culture substrates. Upon removal of the growth medium (the supernatant), the target cells can be isolated. It should be noted that cells can be removed from the culture flask and re-cultured multiple times to further enhance the purity of separation. Unfortunately, the culturing technique suffers from several shortcomings: (a) while the cell suspension is certainly enriched in target cells, the enrichment process is generally inefficient, due to the unavoidable adhesion of the non-target cells to the substrate surface; (b) culture-based cell separation is not systematic due to the lack of controllable process parameters such as cell growth rate, cell adhesion strengths, and cell settling dynamics; and lastly (c) further separation of the target cell populations that have adhered to the tissue culture plate surface during the separation process is difficult. As a result of both (a) and (c) aspects, cultures with a high non-target cell content can require 3-7 days of additional proliferation to achieve preferential growth of desired cells. This extra time can result in experimental delays and possible loss of cell function and gene expression.
2.1.2. Separation Techniques Based on Physical Properties of Cells
While there are a large number of cell separation techniques based on the physical attributes of cells, their efficacy and applicability can vary widely. Numerous external forces may be applied to separate cells based on their physical properties, including acoustic waves, hydrodynamic flow, and electric and or/magnetic fields. Since these techniques often do not require the addition of an additive (such as a fluorescent or magnetic particle tag), they are attractive methods when affinity ligands are not available. These techniques are also desirable because they can be performed under high-throughput continuous flow conditions, with minimal sample preparation before or after separation.
2.1.2.1 Density-Gradient Centrifugation
Centrifugal separation is an operation that relies upon sedimentation (the tendency for particles in suspension to settle out of the fluid to rest against a barrier) that is accelerated by centrifugal force and requires a difference in density between the constituent phases. In this technique particles or cells of different densities/volume in a suspension will settle at different rates, with the larger and denser particles settling out of suspension more rapidly based on their sedimentation velocity, ; where m is mass, D is diffusion coefficient, g is the gravitation constant, kB is the Boltzmann constant, and T is the temperature (Berg, 1993). There are several different centrifugation techniques of relevance to cell sorting, including differential centrifugation, rate-zonal centrifugation, and equilibrium centrifugation, briefly described below (Axelsson, 2002).
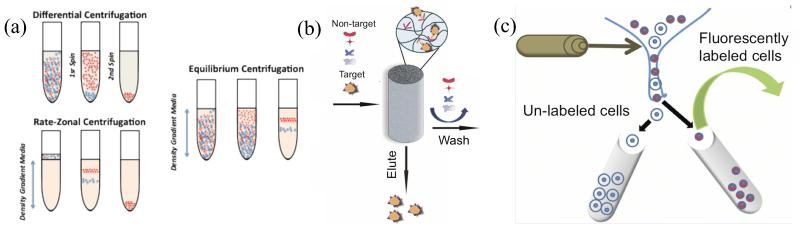
Differential centrifugation (Figure 2) subjects cell suspensions to a series of increasing centrifugal force cycles to yield cell population groups of decreasing sedimentation rate (i.e., decreasing mass). Due to the heterogeneity of cells characteristics in typical biological suspensions, products from differential centrifugation suffer from contamination and poor recoveries. Contamination can be addressed by breaking up the resulting cell pellet, re-suspension, and repeating the centrifugation steps (i.e., washing the pellet). Rate-zonal centrifugation uses a density gradient to effectively separate cells into different zones according to their shape and mass. Rate-zonal centrifugation (shown in Figure 2) mitigates cross-contamination problems by layering the sample on top of a density gradient fluid (such as Ficoll-Paque™), which controls the diffusion coefficient in the sedimentation velocity equation. Thus, rapidly-sedimented cells are not contaminated by the slowly-sedimented cells. Finally, equilibrium (or isopycnic) centrifugation (Figure 2) separates based solely on density. In this technique, the mass of the cell affects the rate at which cells transport through the gradient medium; transport takes place until the granularity of the cell population is the same as that of the surrounding gradient medium added to the cell suspension (i.e. the equilibrium state). It is to be noted that the cell concentration zones produced in all three of these techniques are very sensitive to mechanical disruption, and it is common to inadvertently mix two or more phases during removal from the centrifuge that had been separated using this process. Effective centrifugation can also be constrained by the fact that numerous cell populations, especially leukocytes, are highly sensitive and reactive to changes in the environment and therefore centrifugation may alter their immunophenotype (Lundahl et al., 1995, Fukuda and Schmid-Schönbein, 2002).
Figure 2. Traditional modes of cell separation currently used in the laboratory and clinic.
(a) The major modes of centrifugation include differential centrifugation, rate-zonal centrifugation, and equilibrium centrifugation. (b) Affinity chromatography allow for separation of a target cell(s) from heterogeneous cell slurry via interactions with a porous matrix conjugated with capture ligands. Adapted from (Kumar and Srivastava, 2010). (c) Traditional set-up of a FACS instrument where cells labeled with fluorescent tags can be separated form un-labeled cell populations. Adapted from (Kang et al., 2011).
2.1.2.2 Acoustophoretic Cell Sorting
The application of acoustics in cell and cell manipulation, including separation methods, has been extensively reviewed by the journal Lab on a Chip in a themed collection titled “Acoustofluidics”. Acoustophoresis devices are based on cell migration in a sound field. Exposing cells or microparticles to an acoustic field in a fluid creates an acoustic radiation force that acts on the cells and controls the spatial migration of the cells. The acoustic radiation force arises from the differences, or contrast factor, in density and compressibility of the cells and particles, compared to the surrounding fluid medium. In the presence of an acoustic standing wave, particles (or cells) with positive and negative contrast factors migrate to pressure nodes and pressure anti-nodes (Gupta et al., 1995), respectively, and can then be concentrated and collected. The acoustic field that enables acoustophoretic cell separation systems is easily tunable, lending high adaptability to the system. Although acoustophoretic sorting has shown some promise with particles (Harris et al., 2010, Liu and Lim, 2011, Petersson et al., 2007) and with some cell applications (Kumar et al., 2005, Petersson et al., 2007, Yang and Soh, 2012, Petersson et al., 2005, Augustsson et al., 2012, Ding et al., 2012, Lenshof and Laurell, 2011, Shi et al., 2009), its appropriateness for clinical application has never been validated as most mammalian cells have contrast factors of the same sign, and therefore are not amenable to separation via this technique.
2.1.2.3 Size-based Cell Separation: Filter, Hydrodynamic, Inertial Separation
The current field of size-based cell separation in a fluid carrier employs a number of platforms that manipulate the system fluid dynamics; these techniques include hydrodynamic filtration, field-flow fractionation, fluid dynamics modified by structures, and inertial microfluidics (Gossett et al., 2010). An intuitive approach for cell separation based on size exclusion is filtration. While fibrous membrane filters typically exhibit a wide range of macroscale pore size and are therefore insufficiently selective, microfabricated filter designs have pores that are precisely controlled through synthesis parameters and are thus an appropriate for many heterogeneous tissue samples, such as blood. Four types of microfilters have been reported: weir, pillar, cross-flow, and membrane; the cell separation performance of all these filters has been experimentally validated and reviewed by Ji et al. (Ji et al., 2008).
Hydrodynamic cell separation techniques, including pinched-flow fractionation and hydrodynamic filtration approaches, operate on the principle that at low Reynolds number conditions (Re ~0.1), characteristic of laminar flow behavior with the center of a particle or cell following fluid streamlines. In these techniques, the characteristics of the fluid flow alone are used to determine the size-based sorting; therefore, parameters such as flow rate control through one or more inlets, the channel geometry, and the configuration of outlets dictates the flow character and, ultimately, the cell isolation performance. As laminar flow is required for proper control of the fluid dynamics in this technique, both pinched flow fractionation and hydrodynamic filtration platforms utilize microscale flow (i.e. microfluidics).
The final method for cell separation based on size exclusion described here is the technique of inertial separation. As exploited for the above-described techniques, the inertial separation technique requires a laminar flow regime to be maintained for achievement of significant cell separation; however for this technique the flow rate is significantly higher (Re ~1 – 100) than most size-based separation techniques. In the upper range of the laminar region of Reynolds numbers, inertial effects have been shown to become significant, and thus the assumption that particles (or cells) will follow fluid streamlines is no longer valid (Di Carlo, 2009). At these flow rates a focusing phenomenon, attributed to the balance of two inertial lift forces: the shear gradient lift and the wall effect lift (Di Carlo et al., 2007, Gossett and Di Carlo, 2009), occurs. While the inertial lift forces depend on particle diameter, the equilibrium flow positions in straight channels are roughly the same for all particle diameter as long as the length of the channel is sufficiently long to allow particles to travel to these equilibrium positions. Creation of distinct equilibrium positions tailored to specific particle sizes requires introduction of an additional, size-dependent force, the so-called Dean drag force (Dean, 1928), that is on the order of the inertial lift force but directed in the opposite direction (Seo et al., 2007).
2.1.2.4 Electrophoresis and Dielectrophoresis
Cell separation techniques that rely on manipulation of electrical forces in the system are based on two main electrical phenomena: electrophoresis (EP) and dielectrophoresis (DEP). Electrophoresis describes the motion of dispersed particles relative to that of a fluid under the influence of a spatially uniform electric field. This technique has little application in current cell separation methods due to the lack of sufficient resolution in cell electrical properties. However, the phenomenon of dielectrophoresis, in which a force is exerted on a dielectric particle under the influence of a non-uniform electric field, allows control of motion of both charged and uncharged but dielectrically-active biological entities, such as cells and bacteria. Details of the electrical polarization of the cell are determined by the dielectric properties of the cell, which are influenced by its membrane characteristics, diameter, and internal structure (Pethig, 2010), including the cytoplasmic characteristics. Extensive theory, experimental and review articles, well beyond the scope of this review, are available in the literature that examines phenomena that influence and control the electrophoretic and dielectrophoretic mobility of cells (Demircan et al., 2013, Gagnon, 2011, Gascoyne and Vykoukal, 2002, Hughes, 2002, Lei and Lo, 2011, Pethig, 1996, Pethig, 2010, Bruus, 2008, Kulkarni and Dalal, 2011). The DEP technique can be very selective in cell sorting, as it is highly sensitive to the specificity of the dielectric phenotype of cells. This sensitivity has given rise to a number of devices that utilize the dielectrophoretic force for cell separation that do not require biochemical labeling. Furthermore, viable, culturable cells can be isolated by DEP with minimal or no biological damage because of the passive nature of DEP isolation. However, despite their relatively wide applicability, DEP approaches are time consuming and are thus rarely used to analyze real clinical samples compared with other separation approaches such as magnetophoresis and fluorescence-based approaches. Another drawback of using DEP-based techniques for biomedical applications is that, ideally, the dielectric force should be exerted in an electrically-insulating environment. However, many biological environments and body fluids such as blood and urine have high salt concentrations, creating a high electrical conductivity environment.
2.1.3 Separation Methods Based on Biochemical Affinity
An important class of cell separation technology is based on biochemical affinity. In this technique, affinity ligands for cell surfaces can be used either to provide an intermolecular force for separation, such as in cell affinity chromatography, or as a label in the techniques of fluorescent-activated cell sorting (FACS) or magnet-activated cell sorting (MACS). Affinity ligands are molecules that can form a complex, or non-covalent bond, with a biomolecule; in the case of cell separation, this chemical complex is bonded to the surface of a cell, with complex-cell interactions that can be made to be highly specific for a particular targeted biomolecule. The complex-cell binding typically occurs via intermolecular forces, such as ionic bonds, hydrogen bonds and van der Waals forces. Numerous affinity ligands have been reported in the literature, including antibodies, peptides, and nucleic acids (Grinnell et al., 1972, Gumbiner, 1996). Cell separations based on the affinity of selected ligands to the surface of cells often offer more selectivity for a given cell type when compared with that provided by other physical separation techniques. However, ligand availability and performance continues to be a limitation for cell affinity separations. Since most affinity-based methods require binding of cells to antibodies or other ligands, nonspecific binding is also an issue that must be minimized for successful separations of cell populations. Affinity-based separations are particularly well suited to cell types that are physically similar to the background cells in the sample.
2.1.3.1 Cell-affinity Chromatography (CAC)
One form of affinity chromatography (also called affinity purification) makes use of specific binding interactions between affinity-based molecules that are located on the cell surfaces. In this technique a particular ligand is chemically immobilized or “coupled” to a solid support within in a packed column, such as glass or polymer microbeads. When a complex mixture, such as a cell suspension, is passed over the column those molecules or cells that possess the specific binding affinity to the ligand become bound. For cell affinity separations, separation occurs when cells have different affinities to surface-immobilized molecules. The first example of cell affinity chromatography was by demonstrated by Wigzell et al. (Wigzell and Andersson, 1969) in 1969 – opening up the field of affinity-chromatography to cell separation.
Since this seminal publication the field of affinity-based cell chromatography has rapidly expanded and evolved from employing a simple batch-like process to utilizing a high throughput dynamic separation platform. The use of multiple capture molecules arranged in an array format allows separation of more than one cell type or fosters the ability to assay two or more parameters on the same cell type. Array formats use minimal sample to generate a wealth of information, but are not routinely used to elute cells for other use. Another advantage of using array separation devices is that the volume of the array fluidic chamber is typically well known, allowing for absolute cell counting and eliminating the need for counting beads or an additional counting step – a large bottle neck in separations today. Affinity arrays have been extensively developed for characterization of blood cells and other cells based on antibody–antigen capture (Barber et al., 2009, Kaufman et al., 2010, Kohnke et al., 2009, Rahman et al., 2012, Zhou et al., 2010).
As an extension of macroscale affinity chromatography columns, recent work has primarily focused on miniaturizing the channels to minimize samples volumes and enhance the throughput. Briefly, microfluidic channels can be functionalized with affinity biomolecules and, similar to the initial work by Wigzell et al. (Wigzell and Andersson, 1969), cells can be selectively captured from a flow channel (Didar and Tabrizian, 2010). Numerous examples exist in the literature that have illustrated the effectiveness of microfluidic cell affinity chromatography for the isolation of circulating tumor cells (Gleghorn et al., 2010, Stott et al., 2010a, Nagrath et al., 2007, Adams et al., 2008a, Du et al., 2006), endothelial progenitor cells (Hansmann et al., 2011, Plouffe et al., 2009b, Hatch et al., 2011, Hatch et al., 2012), endothelial and smooth muscle cells (Plouffe et al., 2009b, Green and Murthy, 2009, Plouffe et al., 2007, Plouffe et al., 2008), skin stem cells (Zhu et al., 2013), white blood cells (Murthy et al., 2004, Sin et al., 2005, Xu et al., 2009). Although microfluidic capture channels have illustrated excellent recoveries (> 90%) and purities (> 95%) (Didar and Tabrizian, 2010) of very rare cells versus many alternative approaches the difficulty in gently removing trapped cells from the surface of the affinity substrate has limited the use in many biological fields (Murthy and Radisic, 2008).
Recently, Karnik and coworkers (Bose et al., 2013, Choi et al., 2012, Karnik et al., 2008, Lee et al., 2011) have illustrated that combination of an affinity-based capture methodology with precise hydrodynamic control allows cell separation via a “rolling” mechanism. Briefly, these authors modify flow channels with a unique pattern of antibodies whereby the specific cells, through their affinity to the antibody patterns, follow the patterns. It is demonstrated that precise manipulation of white blood cells is possible to very effectively isolate them from whole blood (Bose et al., 2013). This technique does not trap the cells and thus the isolated cells remain label-free for post-separation applications.
2.1.3.2 Fluorescence-Activated Cell Sorting (FACS)
The fluorescent-activated cell sorting (FACS) technique harnesses the ability to label a target cell(s) with fluorescent dyes tags, which allows for cell sorting based on the individual labeling profile of a particular cell population. Each labeled cell is individually entrapped in a droplet of buffer solution and is passed through one or multiple laser beams at high speed (Crosland-Taylor, 1953) – thus probing the cells on an individual, one-by-one, basis. Prior to fluorescent probing, the labeled cells are first identified by detectors that are sensitive to cell size (a process known as forward scattering) and granularity (a process known as side scatter). Second, the cells are then probed for their unique fluorescent profile via precise fluorescent filters and detectors. Depending on predefined sorting criteria, each droplet is then given an electric charge and then sorted using electrostatic deflection plates. Current state-of-the-art sorting devices typically use up to seven lasers, can sort six different types of cell per pass and can manage up to 70,000 sorting decisions per second (MoFlo Astrios™, Beckman Coulter). In theory, through the application of sequential sorting, higher orders of separation can be achieved. The MoFlo Astrios™ instrument has a sort purity of < 99% and a 90% of theoretical sort yield; viability was also shown to not be influenced by the sorting technique (Davies, 2012). Recently significant advances in optics, detectors, and software have allowed for a significantly larger number of colors to be analyzed (> 80 colors) (Nolan et al., 2013, Nolan et al., 2012, Nolan and Sebba, 2011). Although still one of the most highly-used cell isolation platforms, a serious limitation to FACS systems remains their price and complexity. A typical FACS instruments can cost upwards of $250 K for three-channel sorting and over $1,000 K for a seven-channel sorting instrument. Furthermore, the complexity of operation requires dedicated highly-trained personnel to ensure reliable cell sorting efficiency and purities. FACS systems are susceptible to cross-contamination, clogging in the nozzle and require high reagent consumption. Additionally, these high-end systems only deliver limited throughput for direct separation of rare cells from whole blood, requiring hundreds of hours to sort the billions of red blood cells present in a sample tube. This limitation is addressed, in part, through the application of lysis or sample pre-treatment to facilitate sample analysis time of a few hours or minutes by first removing the red blood cells from the sample.
2.1.3.3 Magnet-Activated Cell Sorting (MACS)
In 1977, Rembaum and co-workers (Molday et al., 1977) introduced a novel immunomagnetic technique, now commonly known as magnetic-activated cell sorting (MACS). In the field of cell separation, MACS (Miltenyi et al., 1990) is one of the most standard separation techniques, harnessing functional micro- or nanoparticles that are conjugated with antibodies corresponding to particular cell surface antigen. Extensive detail of MACS is provided in Section 4.0 of this review article that addresses specific cell-separation platforms. Magnetic cell isolation platforms can utilize either an intrinsic magnetic character (e.g., the iron-containing hemoglobin in erythrocytes ((Melville et al., 1975b, Melville et al., 1975a)) a topic discussed in Section 5.2, or can utilize extrinsic magnetic character (e.g., cells labeled with magnetic nanoparticles). Under application of a magnetic field gradient, the magnetically targeted cells can be separated in either a positive or a negative fashion with respect to the particular antigen employed. As outlined earlier, this type of technique typically requires a relatively large volume, a few milliliters, of suspension. Maximum flow rates within a magnetic sorting device are limited by the achievable magnetic field strength as well as the magnetic response of the cell. In many early MACS devices, cell suspension flow rates were limited to about 1 mm s-1 that provided a rates of few hundred microliters per hour, not practical for a clinical application. In recent years, more sophisticated configurations have been employed that now allow processing of sufficient volumes of cell suspension in shorter time periods. It has been demonstrated that current MACS platforms can provide extremely high cell purities (> 95%) at high throughput (~1010 cells/hr), presenting a more cost-effective ($10 K vs. $250 K) device option as compared to fluorescence-activated cell sorting (FACS) methods (Thiel et al., 1998).
More recently, magnetic cell sorting techniques have been successfully integrated with microfluidic techniques (Pamme, 2006, Yun et al., 2013, Radisic et al., 2006, Pamme, 2007). For example, a microfluidic MACS system was developed to sort target cell types in the continuous flow-manner (Adams et al., 2008b, Plouffe et al., 2011a, Plouffe et al., 2012). By employing either permanent magnets (Adams et al., 2008b) or electromagnets (Plouffe et al., 2012), cells of interest can be rapidly isolated (> 250 μL/min) from large sample volumes (> 10 mL). More recently, while most magnetic cell manipulation techniques utilize labeling methods that allow magnetic nanoparticles to bind to antigens on the cells, a new label-free separation strategy has been illustrated that relies upon magnetic nanoparticles internalized within the cells of interest. This technique exploits the different internal absorption capacity of cells (known as endocytosis) with the result that monocytes with low absorption capacity and macrophages with high absorption capacity were successfully separated via on-chip magnetophoresis. From this study, it is demonstrated t hat cells can be internalized by different amounts of magnetic nanoparticles according to their own capacity (Robert et al., 2011). It should be noted, though, that internalization of magnetic particles has been shown to adversely influence cellular function (Liu et al., 2013, Pisanic II et al., 2007, Sharifi et al., 2012, Soenen and De Cuyper, 2010) and is not the most favorable methodology for cell labeling.
Techniques that rely upon magnetic forces to manipulate cells are not limited to those that use particles as the magnetic source (Sofla et al., 2013). The intrinsic magnetic properties of select cells allow for label-free manipulation without the potential interference of attached particles. As an example, the presence of hemoglobin in erythrocytes enables the ability to isolate erythrocytes from leukocytes by the application of high magnetic fields (Melville et al., 1975b, Melville et al., 1975a, Han and Frazier, 2004, Han and Frazier, 2005, Han and Frazier, 2006b). Furthermore, under an exposure to a high magnetic field, it is disclosed that the migration velocity of erythrocytes tends to increase with increased concentration of intrinsic magnetic content (Zborowski et al., 2003) – a property that has allowed for separation of malaria-infected red blood cells from healthy red blood cells (Kim et al., 2012, Nam et al., 2013, Paul et al., 1981a)
Target cell concentration via isolation is another important application enabled by magnetic cell manipulation techniques. For example, circulating tumor cells (CTCs) were separated from blood cells using a microfluidic device consisting of a single inlet/outlet that was placed alongside magnet (Kang et al., 2012). In another manifestation, target CTCs conjugated with magnetic nanoparticles in a blood sample are trapped at the bottom of a microchannel that is integrated with a permanent magnet (Hoshino et al., 2011). Along similar lines, microfluidic devices containing magnetic micropillar structures can be used to capture specific target cells (Liu et al., 2009c). One example is a microfluidic device that featured a strong induced magnetic field derived from an array of hexagonal nickel micropillars captured target cancer cells for subsequent on-chip sample preparations (Liu et al., 2007). While it is true that in-situ analysis can be performed with high sensitivity using small sample volumes in a complex manner in lab-on-a-chip devices that employ magnetic cell separation, this technology is still limited by time-consuming and labor-intensive procedures such as magnetic bead labeling (Whitesides, 2006).
2.2 Major Advantages of Magnet-Activated Cell Sorting (MACS) Relative to Other Techniques
Compared with other cell enrichment methodologies, an immunomagnetic approach that combines magnetic forces with biochemically-labeled magnetic nanoparticles to direct cell motion in a sample has several advantages that make it especially suitable for targeted rare cell separation. As magnetic separation platforms harness the unique ability to control cells from a distance, MACS is traditionally considered a user-friendly method to separate target cells. A few figures of merit that distinguish MACS from other cell separation modalities are described below:
-
(a)
Selectivity: similar to adhesion-based approaches, magnet-based separations have good sensitivity that arises from robust antibody–antigen binding between the cell and the magnetic particle label.
-
(b)
Specificity: using magnetic force as the retaining force, an immunomagnetic assay fosters good contrast between target and non-target cells in terms of the surface attachment. Towards the end of the cell separation process, it is possible to apply a high shear stress to the sample during flushing to remove the non-target cells from the suspension, leaving behind enriched cells of interest.
-
(c)
Throughput: in comparison to cell-affinity chromatography, where direct contact between cells and surface molecules is essential for successful cell capture, magnetic assays can attract cells over a wider spatial domain. In this scenario, the separation throughout is not compromised by larger separation chamber spaces and higher flow rates (up to tens of ml h−1).
-
(d)
Tunability: compared to techniques that feature a fixed filtration structure or surface molecule immobilization, the magnetic field component of MACS can be easily and accurately controlled, especially when an electromagnet is used as the magnetic field source. The field intensity and flux distribution can be optimized for specific cell types and the magnetic tag properties based on models when possible.
-
(e)
Integration: a magnetic field acts at a distance and can be introduced without direct contact with cells. Furthermore, the MACS separation platform can be integrated easily with other separation methods. Recent, work by Toner and co-workers illustrated that a magnetic separation platform can be integrated with a size-based cell separation approach to increase the target cell separation efficiency by removing red blood cells (RBCs) and platelets in advance (Ozkumur et al., 2013, Karabacak et al., 2014).
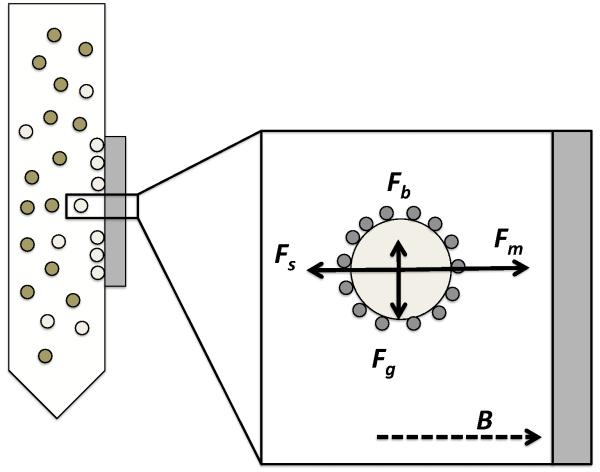
3. Magnetophoretic Cell Separation: Theory and Phenomena
Magnetic forces are unique in that they allow action at a distance, providing the ability to control objects without external wires or contacts. While not the only force that acts at a distance, i.e. gravity, electric forces, optical forces, and acoustics, the magnetic force underlying magnetic cell separation provides for action at a distance based on cell-marker affinity. While the intertwined fields of magnetism and magnetic materials are immense and very old, they have expanded to include biomedicine only rather recently (Krishnan, 2010, Murthy, 2007, Frimpong and Hilt, 2010, Mout et al., 2012, Pankhurst et al., 2009, Roca et al., 2009). The phenomenon of magnetophoresis is the controlled migration of particles, in this case biological cells, upon the application of an inhomogeneous magnetic field. Magnetophoresis may be employed to separate out specific cells from a heterogeneous cell population, with high selectivity, high sensitivity, and good throughput. In this section a brief overview of phenomena and terminology of relevance to magnetophoretic cell separation is provided. This section describes the categories of magnetic materials, the governing forces responsible for the separation and isolation of a target cells population, and the materials and methods choices that impact the overall operation of the desired platforms. More detailed information magnetic force theory and magnetophoretic principles may be found in a selection of excellent textbooks (Aharoni, 1996, Coey, 2010).
3.1 Magnetic Phenomena for Cell Sorting: Allowing Specific Action at a Distance
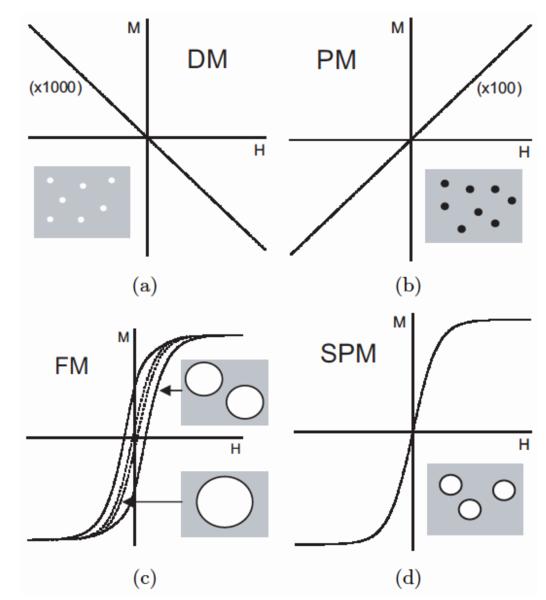
Among all functional materials, magnetic materials are singular by virtue of their ability to transfer energy and force through air, vacuum or intervening materials without wires or contacts. This property bestows these materials with a key technological role to enable devices of all types. In particular the magnetic force is well suited to many non-invasive biomedical applications through the phenomenon of magnetophoresis, which is the basis of magnetic-activated cell sorting. The magnetic response of materials systems with relevance to biological magnetophoresis and cell sorting may be classified into four main categories: diamagnetism, paramagnetism, ferromagnetism and superparamagnetism. The last category of superparamagnetism is of paramount significance in cell sorting applications. This overall categorization scheme describes responses derived from the fundamental electronic structure of the atomic (electronically-localized systems) or collective (electronically-itinerant systems) constituents of the materials under examination in a magnetic field; simple examples are provided in Table 2, with graphical representatives of their field-dependent character provided in Figure 4. As biomaterials are typically non-metallic, the origins of magnetic phenomena described here are ascribed to the number and configuration of electrons in matter, with the intrinsic angular momenta associated with unpaired electrons donating a magnetic moment that determines the magnitude of the functional response. Depending upon the conditions of the biological system to be probed, the phenomenon of magnetic hysteresis may become important. Magnetic hysteresis, whether it is found in thermal cycles as thermal hysteresis or under cyclic applied magnetic field conditions as field hysteresis, signals irreversible processes within the system that may enhance or degrade functional effects.
Table 2.
Properties and examples of magnetic materials.
| Material Class | Examples | Typical (SI) χm | B-H relationship | Comments |
|---|---|---|---|---|
| diamagnetic | water | −l × 10−6 | linear (constant χm) | no hysteresis |
| paramagnetic | aluminum | 2 × 10−5 | linear (constant χm) | no hysteresis; becomes ferromagnetic below Curie temp |
| ferromagnetic | Iron | 3 × 103 | nonlinear (χm is f(B)) | shows hysteresis |
| superparamagnetic | Fe2O3 and Fe3O4 | 2.5 × 10−3 | nonlinear (χm is f(B)) | shows hysteresis |
Figure 4.
M – H curves for (a) diamagnetic, (b) paramagnetic, (c) ferromagnetic and (d) superparamagnetic beads. Adapted from (Pankhurst et al., 2003).
When a material is placed in a magnetic field , a magnetization (magnetic moment per unit volume) is induced in the material which is related to by the relationship , where χ is the volumetric magnetic susceptibility. In SI units1, the magnetic susceptibility represents a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field, with the direction and magnitude of χ indicative of the class of magnetic material (as described in Table 2). In the absence of an existing magnetic moment, it should be noted that the magnetic field can be related to the magnetic flux density by the relationship , where μ is the magnetic permeability. The magnetic permeability is directly related to the magnetic susceptibility by μ = μ0(1 + χ), where μ0 is the permeability of vacuum. Overall, magnetophoresis is driven by the magnetic flux density, rather than magnetic field strength, and thus this relationship play an important role in MACS systems.
Diamagnetism describes the negative, typically linear magnetization response, or negative magnetic susceptibility, upon application of a positive magnetic field. The origin of this effect is the pairing of all electrons to create a completed electronic shell configuration within the atoms comprising the material. This effect is nearly independent of temperature, and when the magnetic field is removed, the magnetic moment of the system becomes zero. All materials exhibit small but finite diamagnetism; as biologically-relevant materials are usually formed from organic compounds or structures with closed-shell electronic configurations, diamagnetism is an important consideration. Under the application of low fields, diamagnetic effects are generally sufficiently small to be neglected for the purposes of cell separation, although an example of where diamagnetism can be harnessed for red blood cell isolation is described in Section 5.2.
Paramagnetism provides an opposite response in materials to the application of a magnetic field; it describes the positive linear magnetization response upon application of an external positive magnetic field to systems of atoms or materials that contain unpaired electrons in their electronic structure. These unpaired electrons are characterized by a non-zero spin angular momentum that results in electronic magnetic moments or “spins”. In the absence of an applied magnetic field, paramagnetic materials have zero magnetization as the spins have negligible interelectronic correlation. Upon application of an applied magnetic field, the unpaired electron spins (and the resulting magnetic moment) align along the direction of the external magnetic field. The paramagnetic susceptibility, which describes the magnitude of the response of a paramagnetic spin to an applied magnetic field, is constant, positive and small, on the order of 10-4 to 10-5.
Ferromagnetism is a quantum-mechanical manifestation of a Coulombic-type of interelectronic interaction and is identified by the existence of a spontaneous magnetic moment in the absence of a magnetic field. Ferromagnetic effects occur in a subset of materials that contain unpaired electrons with a a non-zero spin angular momentum that results in the formation of electronic magnetic moments or “spins”. The wavefunctions associated with these electrons of finite spin are correlated to provide interatomic magnetic coupling — the so-called “exchange coupling” — and associated spin alignment that donates a volume magnetization to the material. The unpaired electron spins (and the resulting magnetic moment) align in the same direction as an external applied magnetic field and remain aligned in the absence of a magnetic field to produce ferromagnetism. Ferromagnetism is temperature-dependent; the systems’ magnetic moment decreases with increasing temperature, typically in accordance with the Brillouin function and essentially disappears at the Curie temperature TC. (Coey, 2010).
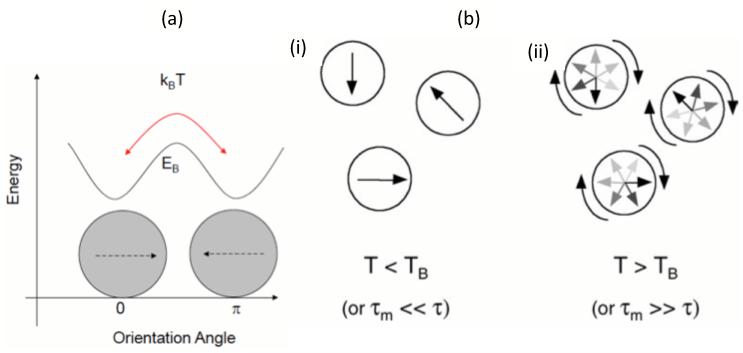
As a subclass of ferromagnetism, the phenomenon of superparamagnetism (SPM) may arise when a ferromagnetic material is subdivided into very small volumes such that magnitude of the volume magnetic energy is on the same order as that of the ambient thermal energy (Aharoni, 1996, Bean and Jacobs, 1956). In this circumstance the ensemble magnetic moment of the small volume cannot retain its physical direction in space due to thermal fluctuations. The magnetic entity or cluster behaves in a paramagnetic fashion, with a sharply increasing and linear response to an applied magnetic field but with an immense magnetic moment, hence the moniker, “superparamagnetism”. Superparamagnetic materials exhibit a large ferromagnetic response in the presence of a magnetic field but have zero response in the absence of a field. This property can be very useful in applications that require strong forces that can be “turned on and off” by a magnetic field, such as cell separation. Superparamagnetism arises by design in ferromagnetic nanoparticles with diameters that range from nanometers to a couple of tenth of nanometers, depending on the magnetic attributes of the specific material.
The relationship between the specific magnetic character and the size of the magnetic particle in magnetophoretic cell separation is very important. In all cases, the magnetic character of the particle must be superparamagnetic, to allow for selective motion of a particle under a magnetic field – in the absence of a magnetic field, superparamagnetic particles exhibit zero moment and thus are indistinguishable from other types of entities in a cell suspension. Ferromagnetic particles, on the other hand, maintain a permanent or spontaneous magnetization in the absence of an applied field. While the size of the magnetic entity itself must be in the superparamagnetic realm, the size of the particle that contains the superparamagnetic material is tailorable to meet specific applications. In some cases, the magnetic entity constitutes the magnetic particle itself, with diameters in the range 1-50 nm. In other instances, the magnetic particle is comprised of an encapsulated volume of superparamagnetic nanoparticles: in this instance the particle matrix, often consisting of a polymeric material, may have a diameter of 100 nm to 50 μm but will contain approximately 10 – 40 vol % superparamagnetic phase.
Applied Aspects of Superparamagnetism: The following is a brief exposition on the phenomenology and controlling parameters of superparamagnetism, as this type of magnetic behavior forms a framework for magnetic particle synthesis goals and selection, as well as for cell separation applications. In a simple approximation, the total magnetic moment of a superparamagnetic nanoparticle, typically comprised of 10,000 – 100,000 atoms that form the nanoparticle, can be regarded as one giant magnetic moment. The size range of superparamagnetic nanoparticles, with typical diameters in the range 1-50 nm (See Table 2), is smaller than or comparable to that of biological entities, such as cells (10-100 μm) and proteins (5-50 nm). A useful characteristic of superparamagnetic particles is that they exhibit a large magnetic moment and a high susceptibility in the presence of a small field; upon removal of the field they ideally release all residual magnetization – similar to paramagnetism; hence the nomenclature, “superparamagnetism”. In the zero-field condition, superparamagnetic nanoparticles exhibit zero coercivity, i.e. zero resistance to magnetic reversal, which makes them highly manipulateable. The sum total of these properties makes magnetic nanoparticles the best candidate for cell separation applications in magnet-based cell separation platforms.
The superparamagnetic state is distinguished by its thermal response. The thermal stability of the vector ferromagnetic moment of an ensemble of uniform spherical magnetic nanoparticles, each comprised of n atoms with the moment magnitude mn, such that , be quantified by the thermal relaxation time τ. The thermal relaxation time describes the average time the ensemble magnetic moment at a given temperature T reverses from one direction in space to another over a uniaxial activation energy barrier EB:
| Eq.(1) |
where 1/τo is the reversal attempt frequency and EB is equal to K·V, where K is the effective uniaxial magnetocrystalline anisotropy energy per volume, an intrinsic attribute of the material, and V is the particle volume.
As illustrated in Figure 5, for measurement times τm <<τ, the average time between magnetic reversals is much larger than the measurement time. This condition allows the magnetic particle to reside in a well-defined, quasi-stable state that is referred to as the blocked state of the system (Figure 5). In the blocked state, the particles exhibit a finite coercivity (i.e., resistance to magnetic reversal). For measurement times τm >>τ, the average time between magnetization reversals is much smaller than the measurement time; in this circumstance the measurement probes a magnetically-fluctuating state of different unresolved magnetization spin directions. In the absence of an external magnetic field, a time-averaged net magnetic moment of zero magnitude is measured and the system is thus in the superparamagnetic state. As indicated in Eq. (2), the temperature at which the ambient thermal energy becomes equal to the volume magnetic anisotropy energy is defined as the blocking temperature:
| Eq.(2) |
It can be seen that the superparamagnetic blocking temperature provides an intuitive indication of the size of the nanoparticles, by virtue of the intrinsic magnetocrystalline anisotropy constant: for a given nanoparticle composition, a lower blocking temperature implies a smaller nanoparticle radius. To a first approximation, two magnetic states of the ensemble can be distinguished as follows: the block state is defined as τm << τ or T < TB; the superparamagnetic state is defined as τm >> τ or T > TB. In practice, distributions of nanoparticle sizes and compositions inevitably result in distributions of blocking temperatures that can create a broad blocking temperature profile, which must be considered when magnetic nanoparticles are incorporated into applications.
Figure 5.
(a) Schematic of the energy barrier (EB) required for the magnetic moment to flip between their easy axis (b) Illustration of particles in a (i) quasi-stable blocked state with a fixed coercivity behaving in a pseudo-ferromagnetic state and (ii) an unblocked freely rotating state, where the magnetic coercivity is rotating randomly making the net magnetic moment to be zero. Adapted from (Pankhurst et al., 2003).
3.2 Governing Forces in Magnetic-Based Cell Separation
In this section the governing forces responsible for the motion of a spherical magnetic particle traveling through a medium under the influence of an applied magnetic field will be described. In this manner the mechanism of magnetically-activated cell sorting, with cells attached to magnetic particles flowing in a device and diverted to specific selection channels, will be developed. Biochemical aspects of cell-nanoparticle attachment and detachment will be addressed in Section 3.3.
The magnetic particle under consideration possesses a finite magnetic moment, has zero electric charge and possesses density ρp, radius Rp, volume Vp=4/3πR3, with mass mp=ρp·Vp. Under the influence of an applied magnetic field, the particle seeks to reduces its energy by moving towards the magnetic field source. The trajectory of motion of this particle is governed by the interaction of a number of forces and phenomena including (a) the magnetic force , due to all magnetic field sources, (b) the fluidic drag force , (c) forces describing particle-fluid interactions (i.e., perturbations to the flow characteristics), (d) inertia, (e) buoyancy (f) gravitational forces , (g) thermal kinetic energy (Brownian motion), and (h) interparticle effects that include magnetostatic (i.e., dipole-dipole) interactions (Furlani, 2010). Employing classical Newtonian dynamics , the total force balance on the particle may be written as:
| Eq.(3) |
where is the acceleration experienced by a particle traveling with . Unless the particles under consideration have diameters less than a few tens of nanometers, particle diffusion due to Brownian motion may be neglected (Sinha et al., 2007). Gerber and coworkers (Gerber et al., 1983) formulated a criterion to estimate the diameter (Dp) in which Brownian motion influences magnetic manipulation:
| Eq.(4) |
where |F| is the magnitude of the total force acting on the particle, kB is Boltzmann’s constant, and T is the absolute temperature. For example, Fe3O4 particles in water has a critical diameter of Dp = 40 nm. For particles with diameters smaller than Dp, one needs to solve an advection-diffusion equation for the particle volume concentration. For detail on solving these problems see the initial work by Gerber et al (Gerber et al., 1983) and Fletcher (Fletcher, 1991). Additionally, the inertial force contribution to the total force balance is often ignored in situations that involve submicron-sized particles, as their small mass makes this force negligible relative to other forces acting on a given particle (Sinha et al., 2007, Sinha et al., 2009). Under these conditions, the trajectory of the particle motion at constant velocity may be determined from a simple force balance:
| Eq.(5) |
(For the case of a large cell or particle in motion, the inertial term may need to be included; this case will be explored later in this section)
In the following sections these individual force terms will be examined in more detail and first-principle calculations of the motion of a single magnetic particle in these conditions will be described. These results may be directly applied to the conditions underlying magnet-based cell separation. Additionally, although less significant, the effects of additional forces (such as gravitational forces and Coulombic forces) will also be described below.
Magnetic Forces
A brief review of elements of magnetic vector theory will facilitate understanding of how a magnetic field may be used to manipulate magnetic nanoparticles in a fluidic system. Advanced details are available in an excellent review (Zborowski, 1997). A magnetic field gradient is required to exert a force at a distance; a uniform field gives rise to a torque, but does not contribute to translational action. The magnetic force acting on a point-like magnetic dipole moment of magnitude in a magnetic field gradient is given by:
| Eq.(6) |
In the case of a magnetic nanoparticle residing in a diamagnetic medium, the total magnetic moment of the particle is which depends both on the volume of the particle (Vp) and on the volume magnetization of the particle . Assuming uniform magnetization, and the magnetization is a linear function of the field intensity up to saturation, , where Δχ is the difference in magnetic susceptibility between the particle (χp) and the surrounding medium (χmed). Above saturation, , which is a case explored below in Eqs. (9-10). Specifically, below saturation, the overall response of a magnetic particle in a fluid to an applied magnetic field, as described earlier, is then determined by the strength and gradient of the applied magnetic field (; when ), yielding (Boyer, 1988, Lee et al., 2004):
| Eq.(7) |
where μo is the permeability of vacuum equal to .
As the magnetic susceptibility of the surroundings of an ensemble of magnetic particles is typically 5-6 orders of magnitude lower than that of the particles themselves (Pamme, 2006), Δχ is determined primarily by the susceptibility of the magnetic particle, χp. By way of example, the magnetic susceptibility of phosphate buffer saline is on the order of 10-7 and that of blood is on the order of 10-6, while the susceptibility of commercial magnetic oxide particles is generally on the order of 100 – 10-1 (Hayes, 1914, Melville et al., 1975b).
The above equation Eq. (7) is directly proportional to the square of the magnetic flux field as well as directly proportional to particle-specific terms (i.e. volume and susceptibility), confirming intuitive conclusions that more force is necessary to direct larger particles of comparable magnetic susceptibilities. In a standard commercial magnetic cell separation system, the applied magnetic field magnitude, and hence the available force for separation, is determined by the equipment; the operator often has little influence over this parameter. On the other hand, the magnetic particle component attributes can be easily improved via implementation of beads with a larger magnetic moment.
To label a specific cell with magnetic particles, as described in Section 3.3, the magnetic nanoparticle or microbeads are typically conjugated to antibodies that target a specific cell surface marker. Therefore, the magnetic force equation Eq. (7) must be somewhat modified to reflect the tagging of a single cell with a number of individual, much smaller, magnetic particles:
| Eq.(8) |
where β is the number of magnetic particles on the cell surface that are conjugated to the desired antibody, n is the number of cell surface markers targeted, θ in the fraction of the cell surface marker bound and λ is the number of antibodies that can bind to a specific cell-surface marker (McCloskey et al., 2000).
Eqs. (7-8) apply below saturation, but many magnetic separators operate in the saturation region and thus the magnetic force equation must be augmented to account for this case. When the particle is saturated, , both conditions of saturation and below saturation must be accounted for by expressing magnetization as:
| Eq.(9) |
where
| Eq.(10) |
and . As noted earlier the definition that links can be used to rewrite these equations in terms of . More details on the magnetic force equations for superparamagnetic particles in separation are described by Furlani (Furlani, 2010).
Viscous Forces
In addition to the magnetic force acting on the magnetic particles due to the presence of a magnetic field gradient, there exists a viscous drag force acting on the particle in the direction opposite to the particle motion (Bird et al., 2002). This drag force, or Stokes force , is a function of the suspension medium viscosity (η), the radius of the particle (Rp), and the relative velocity of the particle in the direction of the magnetic force versus the carrier fluid (i.e. Δν):
| Eq.(11) |
To correlate the viscous force to a cell moving in a carrier fluid it can be assumed that the radius of the cell (Rc) is significantly larger than those of the bound particles (Rp), Fig. 6, and thus this term dominates the drag force resistance and that the velocity of interest is that of the cell body .
Figure 6.
Schematic representation of the dominant forces in immunomagnetic cell separation system. Cell manipulation is a function of magnetic force, gravitational force, buoyancy force and hydrodynamic (Stokes’ drag) force. To achieve successful cell isolation, the magnetic force must be greater than the opposing drag force and able to overcome the gravitation and buoyancy forces.
Other Forces in Magnet-Based Cell Separation
In addition to the magnetic and viscous drag forces, gravitational forces exist and act on the cell during the separation process. The gravitational force and the buoyance forces, respectively, are given by:
| Eq.(12) |
| Eq.(13) |
where ρc and ρf are the densities of the cell and the fluid, respectively, and g = 9.8 m s-1 is the acceleration due to gravity. These forces are often ignored for scenarios that depict the actions of submicron and nanoscale particles employed in magnetophoretic separation processes, as they are usually much weaker than the magnetic force. However, in the case of larger particles, such as magnetic microbeads employed in some cell separation platforms (with Rc > 5μm), the buoyancy force is on the same order of magnitude (in the range of 10 – 100 pN) as the gravitational and buoyance forces and thus cannot be neglected. Eq. (14) describes the equation of motion detailing the separation forces appropriate to large cells of sufficient mass to contribute a finite inertial force term to the separation dynamics. Inclusion of the cell inertial term yields the overall force balance:
| Eq.(14a) |
| Eq.(14b) |
where Eq. (14a) and Eq. (14b) represent the case where the particle is below saturation and at saturation, respectively. Although cell properties, such as mc, Vc, and ρc, are inherit properties, these relationships demonstrates that the overall force balance may be easily tuned via changes in particle properties (Vp, χ, Ms), particle chemistry (λ, β), the magnetic source (), and in the fluid properties (η, ρf), and thus magnet-based sorting serves as a robust separation platform for targeted cell enrichment. By rational selection of particle characteristics, antibody choices, flow rates, magnetic field generator, and fluid characteristics one can optimize a magnet-based separation platform without significantly instrumentation changes.
A final force that contributes to the overall motion of a magnetic particle or magnetic particle-decorated cell under the influence of an applied magnetic field is derived from the electrostatic forces inherent in the system. It is well know that the surfaces of magnetic particles, cells, and separation devices normally possess a surface charge when residing in an electrolytic solution (e.g. buffers, cell culture medias, blood, and urine) (Stoker, 2011). The interaction between charged surfaces is described by the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory (Hiemenz, 1997). The DLVO force is simply the sum of the van der Waals force and the electrostatic force due to the so-called double layer of counterions, both of which are large when the interparticle separation is small or if particles are close to the surface of the cell separation device or container (distances in the vicinity of 10-9-10-10 m) (Hiemenz, 1997). As these forces are negligible at distances greater than several hundred angstroms, they are only rarely considered to be significant for cellular interactions (Bartlett, 2008).
3.3 Materials and Methods Considerations in Magnetophoretic Cell Separation
Once the forces that contribute to the motion of a magnetic particle or magnet particle-decorated cell within a suspension have been considered (Eq. (12)), parameters that control the particle motion can be identified and manipulated to optimize a magnet-based cell separation platform. These parameters include the choice of magnetic bead, including size, magnetic moment and density, the magnitude of the applied field and the source to provide the field, and the carrier fluid characteristics. The trade-offs in selection and tuning of each of these parameters is discussed in this section.
Magnetic Beads for Cell Separation Applications
The first parameter that will be examined in the context of optimizing magnetic cell separation platforms is the magnetic beads themselves, with factors such as materials selection, synthesis methods, and particle size to be considered. In addition to materials selection and synthesis techniques, coating of the magnetic bead to promote favorable chemistries to enhance application and improve functionality and usability is also described here.
As magnetic particles are intended for use in biological in vivo applications, there are strict governmental guidelines concerning the use of materials that are non-toxic (Soenen et al., 2011). Therefore the most widely-used in vivo magnetic nanoparticles are the iron oxides of magnetite Fe3O4 and maghemite γ-Fe2O3. However, pure ferromagnetic metals such as Fe, Ni and Co, oxide ferrites of the form MeO·Fe2O3 (where Me = Mg, Zn, Mn, Ni, Co, …), and compounds such as CoPt3 and FePt may also be used for in vitro applications, including cell separation, where somatic toxicity is not a concern (Pankhurst et al., 2003, Pankhurst et al., 2009). Magnetic nanoparticles typically range from 1–100 nm in diameter. However, larger particles with diameters of several hundred nanometers or even micron-scaled can be fabricated and biologically isolated by creating clusters of magnetic nanoparticles and encapsulating them in organic (e.g., polymeric) or inorganic coatings. Methods for synthesizing magnetic nanoparticles have evolved over several decades, and new methods continue to be developed and refined (Mok and Zhang, 2013, Wang and Su, 2011, Kolhatkar et al., 2013). There are two basic approaches to nanoparticle synthesis: the so-called “top-down” and “bottom-up” synthesis techniques.
The top-down approach involves the reduction of larger-scale matter to desired nanoscale dimensions, and is generally subtractive in nature. Top-down methods include photolithography, mechanical machining/polishing, laser beam and electron beam processing, and electrochemical removal (Thakkar et al., 2010). The top-down method starts from the bulk material, which is typically decomposed by mechanical influences into decreasingly smaller fragments. The resulting fragments have a typical diameter of about 100 nm and may exhibit a wide size distribution. Such an approach is usually not suitable for the large-scale manufacturing of particles that must possess a well-defined geometrical configuration and therefore has shown little promise in magnet-based cell separation motifs.
Utility of Nanoparticles versus Microparticles in Magnetic-based Cell Separation
Selection of particle size influences the dynamics of the cell separation process as well as the characteristics of the cells themselves. Due to the necessity of utilizing superparamgnetism for cell separation, two size ranges of particles are generally studied and reported. Research has focused most efforts on the fabrication and application of magnetic cell labels comprised either of individual nanoparticles (10 – 50 nm) or of microparticles consisting of individual superparamagnetic nanoparticles embedded in a larger particle body (1 – 100 microns). Therefore, research employing particles in the size range between these two categories, known as sub-micron particles, is far less common. Applications that require a large number of magnetic particles bound to a given cell surface require smaller particles. For applications that do not required extensive particle labeling, larger particles are needed. Performance and efficacy tradeoffs between smaller versus larger particles vary for specific applications. For example, the magnetic force exerted on a given magnetic particle by an external guiding magnetic field decreases linearly with the magnetization per particle, which is a product of the volume and the magnetization per volume of a given particle, as per Eq. (12). While the magnetic properties of magnetic particles can be improved by increasing their magnetic material content (such as polymer microparticles embedded with magnetic nanoparticles; synthesis described in Section 3.3), they become more prone to sedimentation in a carrier solution as the density of the particles increase. Thus a compromise between these two aspects must be considered to simultaneously optimize the specific cell-particle surface area and the magnetic response (Borlido et al., 2013). A third factor in the selection of magnetic particles for cell separation applications that has caused some debate amongst researchers and commercial particle vendors is the influence of particle size on cell fate and characteristics. It is well know that nanoparticles attached to cells will, over a span of a few hours to days, will be taken into the cell cytoplasm through a process called endocytosis (Iversen et al., 2011, Chalmers et al., 2010, Jing et al., 2008). This phenomenon results in the accumulation of a population of nanoparticles within the cells, affecting the internal biology of the cell. On the other hand, microparticles are too large for the endocytosis process to occur and thus the microparticles tend to remain on the cell membrane, potentially affecting post-process analysis. Much research has been conducted on the toxicity of magnetic micro- and nanoparticles (Sharifi et al., 2012, Soenen et al., 2011) with the generalized conclusion that the labeled cells’ viability is compromised at high particle concentrations (> 0.03 μg/mL) (Huang et al., 2008a) and long exposure times, in excess of 12 hrs. (Ge et al., 2009). Additionally, the intracellular presence of inorganic materials (noted for particle diameters < 100 nm) has been found to generate reactive oxygen species (ROS) (Soenen and De Cuyper, 2010, Shubayev et al., 2009). High ROS levels can damage cells by oxidizing cell membrane structures, disrupting DNA, disrupting gene transcription, modifying proteins and resulting in a decline in physiological function and in cell apoptosis/death (Sharifi et al., 2012, Liu et al., 2013). Labeling cells with larger microparticle have also produced decreases in cell viability (McGuckin et al., 2008, Tiwari et al., 2003), reduction in proliferation and in metabolic activity (Tiwari et al., 2003), and changes in gene expression (Woelfle et al., 2005).
Synthesis of Superpapamagnetic Particles
The most commonly-used methods for preparing magnetic nanoparticles involve some form of bottom-up chemical approach. In a bottom-up approach, elemental building blocks such as atoms, molecules or clusters are assembled into nanoparticles. This approach relies on the energetics of the process to guide the assembly. Over the last decades, much research has been devoted to the synthesis of magnetic nanoparticles. Especially during the last few years, many publications have described efficient synthetic routes to realize shape-controlled, highly stable, and monodisperse magnetic nanoparticles. Several bottom-up chemical methods including co-precipitation (Zhao et al., 1990, Chen et al., 1999, Lee et al., 2004), thermal decomposition (O’Brien et al., 2001, Sun et al., 2004, Redl et al., 2004), microemulsions (Carpenter et al., 1999), and hydrothermal reactions (Wang et al., 2005, Euliss et al., 2003) have shown promise as viable syntheses processes for magnetic nanoparticles. Instead of summarizing the results of this body of literature, which would by far exceed the scope of this review, a brief overview of each technique is provided here. The techniques of co-precipitation, thermal decomposition, microemulsion and hydrothermal synthesis are described in the following sections.
The chemical synthesis method of co-precipitation is the most facile and convenient way to synthesize iron oxide nanoparticles from an aqueous solution. Briefly, alkyline salt solutions containing a mixture of Fe2+/Fe3+ ions are precipitated under an inert atmosphere. The size, shape, and composition of the resultant magnetic nanoparticle suspension can be tuned via the type of salts employed (i.e. FeCl2/FeCl3, FeSO4/Fe(NO3)3, etc.), the reaction temperature, the pH value of the base and the ionic strength of the media (Lu et al., 2007). Major challenges in the synthesis of iron oxide nanoparticles using the co-precipitation method are control of the particle size and of the particle size distribution. Particles prepared by the co-precipitation technique unfortunately tend to be rather polydisperse. As the magnetic blocking temperature of a given particle is a function of the volume of the particle (Section 3.1), an inhomogenous particle size distribution will result in a wide range of blocking temperatures that provides non-ideal magnetic behavior for many applications. During the co-precipitation process, it is well known that a short burst of nanoparticle nucleation and subsequent slow controlled growth is crucial to the production of a population of monodisperse particles. Controlling nucleation and growth is therefore key for successful synthesis of a batch of monodisperse magnetic nanoparticles. Recently, several organic stabilizers and/or reducing agents have been used to improve nanoparticle size dispersity during synthesis. For example polyvinylalcohol (Lee et al., 1996b), citric acid (Bee et al., 1995), and oleic acid (Willis et al., 2005, Cushing et al., 2004) have all shown promise in controlling nanoparticle size and stabilizing the individual particle, thus preventing aggregation.
One important chemical synthesis method for the production of magnetic nanoparticles is the thermal decomposition of organometallic compounds in high-boiling organic solvents containing stabilizing surfactants (O’Brien et al., 2001, Sun et al., 2004, Redl et al., 2004). The thermal decomposition technique, which can produce fairly monodisperse products, is similar to the seed-mediated particle growth process understood within the context of the classical LaMer mechanism (LaMer and Dinegar, 1950). Briefly, under the LaMer mechanism, a short burst of seed nucleation from a supersaturated solution is followed by the slow growth of particles without any significant new nucleation; this feature allows decoupling of the particle nucleation and growth phases, fostering a high degree of size and composition uniformity (LaMer and Dinegar, 1950).
The reverse micelle method exploits the thermodynamically stable, isotropic dispersion of two immiscible liquids to form nanoparticles, where either or both liquids form microdomains that are stabilized by an interfacial film of surfactant molecules (Langevin, 1992). In reverse micelles, or water-in-oil microemulsions, the aqueous phase is dispersed as microdroplets (typically 1–50 nm in diameter) that are surrounded by a monolayer of surfactant molecules in the hydrocarbon phase. The size of the reverse micelle is determined by the molar ratio of water:surfactant, with higher surfactant ratios yielding larger particles (Paul and Moulik, 2001). By mixing two identical water-in-oil microemulsions containing the desired reactants, under the action of agitation microdroplets will continuously collide, coalesce, and break up again, and finally forming a precipitate within the micelles which constitutes the nanoparticle (Gupta and Gupta, 2005). Although many types of magnetic nanoparticles have been synthesized in a controlled manner using the microemulsion method, the resultant particle size and shapes usually vary over a relative wide range (1 – 100 nm). Moreover, the yield of nanoparticles is low compared to those obtained other from other nanoparticle synthesis methods, such as thermal decomposition and co-precipitation. Large amounts of solvent are necessary to synthesize appreciable amounts of material with the microemulsion technique, compromising efficiency and manufacturing scale-up.
The hydrothermal synthesis technique allows a wide variety of nanoparticulate shapes and compositions to be synthesized (Wang et al., 2005). Hydrothermal synthesis requires the combination of solid metal acid, an ethanol-linoleic acid liquid phase, and a water-ethanol solution under hydrothermal conditions. This nanoparticle synthesis strategy is based on a general phase transfer and separation mechanism that occurs at the interfaces of the liquid, solid and solution phases. As an example, Deng et al (Deng et al., 2005) utilized a hydrothermal synthesis method to fabricate monodispersed magnetic spheres of ferrite oxide with diameters in the range of 200 – 800 nm by tuning the chemical constituents of the reaction, which included FeCl3, ethylene glycol, sodium acetate, and polyethylene, at 200 °C for 8–72 h. In this work, ethylene glycol was used as a high-boiling-point reducing agent — known from the polyol process to produce monodisperse metal or metal oxide nanoparticles — and sodium acetate and polyethylene glycol were used as surfactant against particle agglomeration. While to date the mechanism of nanoparticle synthesis is not fully understood, the multicomponent approach seems to be powerful in directing the formation of nanoparticles (Lu et al., 2007).
Advantages and disadvantages of the four above-described synthesis methods are summarized in Table 3. Co-precipitation is the overall preferred nanoparticle synthesis method for ease of use and high throughput, but, in terms of size and morphological control of the resultant nanoparticles, thermal decomposition is the best the method developed to date. Due to the large amount of solvent needed for micelle formation, the microemulsion technique is not generally scalable nor is it cost effective. Finally, the hydrothermal synthesis route still requires far more experimental validation, although to date this technique seems be able to produce high-quality nanoparticles in medium bulk amounts, on the order of 0.1 to 1 kg. Overall most common methodologies for nanoparticle production are co-precipitation and thermal decomposition, as they are commercially scalable.
Table 3.
Comparisons of the Major Synthesis Method
| Method | Description |
Typical Reaction Temp. (°C) |
Reaction
period |
Solvent |
Particle Size
distribution |
Particle
Shape Control |
Yield |
|---|---|---|---|---|---|---|---|
| Co-precipitation | Very simple, ambient conditions |
20-90 | Minutes | Water | Broad | Not good | High |
|
Thermal
decomposition |
Complicated, inert atmosphere required |
100-320 | Hours to days |
Organic | Very narrow | Very good |
High |
| Microemulsion | Complicated, ambient conditions |
20-50 | Hours | Organic | Relatively narrow |
Good | Low |
|
Hydrothermal
synthesis |
Simple, requires high pressure |
220 | Hours to days |
Water- ethanol |
Very narrow | Very good |
Medium |
Ligand Exchange of Surface Chemistries for Use in Aqueous Systems
As a means of isolating individual nanoparticles within a suspension, many of the above-described synthesis techniques utilize stabilizers. These stabilizers ensure realization of a solution containing well-dispersed particles, but in many cases the stabilizers directly impede the use of labeling. As outlined above, biocompatible iron oxide nanoparticles can be made using an iron precursor, such as iron oleate (Zhao et al., 1990, Chen et al., 1999, Lee et al., 2004) heated together (250–350 °C) with a complexing agent in an organic solvent. Typically these particles are hydrophobic and are thus incompatible with many biomedical applications. Most biomedical applications using iron oxide nanoparticles, including cell separation, require water-dispersible and biocompatible systems (Kim et al., 2005, Plouffe et al., 2011b, Tartaj et al., 2003). To address this issue, hydrophobic particles must be modified for applications in aqueous systems by adding subsequent surfactants or adsorbing polymers to achieve a bilayer stabilization, or by undergoing a surface ligand exchange process (Michalet et al., 2005). Many stabilizing techniques to donate hydophilicity have been described in the literature (Hultman et al., 2008, Sun et al., 2004, Selvan et al., 2007, Yi et al., 2005, Sun et al., 2008b, Zhang et al., 2002, Zhang et al., 2004, Kohler et al., 2004, Aqil et al., 2008, Casula et al., 2010). This stabilization can have an effect on the surface of the magnetic particle themselves; recent research has shown that these exchanges of the stabilizing ligand can actually alter the magnetic moment of the nanoparticle (Crespo et al., 2004, Nagesha et al., 2009, Daou et al., 2008). This effect is hypothesized to be attributed to modification in the magnetically-inactive (“dead”) layer on the surface of the magnetic particle induced by ligand exchange. Therefore, it is critical to interrogate magnetic characteristics changes as a result of chemistries manipulations. Ultimately, exploitation of the encouraging properties of monodisperse iron oxide particles synthesized by high temperature methods (i.e. thermal decomposition) for applications in the life sciences is still limited by the fact that a surface ligand exchange process is often impeded by the strong binding carboxylic acid stabilizers used, such as oleic (Hyeon et al., 2001, Park et al., 2004) or stearic acids (Jana et al., 2004). In addition to stabilization, the ligand exchange may impart a functional group into the outer sphere of nanoparticles that permits surface functionalization with highly specific biomolecules (Sun et al., 2008a, Hainfeld and Powell, 2000).
Matrix Encapsulation of Magnetic Nanoparticles
An additional methodology for ensuring the attainment of biocompatible, water-soluble particles is the encapsulation of the magnetic nanoparticle within a polymeric matrix (Molday and Mackenzie, 1982, Roca et al., 2009, Tartaj et al., 2003, Tartaj et al., 2005). Several natural polymers have been successfully used in the formation of stable magnetic particles (An et al., 2003, Gupta and Gupta, 2005, Kim et al., 2003, Ma et al., 2007, Villanueva et al., 2009, Williams et al., 2006, Wotschadlo et al., 2009).Different combinations of styrene-sulfonic acid, vinyl-sulfonic acid, and acrylic acid have also been used to coated magnetic particles to donate a variety of electrostatic and hydrophobic surface properties (Ditsch et al., 2005). Despite the possibility to control polymer properties, homopolymers are still the most commonly used for coating (Lee et al., 1996a, Mendenhall et al., 1996, Zhang et al., 2010, Al-Deen et al., 2011). Overall, despite the simplicity of coating the magnetic nanoparticle core with a polymer, the particles that are thus formed, either as a single domain or as a cluster, tend to be irregular in shape and mechanically soft, making them sensitive to mechanical disruption. Ideally, particles should be spherical, as they possess better hydrodynamic properties and lower tendency to breakage due to mechanical stresses (Borlido et al., 2013). It is clear that the attributes of the cellular body generally dominate the system hydrodynamic properties. However, the mechanical robustness of spherical nano- or micro-beads is advantageous during the cell labeling protocol, where high mechanical forces can present problems with bead integrity. Furthermore, several polymers are only weakly bound to the magnetic cores, dissociating over time or under harsher conditions that are found in processing such as mixing, exposure to high or low pH environments, to ionic solutions, heat, and enzymes. To circumvent such problems a crosslinking agent (e.g. gluteraldehyde, epichlorohydrin) might be used for overall particle stabilization.
As a means to correct the irregular shape of most polymer-coated magnetic particles and to completely encapsulate the cores inside a polymeric matrix, several heterogeneous polymerization techniques have been used. These include suspension (Ma et al., 2005), emulsion (Liu et al., 2009b), microemulsion (Liu et al., 2006), miniemulsion (Dou et al., 2012, Liu et al., 2004, Zheng et al., 2005) and dispersion polymerization (Horák et al., 2004). The encapsulation of submicron magnetic particles within a polymer coating is usually done using the miniemulsion and emulsion polymerization techniques. Depending on the methods used and often on the experimental conditions employed, the morphology of the magnetic-polymer composites may vary from single or multiple dispersed magnetic cores enclosed in a polymeric matrix to particles with a polymeric core covered with magnetic particles. Each matrix composition will have its own unique advantages and disadvantages. Although all these technique start with a base suspension of superparamagnetic nanoparticles, once the nanoparticles are coated, the resulting coated particle diameters are generally sufficiently large to categorize them as microparticles, with diameters ranging from 100 nm to 5μm.
A completely different approach from those discussed earlier to obtain magnetic beads is to co-precipitate the magnetic oxide in the presence of porous polymer particles. In order to prevent the iron from leaching from the polymer matrix, a final polymeric layer is subsequently added to seal the beads. This approach was first introduced and patented by John Ugelstad in 1979 (Ugelstad et al., 1979) and is the basis of the commercially available Dynabeads® product line. While such particles have numerous advantageous properties, including monodispersity, mechanical robustness, and chemical resistance, their main disadvantage is the usually low amount of magnetite that is encapsulated (<15 wt%), resulting in rather weak magnetic beads.
Biofunctionalization of the Magnetic Particle for Cell Separation
Following nanoparticle synthesis and stabilization, the resulting particles must be functionalized with specific components, such as nucleic acids, peptides, or proteins, to provide biofunctionality. As introduced earlier, magnetic particles are functionalized with select biological agents as a means of targeting and isolated the desired cells of interest. Techniques that allow the modification of the beads and conjugation of the particles to the cell membrane rely on either chemical adsorption or on direct reaction of the molecule to the particle. These mechanisms can be broken down into three approaches: non-covalent interactions, nonspecific covalent conjugation, and selective, orientation-controlled conjugation.
Biofunctionalization via non-covalent assembly can be categorized into physical adsorption and affinity interactions (Ju et al., 2011). Many of the coating polymers and chemistries result in a charged surface on the particles, an effect which can be then harnessed for direct adsorption of the biological agent of interest. For example, a majority of proteins have a net positive charge, although some proteins have a net negative charge (Lodish et al., 2007), therefore a protein can be easily adsorbed to a particle surface simply by controlling electrostatic charge differences. The affinity interaction, on the other, conjugates ligands to particles by virtue of specific and strong complementary recognition interactions such as antigen–antibody and streptavidin–biotin.
The topic of bioconjugation is an exhaustive topic and thus only a few key chemistries will be discussed here; for a thorough background at current methodologies see the textbook by Hermanson (Hermanson, 1996) and Kumar (Kumar, 2005). Most biological target molecules are composed of amino acid or nucleic acid sequences and thus the reactive groups of interest are limited to hydroxyl (-OH), carboxyl (-COOH), thiol (-SH), and amines (-NH2). It should be noted that coating and encapsulation chemistries allow for a larger library of conjugation functionalities to be attached to the particles itself, for example it is known that poly(methacrylic acid) (Mendenhall et al., 1996) imparts carboxyl groups to the particle surface.
In general, three different conjugation techniques exist: zero-length, homobifunctional, and heterobifunctional cross-linking techniques. Zero-length conjugates rely on the formation of an intermediate that allows for the covalent binding of functional groups without the addition of a tertiary species between the initial starting chemistries. By contrast, both hetero- and homobifunctional crosslinkers form linker bridges between the two chemistries of interest. The most popular type of zero-length crosslinker for use in conjugating biological substances is based on carbodiimide chemistries, as they effectively form a linkage between carboxylic acids and amines, EDC [1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride]. It should be noted that the active intermediate formed in the chemistry is subjected to rapid hydrolysis in aqueous solutions (on the order of seconds); this property motivates the addition of NHS [N-hydroxysulfosuccinimide] to stabilize the intermediate species. EDC, with or without NHS, is overall the most popular approach used to achieve covalent conjugation of biomolecules to magnetic particles in the literature (Hermanson, 1996). The most common homobifunctional linker is glutaraldehyde, which crosslinks through the amine groups, i.e. both the primary and secondary molecules both must have a reactive primary amine. The chemistry forms a small five-carbon bridge between the particles and the protein. Several other similar bridging molecule are also available to bridge carboxylates, thiols, and hydroxyls (Hermanson, 1996). These chemistries can be accompanied by a high degree of polymerization, which can lead to beads becoming bound to one another through their amines and to proteins bound to each other without particle binding – both events can significantly reduce separation metrics. To better control the conjugation, heterobifunctional molecules can be used. As an example SPDP [N-succinimidyl 3-(pyridyldithio)propionate] can be used to bind amine containing biomolecules or beads to sulfhydryl (or thiol)-containing biomolecules or beads. These heterobifunctional crosslinkers can also allow for some control over molecule direction, thus reducing steric hindrance and increasing the probability of successful label of the particle to the cells. Summary: Based on a distillation of the information provided in Section 3.0, it is possible to articulate general design rules for the magnetic particles. Although many different synthesis techniques, treatments, and functionalization methodologies have been presented, key parameters that are required for the realization of effective magnetic-particle cell isolation platforms are:
Superparamagnetic behavior, whether manifest in separate nanosized particles or in micron-scale particles that contain a population of embedded nanoparticles;
Narrow particle size distribution and ideally a spherical shape;
High binding capacity (for proteins > 100 mg/g);
Low non-specific binding;
Physico-chemical robustness;
Minimal cell perturbations.
3.4 Selection of Magnetic Field Source to Achieve Magnetic Cell Separation
In addition to the type of magnetic tags attached to the cells of interest for separation, the magnetic field source for the apparatus, either permanent magnets or an electromagnet, must be considered. Aspects to be considered are the desired strength of the magnetic field, the need for variation of the field direction and the degree of sensitivity desired for the magnitude of the field, as well as the cost, the physical size of the overall device incorporating the magnetic field source, and infrastructure/power considerations. Both permanent magnets and electromagnetics, each with advantages and limitations, have been confirmed to provide the necessary magnetic field to facilitate efficient separation of various cell populations,. Electromagnets have two distinct advantages over designs that utilize permanent magnets. The first advantage is that those designs employing electromagnets can be easily switched on/off to facilitate cell capture and release: when the magnetic field is turned off, the superparamagnetic beads lose their magnetization and hence lose their attraction to a particular region of the device. The second advantage of electromagnets is that the strength of the resultant magnetic field may be tuned by varying the input current. In the microfluidic device context, electromagnets have seen limited use because they typically produce rather weak magnetic fields and their implementation into a device usually requires repeated lithographic steps during synthesis of each device. In addition, the bulkiness of the electromagnet and potential Joule heating derived from large currents flowing through the electromagnet coil can quickly become problematic. Other designs utilize permanent magnets, which have a set magnetic field strength and have limited control over the magnitude of the magnetic flux generation. Of course, permanent magnets are readily available and their implementation into a magnetic cell separation scheme is relatively simple. In contrast to the performance provided by electromagnets, which are traditionally located in a fixed location, merely moving the position of permanent magnets with respect to the flow pattern in a device can easily change the direction of the magnetic separation course. Overall, permanent magnets require no current source and thus have little infrastructure associated with their use, allowing them to be relatively easily transported and utilized.
3.5 Carrier Fluid Considerations
All magnetic cell-separation methods require a carrier fluid that provides a medium for the cells and particles to travel in. Effective separation of a population of target cells from the carrier fluid requires consideration of the overall dynamics of the particles within the solution. Particular fluid characteristics that are relevant to cell separation are density and viscosity. In most cases the carrier fluid is inert within the context of the magnetic separation, but there exist many special cases in which the carrier fluid has unique non-Newtonian properties that impact the dynamics of the particle/cell separation process. One case is that of a magnetizable carrier fluid, a situation that can result from an extreme excess concentration of magnetic beads within the system, basically turning the buffer solution into a ferrofluid. Another case where non-Newtonian properties must be considered is where the carrier fluid has a higher viscosity, creating a drag force on the particles that is higher than that exerted by traditional carrier fluid solutions; some examples of high-viscosity fluidic components include tissue digestates, dense protein solutions, and blood.
To address the added complexity of separating cells from a non-Newtonian liquid medium such as blood, the theoretical framework describing drag forces presented in Section 2.1.2 must be augmented to include high viscosity effects of the carrier fluid in which the target cells are located. This viscosity component (η) plays a critical role in the drag force experienced by the cell during magnetophoretic displacement. An additional effect that impacts the efficacy of cell separation from blood is the Fahraeus-Lindqvist effect (Fahraeus and Lindqvist, 1931), a microcirculatory phenomenon that leads to a reduction in the proportion of blood volume occupied by red blood cells (known as hematocrit, Hc) in small arterioles and capillaries of less than 200 microns. It should be noted that, in the circulation and in microchannels, it is not the hematocrit itself that causes this reduction in viscosity, but it is rather the migration of the red blood cells to the center of capillaries (leaving plasma behind at the channel walls) that underlies this viscosity change (Fahraeus and Lindqvist, 1931). Overall, these changes cause blood in a microcirculation system, such as in a microfluidic cell separation device, to exhibit a lower viscosity than that predicted by in vitro blood viscometer measurements. Therefore, the viscosity of blood is often quantified by a parametric relationship in comparison to the viscosity of plasma (ηplasma= 1.5 cP) as a function of channel height or diameter (hch, in microns) and hematocrit, Hc. This description was established by Pries et al. (Pries et al., 1996) from a collection of 18 separate studies of blood viscosity. Assuming velocities higher than 50 channel diameters (or heights) per second, the apparent viscosity of blood was empirically determined to be:
| Eq.(13) |
where:
| Eq.(14) |
| Eq.(15) |
Here, the variable η0.45 describes the apparent viscosity of at a phenotypical hematocrit (Hc=0.45) as a function of channel diameter (in microns) in the cell separation device.
3.6 Other Platform Considerations
In this review, several different tunable parameters and parametric choices that control the dynamics of the cell separation have been considered. The selection of the magnetic particle size (either nanometer or micrometer diameters), the particle synthesis method, and functionalization of the particle to target the cells of interest all play a role in how the applied magnetic force controls the particle trajectory in a device. Additionally, the selection of a permanent magnet or an electromagnetic source to supply the applied magnetic field controls the magnetic flux density and the magnetic forces within the system. The type of carrier fluid of the cell suspension impacts the viscous drag force that opposes particle motion driven by the applied magnetic field and thus is another important consideration for magnet-based cell separation.
In addition to these parameters there are additional factors that play a role in the magnetophoretic platform engineering. As the ultimate aim of many magnetophoretic devices is the application of cell separation in diagnostic and regenerative medicine, most carrier fluids are biological fluid sample analytes, such as blood, interstitial fluid, saliva, vaginal fluid or cellular material, or nasal fluid. As a means of minimizing contact with the analyte, or of contaminating further tests, the separation platform should be disposable in nature (Weigl et al., 2008, Fiorini and Chiu, 2005). Thus it is generally accepted that the fluidic components of a system are isolated from the re-usable magnetic components of the design. Additionally, most researchers would openly acknowledge that bench-space and a researchers’ time is always at a premium, thus the separation platform should be compact and user-friendly. For most researchers, the cell separation step is the first of many pre-process steps in a biomedical project and thus the cell separation device is not considered as a primary instrument analysis tool.
In magnetic separation, the variation of the magnetic field generates a force that varies with distance as shown in Eq. (7). The force is usually very strong at near the magnet itself, where the cells are collected and retained, but decreases quickly with distance. This leads to a few limitations that must be considered. First, although separation may sometimes work well in the initial scale, as soon as the sample is changed (e.g. different well, tube volume is use, flow rate of the sample) the magnetic force experienced by the cells is entirely different. The force over the farthest cells could quickly decrease when the distance from the farthest cell to the magnetic applied field is increase. Thus, increasing losses and separation time almost exponentially. Secondly, the force variation with distance leads to low force farther way and a high increase of the force when cells are approaching the retention area. This can lead to high risk of cell damage and reduced viability of the selected population. Basically, “crashing” into the entrapment area can compromise the cells. This is not a concern in displacement-based approachs. Finally, as the cells are relatively big compared with the magnetic beads, the force changes with the distance have other undesired effects. The different beads attached to the cell experience will experience different forces, both in magnitude and direction. That generates shear over the cell membrane, as each single beads is pulling with different force, with a risk of lysing during the process.
As a final consideration, because many of the separated cells are used for medicinal purposes, in the U.S. the Food and Drug Administration (FDA) regulates the entire cell separation process under the same rules that govern the production of pharmaceuticals; this set of rules is referred to a good manufacturing practice (GMP). Given the vast potential for such cell therapy, the involved instruments, reagents, and other cell separation components and equipment must adhere to GMP regulations, requiring that GMP-certified antibodies, beads, and materials are used in construction of cell separation platforms, and that the cell separation systems be completely closed, to prevent internal and external contamination.
4.0 Examples of Magnetic Particle-Based Cell Separation Platforms
Over the last 35 years, magnetic particles have become standard tools for the isolation of defined cell subsets in modern cell biology, immunology, and clinical medicine. In 1977, Molday et al. first published the use of iron-containing polymeric microspheres conjugated to lectins for the separation of red blood cells and antibody-coated cells (Molday et al., 1977). Today, magnetic cell sorting techniques are well established but improvements are continuing to be made to address ever-growing biomedical and biological needs in the clinic and in the lab. In the following section we outline some of the different approaches that are currently being used to separate target cells from a suspension, including conventional MACS technology, the quadruple high gradient system, and various microfluidic approaches in development. In addition to reviewing current standardized equipment, insight into improvements to enhance the efficacy and efficiency of cell separation will be introduced.
4.1 Conventional Magnet-Activated Cell Sorting (MACS) Systems
The MACS Technology system is a high-gradient magnetic cell separation device that is specifically engineered for the use of antibody-conjugated nanobeads (20 – 100 nm) (Grützkau and Radbruch, 2010). MACS was first developed by Miltenyi Biotec in Germany over 20 years ago (Miltenyi et al., 1990, Radbruch et al., 1994) and Miltenyi’s platforms remain the gold standard in magnetic separation today (Figure 3(d)). High-gradient magnetic cell separation columns are utilized for controlled trajectory of labeled cells in a magnetic field that is generated by a strong external magnet. A very strong magnetic field is necessary in this equipment, due to the small diameter of the labeling particles that only generate small magnetic forces. The separation columns are filled with a matrix of paramagnetic steel wool or with iron spheres, which focus the magnetic flux lines to induce strong magnetic fields (0.4 – 1.0 Tesla), which can attract cells that are weakly labeled with only a few attached magnetic nanoparticles (Radbruch et al., 1994). This dense packing also minimizes the distance of the cells from the applied field. Outside of the magnetic region, labeled cells are no longer retained labeled cells and are eluted as a purified cell solution. Both positive and negative separation approaches have shown promised with conventional MACS systems (Grützkau and Radbruch, 2010).
Figure 3.
There are three different labeling methods that are commonly used in magnetic cell separation (a) extrinsic magnetic bead labeling, (b) intrinsic magnetic moments, and (c) internalization of magnetic nanoparticles via cell encapsulation. (d–e) Examples of different magnet designs currently used for cell sorting. (d) Conventional MACS platform (e) standard quadrapole magnetic flow sorting and (f) deflection of magnetic moieties within a continuous stream flow stream (d) Adapted from (Miltenyi et al., 1990) (e) Adapted from (Nakamura et al., 2001) (a-c, f) Adapted from (Yun et al., 2013).
Miltenyi Biotech commercially provides manual and automated cell enrichment devices that are capable of processing up to 2 × 1010 cells within 5–30 min with enrichment rates that exceed 100-fold. Recently, Miltenyi released a multimagnetic device for simultaneous sample processing of up to eight samples (OctoMACS™), and a fully automated cell separator (autoMACS™ Pro) has been developed that allows up to six separations to take place in parallel. This system complements its current MultiMACS™ Cell24 Separator that handles up to 24 separations but at a lower volume capacity (15 mL versus 5 mL).
4.2 Quadrupole Magnetic Flow Sorter
In 1999, a new model of magnetic cell separation was introduced by Chalmer, Zborowski, et al. that utilized a first-principle-designed magnetic platform (Chalmers et al., 1999). The design, referred to as a quadrupole magnetic flow sorter (QMS), is a cell sorter with operation based on application of a high-gradient quadrupole magnetic field (Figure 3(e)). The magnetic force acting on magnetically-labeled cells in this quadrupole field has a centrifugal character that allows a continuous cell separation process. As described in Section 3.1, the interaction of the magnetic particle label with the external magnetic field is usually a highly complicated function of the spatial coordinates, an aspect which significantly constrains the number of magnetic field geometries suitable for magnetic flow cell sorting. Therefore, the proponents of QMS technology selected a quadrupole magnetic field that efficiently utilizes the available external magnetic field energy and has highly regular dependence of magnetic force on position. In QMS sorting, application of the concept of split-flow thin channel separation technology causes a sample stream to enter a vertical annular flow channel near the channel’s interior wall followed by another sheath flow entering near the exterior wall. A flow splitter initially separates the two flows. These flows pass through the bore of the magnet assembly; the magnetic field draws magnetized cells outward and deflects them into a positive outflow, while negative cells continue straight out via the inner flow lamina (Zborowski et al., 1999). QMS relies upon the cell magnetophoretic mobility, or the velocity of the cell per unit of magnetic force, to achieve cell separation (McCloskey et al., 2003). To date, the QMS technology has been used in a variety of research and clinical separation application. Recently, Chalmers and co-workers used a negative depletion technique to isolate circulating tumor cells from metastatic breast cancer patients. As the isolates were not labeled with beads, the authors illustrated that there was marker heterogeneity in the enriched populations (Wu et al., 2013). If a positive selection approach were used it would be impossible to probed such a vast cell population. In addition to breast cancer cells, QMS has been used for isolation of cancer cells from patients with head and neck cancer (Yang et al., 2009, Balasubramanian et al., 2012) and to separate islet cells for diabetes research (Shenkman et al., 2009). The same research group has illustrated that red blood cell removal, without magnetic tags, is possible using QMS (Moore et al., 2013, Moore et al., 2014, Jin et al., 2012, Zborowski et al., 2003). Using such a negative selection approach, hematopoietic stem cells have been enriched from blood (Jing et al., 2007, Tong et al., 2007, Schneider et al., 2010, Jin et al., 2012). Overall, the high throughput (> 106 cells/s) and high recovery capabilities (> 95%) of the QMS make it a strong magnetic separation technique (Zborowski and Chalmers, 2011, Moore et al., 1998, Schneider et al., 2006, Schneider et al., 2010).
4.3 Microfluidic Magnetic Cell Separation
As illustrated in the previous sections, there has been extensive research conducted in the field of magnet-based microfluidic cell separation device. This technology has offered numerous useful capabilities, such as the ability to use small quantities of samples/reagents, a short time for analysis, and laminar flow, which can provide for good control of the chemical environment (Whitesides, 2006). The combination of a cell manipulation technology and microfluidics offers several new tools and capabilities to benefit both fundamental biological research and clinical medicine. Nowadays, cell manipulation techniques combined with microfluidic technology play a critical role in various applications in cell biology, clinical research and biomedical engineering due to the ability to precisely control the cellular environment, to easily make heterogeneous cellular environments with multiplexing assay, and to analyze cellular information at a near single-cell level. Recently, various cell manipulation techniques based on different phenomena, including optical, magnetic, electrical and mechanical force, have been developed for applications in specific objectives in separating target cells from heterogeneous cell solutions (Bhagat et al., 2010, Liu et al., 2009a, Pamme, 2006, Yun et al., 2013).
Over the past decade, there have been numerous examples of microfluidic-magnetic cell separation devices described and applied. A few prominent examples of platforms that illustrate the advantages of microscale flow for magnet-based isolations are described here. In addition to the microscale channel dimensions providing an advantageous flow regime, the scaling down of ferromagnetic components in microfluidic devices either through the use of microfabricated permanent magnets or electromagnetic wires creates very large magnetic field gradients as compared with those present in macroscale analogs (Berger et al., 2001, Cugat et al., 2003). In one example, a microfluidic MACS system was developed to sort multiple target cell types in a continuous flow manner (Adams et al., 2008b, Inglis et al., 2004). By depositing magnetized metal paths into the flow pattern, magnetically-labeled cells could be spatially directed to specific outlets. Such a platform requires micrometer proximity between the cells and the deposited magnetic field paths. Several different displacement-based separation devices have also been described in the literature (Plouffe et al., 2011a, Plouffe et al., 2012, Shevkoplyas et al., 2007, Xia et al., 2006), which harness strong magnetic fields over short distances to manipulate cells of interest in high throughput flows (~15 mL/hr). Recently, a microfluidic-based magnetophoretic separation technique was demonstrated by using the inherent magnetic property of erythrocytes for directly and continuously separating erythrocytes and leukocytes from whole blood (Han and Frazier, 2004). As this particular mode of separation requires high magnetic fields, the small micron-scale size of microfluidic channels served as an excellent platform for such cell enrichments. More on the topic of label-free separation of blood cells can be found in Section 5.0. Another type of microfluidic device has been developed that generates a strong induced magnetic field mediated by an array of hexagonal nickel micro-pillars in the flow path. This device can capture target cancer cells by using on-chip sample preparation (Liu et al., 2007).
5.0 Non-magnetic particle-based cell separation techniques
In contrast to the current cell separation modes that involve labeling cells with magnetic bead tags, there are two methodologies under development that harness the same magnetic principles used to design magnetic-based cell separation, but employ a label-free approach. Each of these two methods employs the ability to control the magnetic response of a cell set using either a permanent magnet source or an electromagnetic field source to isolate target cells from a heterogeneous fluid mixture. The first method is a ferrohydrodynamic approach, whereby a concentrated solution of magnetic particles allows for manipulation of flow using traveling-wave magnetic field guidance. The ferrohydrodynamic approach exploits differences in cell size and shape, therefore is limited by the same shortcomings described previously in size-based cell separation. Many cells have comparable physical characteristics, thus separation via size and shape cannot generally be effectively utilized. The second technique separates cells based solely on inherit magnetic properties of the cells themselves. Only a few cells have inherit magnetism, thus, to date, only red blood cells and cardiomyocytes have shown any possibility of isolation. Most cells are inherently diamagnetic and thus an applied magnetic field universally displaces un-labeled cells away from the magnetic source (Melville et al., 1975b, Melville et al., 1975a). Magnetic manipulation can also be used to separate non-mammalian cells from a suspension, such as intrinsically-magnetic spores (Melnik et al., 2007).
5.1 Ferrohydrodynamic Cell Separation
To address the perceived limitation of pre-process labeling of a target cell population, Kose et al.(Kose et al., 2009) developed a novel ferrohydrodynamic separation platform that takes advantages of the mobility of magnetic particles versus non-magnetic cells, under the action of an applied AC magnetic field. Briefly, in this technique cells are moved via a “pushing” mechanism; as magnetic particles are moved to a predetermined location, cells are displaced from that location with a desired directionality. For a given particle or cell size, the velocity of movement depends on the local force and torque values along the channel length. At low AC magnetic field frequencies, the magnetic force dominates, pushing the nonmagnetic microparticles up to the ceiling of the channel and into the space between electromagnetic electrodes; at high AC magnetic field frequencies, the rolling microparticles can overcome the diminishing repulsion caused by magnetic force and move continuously along the channel. This rolling and trapping mechanism is dependent on the cell physical characteristics, including size, shape and elasticity. This technique allows for the separation of two or more cell types by trapping one population over a more mobile populations. This platform was tested first with samples of red blood cells and E. coli bacteria; a 95.7% separation efficiency with 76.1% purity was achieved. The authors then tested a more clinical sample of sickle cell red blood cells combined with healthy red blood cells and achieved a 75.2% recovery and a 89.3% purity. Although it is clear that magnetic particles control separation metrics, ferrohydrodynamic isolation is limited by the cell properties in a similar fashion as the size-based approach described earlier (Section 2.1.3.3).
5.2 Manipulation of Cells using Inherit Magnetic Properties
It has been shown that red blood cells (RBCs) have the characteristics of a paramagnetic fluid when deoxygenated (in veins) and are diamagnetic when oxygenated (in arteries) (Pauling and Coryell, 1936). This characteristic allows for a magnetic field to be used to easily separate RBCs from the whole blood. RBCs contain a molecule called hemoglobin, which is an iron-containing oxygen-carrier. Hemoglobin in the blood carries oxygen from the respiratory organs to the rest of the body. Hemoglobin releases the oxygen to burn nutrients to provide energy to power the functions of the organism and collects the resultant carbon dioxide to bring it back to the respiratory organs to be dispensed from the organism. Deoxygenated and reduced-oxide hemoglobin contains four and five unpaired electrons, respectively, making these species paramagnetic. Due to its covalent bonds, oxyhemoglobin has no unpaired electrons and is diamagnetic. These properties allow for the manipulation and control of RBCs under the influence of a magnetic field.
As early as 1975, Meville and coworkers (Melville et al., 1975a) illustrated the ability to directly separate red blood cells from a whole blood sample using a 1.75 T magnet field with an 8000 T m-1 field gradient. Red blood cells have a magnetic susceptibility of 3.86 × 10-6 for erythrocytes versus the magnetic susceptibility of water which is diamagnetic with a susceptibility that is approximately −1 × 10-6 (Zborowski et al., 2003). Using this large electromagnet, approximately 70% of the RBCs were retained within the column. Since 1975, several methods have been developed to reduce the magnitude of the applied magnetic field and gradient, and increase the recovery and purity of the RBCs efficiency (Al-Karmi, 2010, Chen et al., 2013, Furlani and Furlani, 2007, Han and Frazier, 2004, Han and Frazier, 2005, Han and Frazier, 2006a, Han and Frazier, 2006b, Jung and Han, 2008, Moore et al., 2013, Pauling and Coryell, 1936, Zborowski et al., 2003, Furlani, 2007). In order to reduce the magnetic field and gradient requirements, many groups have reduced the distance between the magnetic source and the blood sample, typically by using microfluidic channels or by changing the geometries of the overall design. Additionally, by changing from a trap-based cell isolation methodology many groups have illustrated that continuous, in flow, isolation reduces losses and increases efficiencies.
In addition to general blood fractionation, many researchers have recently demonstrated the utility of using a label-free magnetophoretic approach for the separation of malaria-infected RBCs (Moore et al., 2006, Nam et al., 2013, Paul et al., 1981b, Bhakdi et al., 2010, Ribaut et al., 2008, Bousema et al., 2004, Paul et al., 1981a). Malaria parasites live by feeding off the hemoglobin in RBCs. Through polymerization and oxidation, the parasites convert the hemoglobin, which is toxic to the parasites, into an insoluble crystal known as hemozoin. The iron (Fe3+) in hemozoin has a stronger paramagnetic character than the iron in hemoglobin (Fe2+). The presence of the hemozoin imparts a small and positive magnetic susceptibility of 1.88 × 10-6 to malaria-infected RBCs (Hackett et al., 2009), a value that is significantly higher than that of healthy RBCs (3.86 × 10-6). Therefore, infected RBCs usually behave as paramagnetic particles when exposed to a magnetic field (Bhakdi et al., 2010, Ribaut et al., 2008). Attempts to utilize the magnetic properties of malaria pigment, or hemozoin, for concentration and capture of malaria infected cells date back to 1946 when it was shown that positioning an electromagnet with a field strength of 0.5 T next to a tube containing a suspension of infected blood caused enrichment of the parasitized erythrocyte fraction from 0.17% to over 24% in the course of 6 to 12 h (1946). Improved results were reported in the late 1970s using the technique of high-gradient magnetic separation (HGMS) (Paul et al., 1981b, Melville et al., 1975a). Commercially-developed HGMS columns have been used more recently to synchronize or enrich in vitro human malaria parasite P. falciparum cultures or blood samples (Bousema et al., 2004) and murine malaria parasite P. berghei ookinetes for further in vitro studies (Miao and Cui, 2011). Current research has begun looking at microfluidic techniques to separate infected RBCs from the healthy RBCs (Kim et al., 2012, Nam et al., 2013); the microfluidic channel of these studies places the magnetic field source in close proximity to the blood, causing a strong localized field that improves separation and isolation of the parasitic RBCs.
Recently, Sofia et al. (Sofla et al., 2013) demonstrated that non-magnetic cardiomyocytes (CMs) could be rendered paramagnetic by treating the cells with a solution of NaNO2. They then reported enrichment results obtained from a microfluidic device that relies on the magnetic-force-based manipulation of the settling velocity of the cells. CMs were isolated with a purity of approximately 93 % and separation did not compromise cell viability or function. Overall, the ability to separate such sensitive cells in a label-free manner may lead to significant advances in heart tissue engineering.
6.0 Challenges and Opportunities for Further Research and Commercialization
Despite several decades of progressive development and improvement of the magnet-based cell separation, there remain several areas of research and development that are still ongoing. With the recent surge of interest and research in stem cells, magnetic cell separation techniques have had be re-engineered to address this niche market. The following section describes the current status of work in the areas of particle detachment, cell separation against multiple markers, and the isolation of rare cell population. We also aim to briefly describe the progress in commercialization of the magnet-based cell separation devices for research use and clinical laboratories, including how the different needs of both environments must be uniquely addressed.
6.1 Magnetic Bead Detachment From Cells
Cell isolation is the first of many steps in numerous laboratory workflows, and exposure of target cells to certain foreign substances, such as antibody-functionalized magnetic microbeads used in MACS systems, can influence the results of any downstream experiment or process. Furthermore, magnetic labeling of sensitive cell populations such as stem and progenitor cells is known to negatively impact viability, phenotypic identity, and function (Ugelstad et al., 1992, Farrell et al., 2008, Kostura et al., 2004, Mahmoudi et al., 2011). Overall, the ability to successfully release these magnetic tags from target cell populations following MACS separation has remained one of the largest challenges in the cell isolation field (Kohm et al., 2006, Stemberger et al., 2012, Knabel et al., 2002). Many research groups have attempted to circumvent this issue by using negative separation approaches (i.e. removal of all non-target cells), but such techniques have failed to achieve purities sufficient for clinical application (Zborowski and Chalmers, 2011, Kumar and Bhardwaj, 2008). Three commercial products currently exist that allow for capture and release of cells for application in magnetic separation: DETACHaBEAD®, FlowComp™ (Life Technologies), and Multisort (Miltenyi Biotec). Competitive binding assays, such as DETACHaBEAD and FlowComp, use a saturated protein solution to achieve cell capture/release that could have long-term adverse effects due to cells up taking the extraneous protein in solution. The need to utilize high concentrations of antibodies in these technologies significantly raises the cost of these approaches, making them prohibitive for routine use. Furthermore, these approaches may only work for certain cell types, as evidenced by the limited selection of kits offered by Life Technologies, namely for the targeted separation of T- and B-lymphocytes. On the other hand, Miltenyi Biotec’s product uses enzymes to cleave the bead from the cell of interest. Although Miltenyi has a wide assortment of beads available, these enzymes are naturally temperature- and pH-sensitive, traits which lead to the variable results from user to user (Cornish-Bowden, 2012). In addition, cellular viability is diminished in this technique due to long processing times (>1 hr) and enzyme retention is noted in the isolation process (Fujioka et al., 2003, Jung et al., 1995).
As an alternative to the above-described methods, a temperature-induced cell detachment method, based on the fact that the extracellular matrix generally prefers to adhere to a hydrophobic surface rather than to a highly hydrophilic surface, was also developed (Gurkan et al., 2011, Yamato et al., 2002, Yamato et al., 2001, McAuslan and Johnson, 1987). Although the need for less-invasive cell harvesting methods has been noted in the literature, only a few works, which either require electricity-induction (Yeo et al., 2001, Inaba et al., 2009, Wildt et al., 2010, Zhu et al., 2008), pH change-induction (Chen et al., 2012, Guillaume-Gentil et al., 2011), or light-induction methods (Hong et al., 2013, Pasparakis et al., 2011, Sada et al., 2011, Shin et al., 2011, Kolesnikova et al., 2012, Higuchi et al., 2004) have successfully addressed this issue. Only a few non-invasive cell detachment methods such as those that use aptamers (Wan et al., 2012, Zhang et al., 2012b, Zhu et al., 2012) or hydrogels (Plouffe et al., 2009a, Hatch et al., 2011, Hatch et al., 2012) have been shown to be successful for cell detachment. Although much progress has been made to overcome this need for viable, unperturbed cells, overall, the shortcoming of cell detachment still hinders a broader application of affinity techniques, specifically MACS.
6.2 Separation Against Multiple Markers
Separation of a specific target cell population may require a multiparameter magnetic cell sorting approach. Multiparameter magnetic cell sorting is the strategy for isolating target cells that cannot be defined by a single cell surface marker, but are defined by multiple cell surface antigens (Bosio et al., 2009). Using only magnetic separation, sequential isolation of even complex targets cells can be achieved, combining both depletion (negative) and positive selection steps. There are several different routes for multiparameter magnetic sorting, described as follows.
Commonly, a first step for multiparameter magnetic sorting is “debulking” of the starting cell population by using a panel of magnetic particles directed against the non-target cells, thus depleting the population for several markers simultaneously. It should be noted that removal of the non-target cell population can also be achieved through other separation modalities, such as density-gradient centrifugation (Axelsson, 2002) or size-based separation (Green et al., 2009, Inglis et al., 2011, Long et al., 2008, Zhang et al., 2012a). Non-target cell depletion may be followed by a positive selection for the cell of interest (Estes et al., 2009). The non-retained cells from the first separation are again magnetically labeled and enriched.
An alternative option is sequential positive selection (Bosio et al., 2009, Stemberger et al., 2012) which is accomplished by using colloidal superparamagnetic particles that are rapidly released from the cell. Although this technique is still somewhat immature, there is great potential in such a technique. Once the desired cells are separated from the heterogeneous suspension, the magnetic beads can be detached from the cells’ surface. These cells are then ready for further labeling and another separation cycle. Finally, the option of positive selection followed by depletion is also very attractive to achieve high purity isolations (Stemberger et al., 2012). In this application, cells are separated via magnetophoresis and then the magnetic particles are detached. The resulting cell suspension is then further purified using a negative depletion of the non-target cells that remain in the solution; this procedure greatly enhances purity without sacrificing recovery.
6.3 Isolation of Rare Target Cells
As our understanding of human biology advances, technological capability must likewise advance. As briefly described earlier, clinical applications are steadily progressing towards a customized patient treatment approach, i.e. towards “personalized medicine”. The “personalized medicine” approach increasingly requires effective isolation of specific target cells from each patient, whereby the cells of interest are often rare in number (< 1% of the total cell population in a given sample) (Bhagat et al., 2010, Miltenyi et al., 1990) and are present in a complex mixture of heterogeneous cells. Some examples of rare cells that have potential in the clinical setting include hematopoietic stem cells (HSCs) in blood (Prasongchean and Ferretti, 2012, Chun et al., 2011), circulating endothelial cells (CECs) in blood (Boos et al., 2006), and circulating tumor cells (CTCs) (Cristofanilli et al., 2004).
At its most basic level, cell separation requires a method to select for the targeted cell and a way to isolate the targeted cells from the surrounding cellular suspension. In the field of magnetic particle enrichment, the specificity of separation is directly correlated to the antibody selection; thus improvements in targeted antibodies may lead to better purities and recoveries (Zborowski and Chalmers, 2011). On the other hand, in pursuit of better control of labeled cells, microfluidic systems are under active development as an enabling technology for basic cell separation applications (as described in Section 4.3) (Zborowski and Chalmers, 2011, Pamme et al., 2006, Pamme and Wilhelm, 2006). Magnetic cell separations in particular are very effective when carried out in microfluidic devices as the device microchannels are amenable to the realization of very high field gradients on the microscale (Plouffe et al., 2011a, Sinha et al., 2007, Sinha et al., 2009, Furlani and Furlani, 2007, Furlani, 2007, Furlani and Ng, 2006, Furlani et al., 2007, Saud and Edward, 2013).
While there are many ways to identify and purity a cell, there is a limit to the number of physical or biochemical methods possible for separation of the targeted cell. As described in Section 2.0, numerous techniques are currently employed for cell isolation, each technique with its own specific advantages and shortcomings. Therefore, for the foreseeable future, optimal development of a separation technology for rare cells will most probably involve the combination of one or more of these separation techniques to purify samples. As examples, recent separation devices described by Chung et al. (Chung et al., 2013), Huang et al. (Huang et al., 2008b), and Ozkumur et al. (Ozkumur et al., 2013) utilized a hybrid microfluidic size-based and magnetophoretic separation modality for high efficiency separation of circulating tumor cells.
6.4 Translation of Magnetophoretic Technologies: Research Methods to Clinical Tools
As noted earlier, the presence and concentration of cells within a cell suspension has been shown to be a strong prognosticator of several different diseases (Cristofanilli et al., 2004, Boos et al., 2006, Goon et al., 2006, Krabchi et al., 2001). Additionally, the ability to extract or harvest these key cell types provides clinical researchers the ability to probe an individual patients’ unique disease case, leading to a more personalized medicine approach to treatment (Kim et al., 2013). Finally, isolation of a pure suspension of rare stem and progenitor cells is the first step in providing new-engineered tissues for organ replacement (Haraguchi et al., 2012). All of these biologic/biomedical needs have been continuously illustrated in the literature, but translation of magnetic separation tools to the clinical setting has been very difficult. One of the largest obstacles to clinical use of cell separation platforms is the ability to maintain sterility throughout the process. Secondly, achievement of approval for clinical application from governmental agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), can be a long and expensive process. That said, to date the only two FDA-approved cell separation platforms are immunomagnetic techniques: the CliniMACS® CD34 from Miltenyi and the CELLSEARCH® CTC Test from Janssen Diagnostics Inc. (formerly Veridex LLC). The CliniMACS® CD34 Reagent System was approved by the FDA in late January 2014 as a Humanitarian Use Device for the prevention of graft-versus-host disease (GVHD) in patients with acute myeloid leukemia (AML). Briefly, the CliniMACS® CD34 reagent system is based on the MACS technology described in Section 4.1. Hematopoietic stem cells derived from donor blood (after apheresis) are labeled with magnetic nanoparticles against the CD34 antigen and are positively separated through a sterilized steel wool matrix. This reagent system includes the CliniMACS Plus Instrument and the cGMP approved CD34 antibody reagents. The entire process is enclosed and isolated from the ambient environment and thus the separated cells have been proven to be a viable, clean, source of CD34+ stem cells for treatment. The CELLSEARCH® CTC Test was cleared by the FDA in 2004, 2007 and 2008 as an aid in the monitoring of breast, colorectal, and prostate metastatic cancer, respectively. The CELLSEARCH system operates in a manner similar to that of CliniMACS. CTCs, extracted from buffy coat isolates, are first labeled with nanoparticles against epithelial cell adhesion molecules (EpCAM) and are separated via application of a magnetic field. These cells are then fluorescently labeled with an antibody against a marker specific for the cancer cells, cytokeratin, and an antibody specific for white blood cells, CD45. Using sophisticated imaging techniques, the cancer cells are identified and separated from non-specific white blood cells to yield a cell number sufficient for diagnostic purposes. In comparison to the CliniMACS system, the CELLSEARCH separation tool does not need to maintain sterile conditions, as the isolation is for diagnostic rather than therapeutic purposes. In addition to U.S. federal agency approval, both platforms (CliniMACS® and CELLSEARCH®) have E.U. agency approval and are approved for use in China. Another cell separation platform (Parsortix from ANGLE plc), with an operation based on cell size attributes, has received EMA approval and is currently conducting clinical trials for FDA approval by the end of 2014. The Parsortix system is designed to separate CTCs from whole blood samples based solely on size, through the use of a sieve-based device. In contrast to the CELLSEARCH system, the Parsortix system does not require cells to be labeled with magnetic beads and/or fluorescent stains, resulting in a true living biopsy sample. Both EpCAM+ and EpCAM-cells can also be isolated, as no labeling is required, but it should be noted that not all CTCs have diameters that are larger than those characterizing the surrounding cells population (Maheswaran and Haber, 2010); this aspect limits the cancer cell types that can be targeted with this technique.. However, the unperturbed condition of the biosample is believed to facilitate a more personalized medicine approach to cancer treatment, via unique molecular analyses. It should be noted that these approved CTC platforms are for diagnostic use, an application that requires higher standards and strong evidence of low false-positive and false-negative readouts. Further, adoption of these complex systems in clinical diagnosis settings requires specialized technicians and training according to the Clinical Laboratory Improvement Amendments (CLIA) in the U.S.; ensuring high-quality standards for accuracy, reliability, and timeliness is obviously imperative. Additionally, for regulation of tissue products, the overseeing governmental agencies require that every step in the manufacturing process adhere to current GMP regulations. By mandating such regulations, only recently have current GMP cell separation platforms begun to emerge. As described in Section 1.4, the entire cell separation process must be kept sterile and closed off from the environment. Overall, the translation of research tools to clinically-relevant platforms is still limited in scope, and with only a few options for clinical tools there still remains a need for implementing regulatory requirements into the separation designs.
7.0 Conclusions and Future Directions
Cell separations using magnetic particles have become the most widely used and versatile method for the purification and isolation of key cell populations in a variety of biological fields. Since the initial reports in 1977 of polymeric, iron-containing beads as carriers for cell separation, there has actually been only minor advances in the field (Molday et al., 1977). The state-of-the-art technology continues to rely upon polymeric (either synthetic or natural) iron-oxide-containing particles for labeling of antigens, although the physical and magnetic susceptibility characteristics have significantly improved. Molday et al. placed a permanent magnet against the wall of a glass vial of magnetically labeled white and red blood cells; today, modern instruments employ insights gained from numerous iterations of magnetic manipulation techniques to realize a fully-automated separation platform with 10 – 100-fold greater yields versus that obtained from the first MACS-like device. Application of a permanent magnet and/or an electromagnet to predictably manipulate magnetized entities from a distance will allow cell separation platforms to continue to improve both as a research and a clinical tool. Nevertheless, much still remains to be done with regard to the development of high quality, high efficiency magnetic cell separators for the purification of therapeutic targets, namely in insuring product sterility. Although this current review has detailed the various merits of magnetic cell separation, it must be stressed that MACS will not exclusively be identified as the best methodology for separation. In applications where the labeling of the target cell population cannot be tolerated, such as in tissue engineering, other label-free approaches must be pursued.
In the forty-year history of MACS platforms, various approaches have been developed in parallel to increase cell isolation metrics. These approaches can be categorized into continuous displacement-based techniques and entrapment-based techniques. As described in this review, displacement separation features significantly higher throughputs but the resultant cell purities, due to the lack of a wash step, are generally low. On the other hand, entrapment techniques allow the cell population to be washed free of the non-specific cell populations, thereby increasing the purity but also increased the process steps, consequently lowering throughput. Many researchers are now turning to microfluidic-based cell separation techniques to address these limitations. As detailed earlier, microchannel devices allow for precise control of magnetically-labeled cells with minimal applied fields in the cell vicinity. This proximity of low field minimizes cell damage and decreases the time needed to move the distant cells to the collection zone – either by trapping or displacement. Several examples in the literature of magnetic microfluidic devices have reported high cell throughputs, competitive with state-of-the-art, accompanied by high purities and good efficiency for rare cells applications (Plouffe et al., 2012, Karabacak et al., 2014, Ozkumur et al., 2013, Kang et al., 2012, Yung et al., 2009). These microscale platforms are also inexpensive and are thus disposable. Such advantageous features indicate that the MACS field will, in the next decade, transition from large macroscale channels and magnets to feature microflows and miniaturization, allowing for adoption outside of the clinic and laboratory settings, i.e. in the developing world.
As more evidence is accumulated that exceptional cell separation tools are needed in the field, the specificity, selectivity, and throughput must continue to improve. This improvement will require the development of stronger targeted antibodies as well as better understanding of target cell antigen expressions.. With the rapid development of new discoveries in cell biology and production of new ligands (antibodies, peptides, aptamers, etc.), cell separation yields will concurrently advance. While the physics of magnetic separation is very well understood, and the chemistry and material science of magnetic beads is a mature and established field, the biochemistry and biology specific to separation is still a developing field.
On the whole, cell population purification is the first step in many complex biologic and biomedical workflows and thus improved cell enrichment processes will contribute to an overall simplified experimental protocol. To accomplish this goal, automation of the cell separation process must be improved, along with continued development of hands-free, user-friendly instrumentation. The most recent advances in sterile magnetic-based cell separators have been directly marketed and engineered for clinical use. These tools are poised to advance the field of diagnosis and therapeutic monitoring of numerous diseases, including cancer (Cristofanilli et al., 2007, Budd et al., 2006, Hayes et al., 2006) and cardiovascular disease (Damani et al., 2012). Additional, the ability to viably separate stem cell populations from patients will contribute to the new autologous therapeutics and faster recoveries from disease (Gordon et al., 2003, Schumm et al., 1999). With the recent release of these U.S. Federal Drug Agency-approved and European Medicines Agency-approve magnetic separators, it is anticipated that more economic analogs of these device for basic laboratory use to soon be developed. In summary, with all these developments it is predicted that MACS will continue to enable the unlocking of new biological discoveries, through improved drug delivery, proteomics, genomics, and other fundamental research
Acknowledgements
The authors gratefully acknowledge financial support from the National Institutes of Health through grant R01-EB009327 and the IGERT Nanomedicine and Science Program (NSF-DGE-0504331), and thank Dr. Richard Saferstein for support through the Saferstein Postdoctoral Fellowship.
Footnotes
Magnetic units can be complicated, with selected scientific and technical communities employing the International System of Units (SI or “rationalized” system; defining relationship is , where μ0 is 4π × 10-7 H/m) while others utilize the Gaussian/cgs system of units (defining relationship is ; variables are defined in the main text. In this review paper, SI units are employed. For more detail, see Bennett, L., Page, C. & Swartzendruber, L. 1978. Comments on units in magnetism. J. Res. NBS, 83, 9–12.
References
- [Accessed October 2013 2013];Lab on a Chip Themed Collections [Online] Available: http://pubs.rsc.org/en/journals/journalissues/lc-!themedcollections.
- [Accessed November 22, 2013 2013];The Cost of Cancer [Online] 2012 Available: http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/costofcancer.
- Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Gottert J, Mccarley RL, Nikitopoulos D, Murphy MC, Soper SA. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J. Am. Chem. Soc. 2008a;130:8633–8641. doi: 10.1021/ja8015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JD, Kim U, Soh HT. Multitarget magnetic activated cell sorter. Proc. Natl. Acad. Sci. U. S. A. 2008b;105:18165–18170. doi: 10.1073/pnas.0809795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A. Introduction to the Theory of Ferromagnetism. Clarendon Press; New York: 1996. [Google Scholar]
- Al-Deen FN, Ho J, Selomulya C, Ma C, Coppel R. Superparamagnetic Nanoparticles for Effective Delivery of Malaria DNA Vaccine. Langmuir. 2011;27:3703–3712. doi: 10.1021/la104479c. [DOI] [PubMed] [Google Scholar]
- Al-Karmi AM. Magnetic Properties of Human Erythrocytes. In: Lim CT, Goh JC, editors. 6th World Congress of Biomechanics. 2010. [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- Altelaar AFM, Heck AJR. Trends in ultrasensitive proteomics. Curr. Opin. Chem. Biol. 2012;16:206–213. doi: 10.1016/j.cbpa.2011.12.011. [DOI] [PubMed] [Google Scholar]
- An X, Su Z, Zeng H. Preparation of highly magnetic chitosan particles and their use for affinity purification of enzymes. J. Chem. Technol. Biotechnol. 2003;78:596–600. [Google Scholar]
- Aqil A, Vasseur S, Duguet E, Passirani C, Benoit JP, Roch A, Muller R, Jerome R, Jerome C. PEO coated magnetic nanoparticles for biomedical application. Eur. Polym. J. 2008;44:3191–3199. [Google Scholar]
- Augustsson P, Magnusson C, Nordin M, Lilja H, Laurell T. Microfluidic, Label-Free Enrichment of Prostate Cancer Cells in Blood Based on Acoustophoresis. Anal. Chem. 2012;84:7954–7962. doi: 10.1021/ac301723s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson H. Encyclopedia of Bioprocess Technology. John Wiley & Sons, Inc.; 2002. Cell Separation, Centrifugation. [Google Scholar]
- Balasubramanian P, Lang JC, Jatana KR, Miller B, Ozer E, Old M, Schuller DE, Agrawal A, Teknos TN, Summers TA, Lustberg MB, Zborowski M, Chalmers JJ. Multiparameter Analysis, including EMT Markers, on Negatively Enriched Blood Samples from Patients with Squamous Cell Carcinoma of the Head and Neck. PLoS ONE. 2012;7:e42048. doi: 10.1371/journal.pone.0042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber N, Gez S, Belov L, Mulligan SP, Woolfson A, Christopherson RI. Profiling CD antigens on leukaemias with an antibody microarray. FEBSLett. 2009;583:1785–1791. doi: 10.1016/j.febslet.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Bartlett PN. Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications. John Wiley & Sons; Hoboken, NJ: 2008. [Google Scholar]
- Bean CP, Jacobs IS. Magnetic Granulometry and Super-Paramagnetism. J. Appl. Phys. 1956;27:1448–1452. [Google Scholar]
- Bee A, Massart R, Neveu S. Synthesis of very fine maghemite particles. J. Magn. Magn. Mater. 1995;149:6–9. [Google Scholar]
- Bennett L, Page C, Swartzendruber L. Comments on units in magnetism. J. Res. NBS. 1978;83:9–12. doi: 10.6028/jres.083.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC. Random walks in biology. Princeton University Press; 1993. [Google Scholar]
- Berger M, Castelino J, Huang R, Shah M, Austin RH. Design of a microfabricated magnetic cell separator. Electrophoresis. 2001;22:3883–3892. doi: 10.1002/1522-2683(200110)22:18<3883::AID-ELPS3883>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bhagat A. a. S., Bow H, Hou HW, Tan SJ, Han J, Lim CT. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010;48:999–1014. doi: 10.1007/s11517-010-0611-4. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Ottinger A, Somsri S, Sratongno P, Pannadaporn P, Chimma P, Malasit P, Pattanapanyasat K, Neumann H. Optimized high gradient magnetic separation for isolation of Plasmodium-infected red blood cells. Malaria J. 2010;9:38. doi: 10.1186/1475-2875-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi DW, Mahr A, Zickwolf GK, Houseal TW, Flint AF, Klinger KW. Detection of fetal cells with 47, XY, +21 karyotype in maternal peripheral-blood. Human Genet. 1992;90:368–370. doi: 10.1007/BF00220460. [DOI] [PubMed] [Google Scholar]
- Bird RB, Stewart WE, Lightfoot EN. Transport Phenomena. John Wiley & Sons, Inc.; New York: 2002. [Google Scholar]
- Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GYH, Mancuso P, Sampol J, Solovey A, Dignat-George F. Circulating endothelial cells – Biomarker of vascular disease. Thromb. Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- Boos CJ, Lip GYH, Blann AD. Circulating Endothelial Cells in Cardiovascular Disease. J Am. Coll. Cardiol. 2006;48:1538–1547. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- Borlido L, Azevedo AM, Roque ACA, Aires-Barros MR. Magnetic separations in biotechnology. Biotechol. Adv. 2013;31:1374–1385. doi: 10.1016/j.biotechadv.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Bose S, Singh R, Hanewich-Hollatz M, Shen C, Lee CH, Dorfman DM, Karp JM, Karnik R. Affinity flow fractionation of cells via transient interactions with asymmetric molecular patterns. Sci. Reports. 2013;3:2329. doi: 10.1038/srep02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio A, Huppert V, Donath S, Hennemann P, Malchow M, Heinlein UO. Isolation and Enrichment of Stem Cells. In: Martin U, editor. Engineering of Stem Cells. Springer Berlin; Heidelberg: 2009. [DOI] [PubMed] [Google Scholar]
- Bousema J, Gouagna L, Drakeley C, Meutstege A, Okech B, Akim I, Beier J, Githure J, Sauerwein R. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malaria J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer TH. The Force on a Magnetic Dipole. Am. J. Phys. 1988;56:688–692. [Google Scholar]
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. T-Cell Subsets That Harbor Human Immunodeficiency Virus (HIV) In Vivo: Implications for HIV Pathogenesis. J. Virology. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Iyer RK, Radisic M. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol. Progr. 2008;24:907–920. doi: 10.1002/btpr.11. [DOI] [PubMed] [Google Scholar]
- Bruus H. Theoretical Microfluidics. Oxford University Press; Oxford: 2008. [Google Scholar]
- Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen L, Hayes DF. Circulating tumor cells versus imaging – Predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- Bull TM, Golpon H, Hebbel RR, Solovey A, Cool CD, Tuder RM, Geraci MW, Voelkel NF. Circulating endothelial cells in pulmonary hypertension. Thromb. Haemost. 2003;90:698–703. doi: 10.1160/TH03-04-0251. [DOI] [PubMed] [Google Scholar]
- Bunn HF. Mechanisms of disease – Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J. Am. Soc. Hypertens. 2012;6:85–99. doi: 10.1016/j.jash.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF, Large Scale Collab. Res, P. & Host Response To, I. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Carpenter EE, Seip CT, O’connor CJ. Magnetism of nanophase metal and metal alloy particles formed in ordered phases. J. Appl. Phys. 1999;85:5184–5186. [Google Scholar]
- Casula MF, Floris P, Innocenti C, Lascialfari A, Marinone M, Corti M, Sperling RA, Parak WJ, Sangregorio C. Magnetic Resonance Imaging Contrast Agents Based on Iron Oxide Superparamagnetic Ferrofluids. Chem. Mater. 2010;22:1739–1748. [Google Scholar]
- Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- Chalmers JJ, Xiong Y, Jin X, Shao M, Tong X, Farag S, Zborowski M. Quantification of Non-Specific Binding of Magnetic Micro- and Nanoparticles Using Cell Tracking Velocimetry: Implication for Magnetic Cell Separation and Detection. Biotechol. Bioengin. 2010;105:1078–1093. doi: 10.1002/bit.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JJ, Zhao Y, Nakamura M, Melnik K, Lasky L, Moore L, Zborowski M. An instrument to determine the magnetophoretic mobility of labeled, biological cells and paramagnetic particles. J. Magn. Magn. Mater. 1999;194:231–241. [Google Scholar]
- Chen JD, Chen D, Yuan T, Xie Y, Chen X. A microfluidic chip for direct and rapid trapping of white blood cells from whole blood. Biomicrofluidics. 2013;7:034106. doi: 10.1063/1.4808179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Rondinone AJ, Chakoumakos BC, Zhang ZJ. Synthesis of superparamagnetic MgFe2O4 nanoparticles by coprecipitation. J. Magn. Magn. Mater. 1999;194:1–7. [Google Scholar]
- Chen Y-H, Chung Y-C, Wang IJ, Young T-H. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials. 2012;33:1336–1342. doi: 10.1016/j.biomaterials.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Cheung MC, Goldberg JD, Kan YW. Prenatal diagnosis of sickle cell anaemia and thalassaemia by analysis of fetal cells in maternal blood. Nat. Genet. 1996;14:264–268. doi: 10.1038/ng1196-264. [DOI] [PubMed] [Google Scholar]
- Choi SY, Karp JM, Karnik R. Cell sorting by deterministic cell rolling. Lab Chip. 2012;12:1427–1430. doi: 10.1039/c2lc21225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Byun K, Lee B. Induced pluripotent stem cells and personalized medicine: current progress and future perspectives. Anat. Cell. Biol. 2011;44:245–255. doi: 10.5115/acb.2011.44.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Issadore D, Ullal A, Lee K, Weissleder R, Lee H. Rare cell isolation and profiling on a hybrid magnetic/sizeEsorting chip. Biomicrofluidics. 2013;7:054107. doi: 10.1063/1.4821923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coey JMD. Magnetism and Magnetic Materials. Cambridge University Press; Cambridge: 2010. [Google Scholar]
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen L, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- Collins FS, Morgan M, Patrinos A. The Human Genome Project: Lessons from LargeEScale Biology. Science. 2003;300:286–290. doi: 10.1126/science.1084564. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Fundamentals of Enzyme Kinetics. Wiley-VCH; Weinheim: 2012. [Google Scholar]
- Cowman AF, Crabb BS. Invasion of Red Blood Cells by Malaria Parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Crespo P, Litrán R, Rojas TC, Multigner M, De La Fuente JM, Sánchez-López JC, García MA, Hernando A, Penades S, Fernandez A. Permanent Magnetism, Magnetic Anisotropy, and Hysteresis of Thiol-Capped Gold Nanoparticles. Phys. Rev. Lett. 2004;93:087204. doi: 10.1103/PhysRevLett.93.087204. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Broglio KR, Guarneri V, Jackson S, Fritsehe HA, Islam R, Dawood S, Reuben JM, Kau SW, Lara JM, Krishnamurthy S, Ueno NT, Hortobagyi GN, Valero V. Circulating tumor cells in metastatic breast cancer: Biologic staging beyond tumor burden. Clin. Breast Cancer. 2007;7:471–479. [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen L, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Crosland-Taylor PJ. A device for counting small particles suspended in a fluid through a tube. Nature. 1953;171:37–38. doi: 10.1038/171037b0. [DOI] [PubMed] [Google Scholar]
- Cugat O, Delamare J, Reyne G. Magnetic microEactuators and systems (MAGMAS) IEEE Trans. Magn. 2003;39:3607–3612. [Google Scholar]
- Cushing BL, Kolesnichenko VL, O’connor CJ. Recent advances in the liquidE phase syntheses of inorganic nanoparticles. Chem. Rev. 2004;104:3893–3946. doi: 10.1021/cr030027b. [DOI] [PubMed] [Google Scholar]
- Damani S, Bacconi A, Libiger O, Chourasia AH, Serry R, Gollapudi R, Goldberg R, Rapeport K, Haaser S, Topol S, Knowlton S, Bethel K, Kuhn P, Wood M, Carragher B, Schork NJ, Jiang J, Rao C, Connelly M, Fowler VM, Topol EJ. Characterization of Circulating Endothelial Cells in Acute Myocardial Infarction. Sci. Trans. Med. 2012;4:126ra33. doi: 10.1126/scitranslmed.3003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou TJ, Grene X, Che JM, Pourroy G, Buathong S, Derory A, UlhaqEBouillet C, Donnio B, Guillon D, BeginEColin S. Coupling Agent Effect on Magnetic Properties of Functionalized MagnetiteEBased Nanoparticles. Chem. Mater. 2008;20:5869–5875. [Google Scholar]
- Davies D. Cell Separations by Flow Cytometry. In: Dwek M, Brooks SA, Schumacher U, editors. MetastasisResearch Protocols. Humana Press; New York: 2012. [Google Scholar]
- De Graaf IM, Van Bezouw S, Jakobs ME, Leschot NJ, Zondervan HA, Bilardo CM, Hoovers JMN. First-trimester non-invasive prenatal diagnosis of triploidy. Prenatal Diag. 1999;19:175–177. [PubMed] [Google Scholar]
- Dean WR. Fluid Motion in a Curved Channel. Proc. Roy. Soc. Lond. A Mat. 1928;121:402–420. [Google Scholar]
- Demircan Y, Ozgur E, Kulah H. Dielectrophoresis: Applications and future outlook in point of care. Electrophoresis. 2013;34:1008–1027. doi: 10.1002/elps.201200446. [DOI] [PubMed] [Google Scholar]
- Deng H, Li XL, Peng Q, Wang X, Chen JP, Li YD. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Edit. 2005;44:2782–2785. doi: 10.1002/anie.200462551. [DOI] [PubMed] [Google Scholar]
- Di Carlo D. Inertial microfluidics. Lab Chip. 2009;9:3038–3046. doi: 10.1039/b912547g. [DOI] [PubMed] [Google Scholar]
- Di Carlo D, Irimia D, Tompkins RG, Toner M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18892–18897. doi: 10.1073/pnas.0704958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didar TF, Tabrizian M. Adhesion based detection, sorting and enrichment of cells in microfluidic Lab-on-Chip devices. Lab Chip. 2010;10:3043–3053. doi: 10.1039/c0lc00130a. [DOI] [PubMed] [Google Scholar]
- Diller G-P, Thum T, Wilkins MR, Wharton J. Endothelial Progenitor Cells in Pulmonary Arterial Hypertension. Trends Cardiovasc Med. 2010;20:22–29. doi: 10.1016/j.tcm.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Ding XY, Lin SCS, Lapsley MI, Li SX, Guo X, Chan CY, Chiang IK, Wang L, Mccoy JP, Huang TJ. Standing surface acoustic wave (SSAW) based multichannel cell sorting. Lab Chip. 2012;12:4228–4231. doi: 10.1039/c2lc40751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditsch A, Laibinis PE, Wang DIC, Hatton TA. Controlled Clustering and Enhanced Stability of Polymer-Coated Magnetic Nanoparticles. Langmuir. 2005;21:6006–6018. doi: 10.1021/la047057+. [DOI] [PubMed] [Google Scholar]
- Dou J, Zhang Q, Ma M, Gu J. Fast fabrication of epoxy-functionalized magnetic polymer core-shell microspheres using glycidyl methacrylate as monomer via photo-initiated miniemulsion polymerization. J. Magn. Magn. Mater. 2012;324:3078–3082. [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Du Z, Colls N, Cheng KH, Vaughn MW, Gollahon L. Microfluidic-based diagnostics for cervical cancer cells. Biosens. Bioelectron. 2006;21:1991–1995. doi: 10.1016/j.bios.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- Estes M, Do J, Ahn C. On chip cell separator using magnetic bead-based enrichment and depletion of various surface markers. Biomed. Microdevices. 2009;11:509–515. doi: 10.1007/s10544-008-9257-5. [DOI] [PubMed] [Google Scholar]
- Euliss LE, Grancharov SG, O’brien S, Deming TJ, Stucky GD, Murray CB, Held GA. Cooperative assembly of magnetic nanoparticles and block copolypeptides in aqueous media. Nano Lett. 2003;3:1489–1493. [Google Scholar]
- Fahraeus R, Lindqvist T. The viscosity of the blood in narrow capillary tubes. Amer. J. Physiol. 1931;96:562–568. [Google Scholar]
- Farrell E, Wielopolski P, Pavljasevic P, Van Tiel S, Jahr H, Verhaar J, Weinans H, Krestin G, O’brien FJ, Van Osch G, Bernsen M. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem. Biophy. Res. Comm. 2008;369:1076–1081. doi: 10.1016/j.bbrc.2008.02.159. [DOI] [PubMed] [Google Scholar]
- Fiorini GS, Chiu DT. Disposable microfluidic devices: fabrication, function, and application. Biotechniques. 2005;38:429–446. doi: 10.2144/05383RV02. [DOI] [PubMed] [Google Scholar]
- Fletcher D. Fine particle high gradient magnetic entrapment. IEEE Trans. Magn. 1991;27:3655–3677. [Google Scholar]
- Frimpong RA, Hilt JZ. Magnetic nanoparticles in biomedicine: synthesis, functionalization and applications. Nanomed. 2010;5:1401–1414. doi: 10.2217/nnm.10.114. [DOI] [PubMed] [Google Scholar]
- Fujioka N, Morimoto Y, Takeuchi K, Yoshioka M, Kikuchi M. Difference in infrared spectra from cultured cells dependent on cell-harvesting method. Appl. Spectrosc. 2003;57:241–243. doi: 10.1366/000370203321535187. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Schmid-Schonbein GW. Centrifugation attenuates the fluid shear response of circulating leukocytes. J. Leukocyte Biol. 2002;72:133–139. [PubMed] [Google Scholar]
- Furlani EJ, Furlani EP. A model for predicting magnetic targeting of multifunctional particles in the microvasculature. J. Magn. Magn. Mater. 2007;312:187–193. [Google Scholar]
- Furlani EP. Magnetophoretic separation of blood cells at the microscale. J. Phys. D Appl. Phys. 2007;40:1313–1319. [Google Scholar]
- Furlani EP. Magnetic Biotransport: Analysis and Applications. Materials. 2010;3:2412–2446. [Google Scholar]
- Furlani EP, Ng KC. Analytical model of magnetic nanoparticle transport and capture in the microvasculature. Phys. Rev. E. 2006;73:061919. doi: 10.1103/PhysRevE.73.061919. [DOI] [PubMed] [Google Scholar]
- Furlani EP, Sahoo Y, Ng KC, Wortman JC, Monk TE. A model for predicting magnetic particle capture in a microfluidic bioseparator. Biomed. Microdevices. 2007;9:451–463. doi: 10.1007/s10544-007-9050-x. [DOI] [PubMed] [Google Scholar]
- Gagnon ZR. Cellular dielectrophoresis: Applications to the characterization, manipulation, separation and patterning of cells. Electrophoresis. 2011;32:2466–2487. doi: 10.1002/elps.201100060. [DOI] [PubMed] [Google Scholar]
- Ganshirt-Ahlert D, Borjesson-Stoll R, Burschyk M, Dohr A, Garritsen HSP, Helmer E, Miny P, Velasco M, Walde C, Patterson D, Teng N, Bhat NM, Bieber MM, Holzgreve W. Detection of fetal trisomy-21 and trisomy-18 from maternal blood using triple gradient and magnetic cell sorting. Am. J. Reprod. Immunol. 1993;30:194–201. doi: 10.1111/j.1600-0897.1993.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Gascoyne PRC, Vykoukal J. Particle separation by dielectrophoresis. Electrophoresis. 2002;23:1973–1983. doi: 10.1002/1522-2683(200207)23:13<1973::AID-ELPS1973>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Zhang Y, He S, Nie F, Teng G, Gu N. Fluorescence Modified Chitosan-Coated Magnetic Nanoparticles for High-Efficient Cellular Imaging. Nanoscale Res. Lett. 2009;4:287–295. doi: 10.1007/s11671-008-9239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geifman-Holtzman O, Bernstein IM, Berry SM, Holtzman EJ, Vadnais TJ, Demaria MA, Bianchi DW. Fetal RhD genotyping in fetal cells flow sorted from maternal blood. Am. J. Obstet. Gynecol. 1996;174:818–822. doi: 10.1016/s0002-9378(96)70306-x. [DOI] [PubMed] [Google Scholar]
- Gerber R, Takayasu M, Friedlaender FJ. Generalization of HGMS Theory – The Capture of Ultrafine Particles. IEEE Trans. Magn. 1983;19:2115–2117. [Google Scholar]
- Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Belmonte JCI. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat. Protocols. 2010;5:811–820. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, Giannakakou PA, Kirby BJ. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip. 2010;10:27–29. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomase VS, Kale KV, Tagore S, Hatture SR. Proteomics: Technologies for protein analysis. Curr. Drug Metab. 2008;9:213–220. doi: 10.2174/138920008783884740. [DOI] [PubMed] [Google Scholar]
- Goon PKY, Lip GYH, Boos CJ, Stonelake PS, Blann AD. Circulating Endothelial Cells, Endothelial Progenitor Cells, and Endothelial Microparticles in Cancer. Neoplasia. 2006;8:79–88. doi: 10.1593/neo.05592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PR, Leimig T, Babarin-Dorner A, Houston J, Holladay M, Mueller I, Geiger T, Handgretinger R. Large-scale isolation of CD133+progenitor cells from G-CSF mobilized peripheral blood stem cells. Bone Marrow Transpl. 2003;31:17–22. doi: 10.1038/sj.bmt.1703792. [DOI] [PubMed] [Google Scholar]
- Gossett D, Weaver W, Mach A, Hur S, Tse H, Lee W, Amini H, Di Carlo D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010;397:3249–3267. doi: 10.1007/s00216-010-3721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett DR, Di Carlo D. Particle Focusing Mechanisms in Curving Confined Flows. Anal. Chem. 2009;81:8459–8465. doi: 10.1021/ac901306y. [DOI] [PubMed] [Google Scholar]
- Green JV, Murthy SK. Microfluidic enrichment of a target cell type from a heterogenous suspension by adhesion-based negative selection. Lab Chip. 2009;9:2245–8. doi: 10.1039/b906768j. [DOI] [PubMed] [Google Scholar]
- Green JV, Radisic M, Murthy SK. Deterministic Lateral Displacement as a Means to Enrich Large Cells for Tissue Engineering. Anal. Chem. 2009;81:9178–9182. doi: 10.1021/ac9018395. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Milam M, Srere PA. Studies on cell adhesion II. Adhesion of cells to surfaces of diverse chemical composition and inhibition of adhesion by sulfhydryl binding reagents. Arch. Biochem. Biophys. 1972;153:193–198. doi: 10.1016/0003-9861(72)90436-5. [DOI] [PubMed] [Google Scholar]
- Group HER, Economics OOH, Europe R. Medical Research: What’s it worth? Estimating the economic benefits from medical research in the UK. UK Evaluation Forum; London: 2008. [Google Scholar]
- Grutzkau A, Radbruch A. Small but mighty: How the MACS®-technology based on nanosized superparamagnetic particles has helped to analyze the immune system within the last 20 years. Cytometry A. 2010;77A:643–647. doi: 10.1002/cyto.a.20918. [DOI] [PubMed] [Google Scholar]
- Guillaume-Gentil O, Semenov OV, Zisch AH, Zimmermann R, Voros J, Ehrbar M. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials. 2011;32:4376–4384. doi: 10.1016/j.biomaterials.2011.02.058. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust. Med. J. 1869;14:146–147. [Google Scholar]
- Gupta S, Feke DL, Manas-Zloczower I. Fractionation of mixed particulate solids according to compressibility using ultrasonic standing-wave fields. Chem. Eng. Sci. 1995;50:3275–3284. [Google Scholar]
- Gurkan UA, Anand T, Tas H, Elkan D, Akay A, Keles HO, Demirci U. Controlled viable release of selectively captured label-free cells in microchannels. Lab Chip. 2011;11:3979–3989. doi: 10.1039/c1lc20487d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett S, Hamzah J, Davis TME, St Pierre TG. Magnetic susceptibility of iron in malaria-infected red blood cells. Biochim. Biophys. Acta. 2009;1792:93–99. doi: 10.1016/j.bbadis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Hainfeld JF, Powell RD. New frontiers in gold labeling. J. Histochem. Cytohem. 2000;48:471–480. doi: 10.1177/002215540004800404. [DOI] [PubMed] [Google Scholar]
- Hamburg MA, Collins FS. The Path to Personalized Medicine. N. Engl. J. Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- Han KH, Frazier AB. Continuous magnetophoretic separation of blood cells in microdevice format. J. Appl. Phys. 2004;96:5797–5802. [Google Scholar]
- Han KH, Frazier AB. Diamagnetic capture mode magnetophoretic microseparator for blood cells. J. Microelectromech. Syst. 2005;14:1422–1431. [Google Scholar]
- Han KH, Frazier AB. Paramagnetic capture mode magnetophoretic microseparator for blood cells. IEEE Proc.-Nanobiotechnol. 2006a;153:67–73. doi: 10.1049/ip-nbt:20050019. [DOI] [PubMed] [Google Scholar]
- Han KH, Frazier AB. Paramagnetic capture mode magnetophoretic microseparator for high efficiency blood cell separations. Lab Chip. 2006b;6:265–273. doi: 10.1039/b514539b. [DOI] [PubMed] [Google Scholar]
- Hansmann G, Plouffe BD, Hatch A, Von Gise A, Sallmon H, Zamanian RT, Murthy SK. Design and validation of an endothelial progenitor cell capture chip and its application in patients with pulmonary arterial hypertension. J. Mol. Med. -JMM. 2011;89:971–983. doi: 10.1007/s00109-011-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y, Shimizu T, Yamato M, Okano T. Concise Review: Cell Therapy and Tissue Engineering for Cardiovascular Disease. Stem Cells Trans. Med. 2012;1:136–141. doi: 10.5966/sctm.2012-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N, Boltryk R, Glynne-Jones P, Hill M. A Novel Binary Particle Fractionation Technique. In: Garreton LG, editor. International Congress on Ultrasonics, Proceedings.2010. [Google Scholar]
- Hatch A, Hansmann G, Murthy SK. Engineered alginate hydrogels for effective microfluidic capture and release of endothelial progenitor cells from whole blood. Langmuir. 2011;27:4257–64. doi: 10.1021/la105016a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch A, Pesko DM, Murthy SK. Tag-Free Microfluidic Separation of Cells against Multiple Markers. Anal. Chem. 2012;84:4618–4621. doi: 10.1021/ac300496q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen L. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- Hayes HC. The Magnetic Susceptibility of Water. Phys. Rev. 1914;3:295. [Google Scholar]
- Heidelberger M, Mayer MM, Demarest CR. Studies in Human Malaria: I. The Preparation of Vaccines and Suspensions Containing Plasmodia. J. Immunol. 1946;52:325–330. [PubMed] [Google Scholar]
- Hemberger M. Health during pregnancy and beyond: Fetal trophoblast cells as chief co-ordinators of intrauterine growth and reproductive success. Ann. Med. 2012;44:325–337. doi: 10.3109/07853890.2012.663930. [DOI] [PubMed] [Google Scholar]
- Hermanson GT. Bioconjugate Techniques. Academic Press; Boston: 1996. [Google Scholar]
- Hertz CM, Graves DJ, Lauffenburger DA, Serota FT. Use of cell affinity chromatography for separation of lymphocyte subpopulations. Biotechnol. Bioeng. 1985;27:603–612. doi: 10.1002/bit.260270509. [DOI] [PubMed] [Google Scholar]
- Hiemenz PC. Principles of Colloid and Surface Chemistry. Dekker; New York: 1997. [Google Scholar]
- Higuchi A, Hamamura A, Shindo Y, Kitamura H, Yoon BO, Mori T, Uyama T, Umezawa A. Photon-modulated changes of cell attachments on poly(spiropyran-co-methyl methacrylate) membranes. Biomacromolecules. 2004;5:1770–1774. doi: 10.1021/bm049737x. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hong Y, Yu M, Weng W, Cheng K, Wang H, Lin J. Light-induced cell detachment for cell sheet technology. Biomaterials. 2013;34:11–18. doi: 10.1016/j.biomaterials.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Horak D, Lednicky F, Petrovsky E, Kapicka A. Magnetic Characteristics of Ferrimagnetic Microspheres Prepared by Dispersion Polymerization. Macromol. Mater. Eng. 2004;289:341–348. [Google Scholar]
- Hoshino K, Huang Y-Y, Lane N, Huebschman M, Uhr JW, Frenkel EP, Zhang X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip. 2011;11:3449–3457. doi: 10.1039/c1lc20270g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M, Weber C. Endothelial progenitor cells: Cellular biomarkers in vascular disease. Drug Discov. Today. 2008;5:e267–e271. [Google Scholar]
- Huang G, Diakur J, Xu Z, Wiebe LI. Asialoglycoprotein receptor-targeted superparamagnetic iron oxide nanoparticles. Int. J. Pharm. 2008a;360:197–203. doi: 10.1016/j.ijpharm.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Huang R, Barber TA, Schmidt MA, Tompkins RG, Toner M, Bianchi DW, Kapur R, Flejter WL. A microfluidics approach for the isolation of nucleated red blood cells (NRBCs) from the peripheral blood of pregnant women. Prenatal Diag. 2008b;28:892–899. doi: 10.1002/pd.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MP. Strategies for dielectrophoretic separation in laboratory-on-a-chip systems. Electrophoresis. 2002;23:2569–2582. doi: 10.1002/1522-2683(200208)23:16<2569::AID-ELPS2569>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hultman KL, Raffo AJ, Grzenda AL, Harris PE, Brown TR, O’brien S. Magnetic resonance imaging of major histocompatibility class II expression in the renal medulla using immunotargeted superparamagnetic iron oxide nanoparticles. ACS Nano. 2008;2:477–484. doi: 10.1021/nn700400h. [DOI] [PubMed] [Google Scholar]
- Huntly BJP, Gilliland DG. Cancer biology – Summing up cancer stem cells. Nature. 2005;435:1169–1170. doi: 10.1038/4351169a. [DOI] [PubMed] [Google Scholar]
- Hyeon T, Lee SS, Park J, Chung Y, Bin Na H. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 2001;123:12798–12801. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- Inaba R, Khademhosseini A, Suzuki H, Fukuda J. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials. 2009;30:3573–3579. doi: 10.1016/j.biomaterials.2009.03.045. [DOI] [PubMed] [Google Scholar]
- Inglis DW, Lord M, Nordon RE. Scaling deterministic lateral displacement arrays for high throughput and dilution-free enrichment of leukocytes. J. Micromech. Microengin. 2011;21:054024. [Google Scholar]
- Inglis DW, Riehn R, Austin RH, Sturm JC. Continuous microfluidic immunomagnetic cell separation. Appl. Phys. Lett. 2004;85:5093–5095. [Google Scholar]
- Iversen T-G, Skotland T, Sandvig K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today. 2011;6:176–185. [Google Scholar]
- Jana NR, Chen YF, Peng XG. Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem. Mater. 2004;16:3931–3935. [Google Scholar]
- Ji HM, Samper V, Chen Y, Heng CK, Lim TM, Yobas L. Silicon-based microfilters for whole blood cell separation. Biomed. Microdevices. 2008;10:251–257. doi: 10.1007/s10544-007-9131-x. [DOI] [PubMed] [Google Scholar]
- Jin XX, Abbot S, Zhang XK, Kang L, Voskinarian-Berse V, Zhao R, Kameneva MV, Moore LR, Chalmers JJ, Zborowski M. Erythrocyte Enrichment in Hematopoietic Progenitor Cell Cultures Based on Magnetic Susceptibility of the Hemoglobin. PLoS ONE. 2012;7:e39491. doi: 10.1371/journal.pone.0039491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Mal N, Williams PS, Mayorga M, Penn MS, Chalmers JJ, Zborowski M. Quantitative intracellular magnetic nanoparticle uptake measured by live cell magnetophoresis. FASEB J. 2008;22:4239–4247. doi: 10.1096/fj.07-105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Moore LR, Williams PS, Chalmers JJ, Farag SS, Bolwell B, Zborowski M. Blood progenitor cell separation from clinical leukapheresis product by magnetic nanoparticle binding and magnetophoresis. Biotechnol. Bioeng. 2007;96:1139–1154. doi: 10.1002/bit.21202. [DOI] [PubMed] [Google Scholar]
- Ju H, Zhang X, Wang J. NanoBiosensing. Springer; New York: 2011. Biofunctionalization of Nanomaterials. [Google Scholar]
- Jung J, Han KH. Lateral-driven continuous magnetophoretic separation of blood cells. Appl. Phys. Lett. 2008;93:223902. [Google Scholar]
- Jung K, Hampel G, Scholz M, Henke W. Culture of Human Kidney Proximal Tubular Cells-the Effect of Various Detachment Prodecures on Viability and Degree of Cell Detachment. Cell. Physiol. Biochem. 1995;5:353–360. [Google Scholar]
- Kang JH, Krause S, Tobin H, Mammoto A, Kanapathipillai M, Ingber DE. A combined micromagnetic-microfluidic device for rapid capture and culture of rare circulating tumor cells. Lab Chip. 2012;12:2175–2181. doi: 10.1039/c2lc40072c. [DOI] [PubMed] [Google Scholar]
- Kang N-Y, Yun S-W, Ha H-H, Park S-J, Chang Y-T. Embryonic and induced pluripotent stem cell staining and sorting with the live-cell fluorescence imaging probe CDy1. Nat. Protocols. 2011;6:1044–1052. doi: 10.1038/nprot.2011.350. [DOI] [PubMed] [Google Scholar]
- Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen P-I, Yang J, Hwang H, Morgan B, Trautwein J, Barber TA, Stott SL, Maheswaran S, Kapur R, Haber DA, Toner M. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protocols. 2014;9:694–710. doi: 10.1038/nprot.2014.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik R, Hong S, Zhang H, Mei Y, Anderson DG, Karp JM, Langer R. Nanomechanical control of cell rolling in two dimensions through surface patterning of receptors. Nano Lett. 2008;8:1153–1158. doi: 10.1021/nl073322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman KL, Belov L, Huang P, Mactier S, Scolyer RA, Mann GJ, Christopherson RI. An extended antibody microarray for surface profiling metastatic melanoma. J. Immunol. Med. 2010;358:23–34. doi: 10.1016/j.jim.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Kavanagh DM, Kersaudy-Kerhoas M, Dhariwal RS, Desmulliez MPY. Current and emerging techniques of fetal cell separation from maternal blood. J. Chromatogr. B. 2010;878:1905–1911. doi: 10.1016/j.jchromb.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Kim DK, Mikhaylova M, Wang FH, Kehr J, Bjelke BR, Zhang Y, Tsakalakos T, Muhammed M. Starch-Coated Superparamagnetic Nanoparticles as MR Contrast Agents. Chem. Mater. 2003;15:4343–4351. [Google Scholar]
- Kim J, Massoudi M, Antaki JF, Gandini A. Removal of malaria-infected red blood cells using magnetic cell separators: A computational study. App. Math. Comput. 2012;218:6841–6850. doi: 10.1016/j.amc.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Chen YF, Liu YC, Peng XG. Super-stable, high-quality Fe3O4 dendron-nanocrystals dispersible in both organic and aqueous solutions. Adv. Mater. 2005;17:1429–1432. doi: 10.1002/adma.200401991. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee S, Chen XY. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013;13:257–269. doi: 10.1586/erm.13.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B, Bernhard H, Wagner H, Busch DH. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat. Med. 2002;8:631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- Kohler N, Fryxell GE, Zhang MQ. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J. Am. Chem. Soc. 2004;126:7206–7211. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Mcmahon JS, Podojil JR, Begolka WS, Degutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 Monoclonal Antibody Injection Results in the Functional Inactivation, Not Depletion, of CD4+CD25+ T Regulatory Cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- Kohnke PL, Mulligan SP, Christopherson RI. Membrane proteomics for leukemia classification and drug target identification. Curr. Opin. Mol. Ther. 2009;11:603–610. [PubMed] [Google Scholar]
- Kolesnikova TA, Kohler D, Skirtach AG, Mohwald H. Laser-induced cell detachment, patterning, and regrowth on gold nanoparticle functionalized surfaces. ACS Nano. 2012;6:9585–9595. doi: 10.1021/nn302891u. [DOI] [PubMed] [Google Scholar]
- Kolhatkar AG, Jamison AC, Litvinov D, Willson RC, Lee TR. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013;14:15977–16009. doi: 10.3390/ijms140815977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose AR, Fischer B, Mao L, Koser H. Label-free cellular manipulation and sorting via biocompatible ferrofluids. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21478–21483. doi: 10.1073/pnas.0912138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JWM. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- Kraan J, Strijbos MH, Sieuwerts AM, Foekens JA, Den Bakker MA, Verhoef C, Sleijfer S, Gratama JW. A new approach for rapid and reliable enumeration of circulating endothelial cells in patients. J. Thromb. Haemostasis. 2012;10:931–939. doi: 10.1111/j.1538-7836.2012.04681.x. [DOI] [PubMed] [Google Scholar]
- Krabchi K, Gros-Louis F, Yan J, Bronsard M, Masse J, Forest JC, Drouin R. Quantification of all fetal nucleated cells in maternal blood between the 18th and 22nd weeks of pregnancy using molecular cytogenetic techniques. Clin. Genet. 2001;60:145–150. doi: 10.1034/j.1399-0004.2001.600209.x. [DOI] [PubMed] [Google Scholar]
- Krishnan KM. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. IEEE Trans. Magn. 2010;46:2523–2558. doi: 10.1109/TMAG.2010.2046907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni N, Dalal J. Dielectrophoresis: An Easy Approach for Biotrapping Saarbrucken. LAP LAMBERT Academic Publishing; Germany: 2011. [Google Scholar]
- Kumar A, Bhardwaj A. Methods in cell separation for biomedical application: cryogels as a new tool. Biomed. Mater. 2008;3:034008. doi: 10.1088/1748-6041/3/3/034008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Srivastava A. Cell separation using cryogel-based affinity chromatography. Nat. Protocols. 2010;5:1737–1747. doi: 10.1038/nprot.2010.135. [DOI] [PubMed] [Google Scholar]
- Kumar CSSR. Biofunctionalization of Nanomaterials. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- Kumar M, Feke DL, Belovich JM. Fractionation of cell mixtures using acoustic and laminar flow fields. Biotechnol. Bioeng. 2005;89:129–137. doi: 10.1002/bit.20294. [DOI] [PubMed] [Google Scholar]
- Lamer VK, Dinegar RH. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950;72:4847–4854. [Google Scholar]
- Langevin D. Micelles and Microemulsions. Annu. Rev. Phys. Chem. 1992;43:341–369. [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen YH, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JXJ, Evans S, Chien KR. Postnatal isl1+cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani M, Lu A, Thompson S, Robbins P, Huard J, Niedernhofer L. Isolation of Muscle-Derived Stem/Progenitor Cells Based on Adhesion Characteristics to Collagen-Coated Surfaces. In: Turksen K, editor. Stem Cells and Aging. Humana Press; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Bose S, Van Vliet KJ, Karp JM, Karnik R. Examining the Lateral Displacement of HL60 Cells Rolling on Asymmetric P-Selectin Patterns. Langmuir. 2011;27:240–249. doi: 10.1021/la102871m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Isobe T, Senna M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J. Colloid Interface Sci. 1996a;177:490–494. [Google Scholar]
- Lee JW, Isobe T, Senna M. Magnetic properties of ultrafine magnetite particles and their slurries prepared via in-situ precipitation. Colloids Surf. A. 1996b;109:121–127. [Google Scholar]
- Lee S-J, Jeong J-R, Shin S-C, Kim J-C, Kim J-D. Synthesis and characterization of superparamagnetic maghemite nanoparticles prepared by coprecipitation technique. J. Magn. Magn. Mater. 2004;282:147–150. [Google Scholar]
- Lei U, Lo YJ. Review of the theory of generalised dielectrophoresis. IET Nanobiotechnol. 2011;5:86–106. doi: 10.1049/iet-nbt.2011.0001. [DOI] [PubMed] [Google Scholar]
- Lenshof A, Laurell T. Emerging Clinical Applications of Microchip-Based Acoustophoresis. JALA. 2011;16:443–449. doi: 10.1016/j.jala.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Li JN, Zhang Z, Rosenzweig J, Wang YY, Chan DW. Proteomics and bioinformatics approaches for identification of serum biomarkers to detect breast cancer. Clin. Chem. 2002;48:1296–1304. [PubMed] [Google Scholar]
- Liu C, Stakenborg T, Peeters S, Lagae L. Cell manipulation with magnetic particles toward microfluidic cytometry. J. Appl. Phys. 2009a;105:102014. [Google Scholar]
- Liu G, Gao J, Ai H, Chen X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small. 2013;9:1533–1545. doi: 10.1002/smll.201201531. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu F, Li L, Wang Y, Qiu H. A novel CoFe2O4/polyacrylate nanocomposite prepared via an in situ polymerization in emulsion system. React. Funct. Polym. 2009b;69:43–47. [Google Scholar]
- Liu W, Dechev N, Foulds IG, Burke R, Parameswaran A, Park EJ. A novel permalloy based magnetic single cell micro array. Lab Chip. 2009c;9:2381–2390. doi: 10.1039/b821044f. [DOI] [PubMed] [Google Scholar]
- Liu X, Guan Y, Ma Z, Liu H. Surface Modification and Characterization of Magnetic Polymer Nanospheres Prepared by Miniemulsion Polymerization. Langmuir. 2004;20:10278–10282. doi: 10.1021/la0491908. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lim KM. Particle separation in microfluidics using a switching ultrasonic field. Lab Chip. 2011;11:3167–3173. doi: 10.1039/c1lc20481e. [DOI] [PubMed] [Google Scholar]
- Liu Y-J, Guo S-S, Zhang Z-L, Huang W-H, Baigl D, Xie M, Chen Y, Pang D-W. A micropillar-integrated smart microfluidic device for specific capture and sorting of cells. Electrophoresis. 2007;28:4713–4722. doi: 10.1002/elps.200700212. [DOI] [PubMed] [Google Scholar]
- Liu ZL, Yang XB, Yao KL, Du GH, Liu ZS. Preparation and characterization of magnetic P(St-co-MAA-co-AM) microspheres. J. Magn. Magn. Mater. 2006;302:529–535. [Google Scholar]
- Lodish H, Berk A, Kaiser CA, Krieger M, Scott MP, Bretscher A, Ploegh H, Matsudaira P. Molecular Cell Biology. W. H. Freeman; New York: 2007. [Google Scholar]
- Long BR, Heller M, Beech JP, Linke H, Bruus H, Tegenfeldt JO. Multidirectional sorting modes in deterministic lateral displacement devices. Phys. Rev. E. 2008;78:046304. doi: 10.1103/PhysRevE.78.046304. [DOI] [PubMed] [Google Scholar]
- Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Edit. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- Lundahl J, Hallden G, Hallgren M, Skold CM, Hed J. Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures. J. Immunol. Meth. 1995;180:93–100. doi: 10.1016/0022-1759(94)00303-e. [DOI] [PubMed] [Google Scholar]
- Ma H-L, Qi X-R, Maitani Y, Nagai T. Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 2007;333:177–186. doi: 10.1016/j.ijpharm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ma Z, Guan Y, Liu H. Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups. J. Polymer Sci. A. 2005;43:3433–3439. [Google Scholar]
- Maheswaran S, Haber DA. Circulating Tumor Cells: a window into cancer biology and metastasis. Curr. Opin. Genet. Dev. 2010;20:96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. Effect of Nanoparticles on the Cell Life Cycle. Chem. Rev. 2011;111:3407–3432. doi: 10.1021/cr1003166. [DOI] [PubMed] [Google Scholar]
- Makler MT, Palmer CJ, Ager AL. A review of practical techniques for the diagnosis of malaria. Ann. Trop. Med. Parasitol. 1998;92:419–433. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- Masuda H, Asahara T. Clonogenic assay of endothelial progenitor cells. Trends Cardiovasc. Med. 2013;23:99–103. doi: 10.1016/j.tcm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Masuda H, Kalka C, Asahara T. Endothelial progenitor cells for regeneration. Human Cell. 2000;13:153–60. [PubMed] [Google Scholar]
- Matt P, Fu ZM, Fu Q, Van Eyk JE. Biomarker discovery: proteome fractionation and separation in biological samples. Physiol. Genomics. 2008;33:12–17. doi: 10.1152/physiolgenomics.00282.2007. [DOI] [PubMed] [Google Scholar]
- Mcauslan BR, Johnson G. Cell responses to biomaterials I: Adhesion and growth of vascular endothelial cells on poly(hydroxyethyl methacrylate) following surface modification by hydrolytic etching. J. Biomed. Mater. Res. 1987;21:921–935. doi: 10.1002/jbm.820210708. [DOI] [PubMed] [Google Scholar]
- Mccloskey KE, Chalmers JJ, Zborowski M. Magnetophoretic mobilities correlate to antibody binding capacities. Cytometry. 2000;40:307–315. doi: 10.1002/1097-0320(20000801)40:4<307::aid-cyto6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mccloskey KE, Chalmers JJ, Zborowski M. Magnetic Cell Separation: Characterization of Magnetophoretic Mobility. Anal. Chem. 2003;75:6868–6874. doi: 10.1021/ac034315j. [DOI] [PubMed] [Google Scholar]
- Mcguckin C, Jurga M, Ali H, Strbad M, Forraz N. Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat. Protocols. 2008;3:1046–1055. doi: 10.1038/nprot.2008.69. [DOI] [PubMed] [Google Scholar]
- Mead LE, Prater D, Yoder MC, Ingram DA. Current Protocols in Stem Cell Biology. John Wiley & Sons, Inc; 2007. Isolation and Characterization of Endothelial Progenitor Cells from Human Blood. [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- Melnik K, Sun J, Fleischman A, Roy S, Zborowski M, Chalmers JJ. Quantification of magnetic susceptibility in several strains of Bacillus spores: Implications for separation and detection. Biotechnol. Bioeng. 2007;98:186–192. doi: 10.1002/bit.21400. [DOI] [PubMed] [Google Scholar]
- Melville D, Paul F, Roath S. Direct magnetic separation of red cells from whole blood. Nature. 1975a;255:706–706. doi: 10.1038/255706a0. [DOI] [PubMed] [Google Scholar]
- Melville D, Paul F, Roath S. High gradient magnetic separation of red cells from whole blood. IEEE Trans. Magn. 1975b;11:1701–1704. [Google Scholar]
- Mendenhall GD, Geng Y, Hwang J. Optimization of Long-Term Stability of Magnetic Fluids from Magnetite and Synthetic Polyelectrolytes. J. Colloid Interface Sci. 1996;184:519–526. doi: 10.1006/jcis.1996.0647. [DOI] [PubMed] [Google Scholar]
- Meng G, Liu S, Rancourt DE. Rapid Isolation of Undifferentiated Human Pluripotent Stem Cells from Extremely Differentiated Colonies. Stem Cell Dev. 2011;20:583–591. doi: 10.1089/scd.2010.0400. [DOI] [PubMed] [Google Scholar]
- Miao J, Cui L. Rapid isolation of single malaria parasite-infected red blood cells by cell sorting. Nat. Protocols. 2011;6:140–146. doi: 10.1038/nprot.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S, Muller W, Weichel W, Radbruch A. High-Gradient Magnetic Cell-Separation With MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Mok H, Zhang MQ. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin. Drug. Delivery. 2013;10:73–87. doi: 10.1517/17425247.2013.747507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Mackenzie D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J. Immunol. Meth. 1982;52:353–367. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- Molday RS, Yen SPS, Rembaum A. Application of magnetic microspheres in labelling and separation of cells. Nature. 1977;268:437–438. doi: 10.1038/268437a0. [DOI] [PubMed] [Google Scholar]
- Moore LR, Fujioka H, Williams PS, Chalmers JJ, Grimberg B, Zimmerman PA, Zborowski M. Hemoglobin degradation in malaria-infected erythrocytes determined from live cell magnetophoresis. FASEBJ. 2006;20:747–749. doi: 10.1096/fj.05-5122fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LR, Nehl F, Dorn J, Chalmers JJ, Zborowski M. Open Gradient Magnetic Red Blood Cell Sorter Evaluation on Model Cell Mixtures. IEEE Trans. Magn. 2013;49:309–315. doi: 10.1109/tmag.2012.2225098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LR, Williams PS, Nehl F, Abe K, Chalmers JJ, Zborowski M. Feasibility study of red blood cell debulking by magnetic field-flow fractionation with step-programmed flow. Anal. Bioanal. Chem. 2014;406:1661–1670. doi: 10.1007/s00216-013-7394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LR, Zborowski M, Sun L, Chalmers JJ. Lymphocyte fractionation using immunomagnetic colloid and a dipole magnet flow cell sorter. J. Biochem. Biophys. Methods. 1998;37:11–33. doi: 10.1016/s0165-022x(98)00010-4. [DOI] [PubMed] [Google Scholar]
- Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen L. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology. 2005;65:713–718. doi: 10.1016/j.urology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SK. Nanoparticles in modern medicine: State of the art and future challenges. Int.J. Nanomed. 2007;2:129–141. [PMC free article] [PubMed] [Google Scholar]
- Murthy SK, Radisic M. Cell Adhesion and Detachment. In: Li D, editor. Encyclopedia of Microfluidics and Nanofluidics. Springer; New York: 2008. [Google Scholar]
- Murthy SK, Sin A, Tompkins RG, Toner M. Effect of Flow and Surface Conditions on Human Lymphocyte Isolation Using Microfluidic Chambers. Langmuir. 2004;20:11649–11655. doi: 10.1021/la048047b. [DOI] [PubMed] [Google Scholar]
- Nagesha DK, Plouffe BD, Phan M, Lewis LH, Sridhar S, Murthy SK. Functionalization-induced improvement in magnetic properties of Fe3O4 nanoparticles for biomedical applications. J. Appl. Phys. 2009:105. [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Decker K, Chosy J, Comella K, Melnik K, Moore L, Lasky LC, Zborowski M, Chalmers JJ. Separation of a breast cancer cell line from human blood using a quadrupole magnetic flow sorter. Biotechnol. Progr. 2001;17:1145–1155. doi: 10.1021/bp010109q. [DOI] [PubMed] [Google Scholar]
- Nam J, Huang H, Lim H, Lim C, Shin S. Magnetic Separation of Malaria-Infected Red Blood Cells in Various Developmental Stages. Anal. Chem. 2013;85:7316–7323. doi: 10.1021/ac4012057. [DOI] [PubMed] [Google Scholar]
- Naume B, Borgen E, T0ssvik S, Pavlak N, Oates D, Nesland JM. Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method. Cytotherapy. 2004;6:244–252. doi: 10.1080/14653240410006086. [DOI] [PubMed] [Google Scholar]
- Nolan JP, Condello D, Duggan E, Naivar M, Novo D. Visible and near infrared fluorescence spectral flow cytometry. Cytometry A. 2013;83A:253–264. doi: 10.1002/cyto.a.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JP, Duggan E, Liu E, Condello D, Dave I, Stoner SA. Single cell analysis using surface enhanced Raman scattering (SERS) tags. Methods. 2012;57:272–279. doi: 10.1016/j.ymeth.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JP, Sebba DS. Darzynkiewicz Z, Holden E, Orfao A, Telford W, Wlodkowic D, editors. Surface-Enhanced Raman Scattering (SERS) Cytometry. Recent Advances in Cytometry, Part A: Instrumentation, Methods. (Fifth Edition) 2011 [Google Scholar]
- O’brien S, Brus L, Murray CB. Synthesis of monodisperse nanoparticles of barium titanate: Toward a generalized strategy of oxide nanoparticle synthesis. J. Am. Chem. Soc. 2001;123:12085–12086. doi: 10.1021/ja011414a. [DOI] [PubMed] [Google Scholar]
- Oosterwijk JC, Mesker WE, Ouwerkerk-Van Velzen MCM, Knepfle C, Wiesmeijer KC, Beverstock GC, Van Ommen GJB, Tanke HJ, Kanhai HHH. Prenatal diagnosis of trisomy 13 on fetal cells obtained from maternal blood after minor enrichment. Prenatal Diag. 1998;18:1082–1085. [PubMed] [Google Scholar]
- Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, Yu M, Chen PI, Morgan B, Trautwein J, Kimura A, Sengupta S, Stott SL, Karabacak NM, Barber TA, Walsh JR, Smith K, Spuhler PS, Sullivan JP, Lee RJ, Ting DT, Luo X, Shaw AT, Bardia A, Sequist LV, Louis DN, Maheswaran S, Kapur R, Haber DA, Toner M. Inertial Focusing for Tumor Antigen-Dependent and -Independent Sorting of Rare Circulating Tumor Cells. Sci. Trans. Med. 2013;5:179ra47. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, Kroll T, Jorke C, Hammer U, Altendorf-Hofmann A, Rabenstein C, Pachmann U, Runnebaum I, Hoffken K. Monitoring the Response of Circulating Epithelial Tumor Cells to Adjuvant Chemotherapy in Breast Cancer Allows Detection of Patients at Risk of Early Relapse. J. Clin. Oncol. 2008;26:1208–1215. doi: 10.1200/JCO.2007.13.6523. [DOI] [PubMed] [Google Scholar]
- Pamme N. Magnetism and microfluidics. Lab Chip. 2006;6:24–38. doi: 10.1039/b513005k. [DOI] [PubMed] [Google Scholar]
- Pamme N. Continuous flow separations in microfluidic devices. Lab Chip. 2007;7:1644–1659. doi: 10.1039/b712784g. [DOI] [PubMed] [Google Scholar]
- Pamme N, Eijkel JCT, Manz A. On-chip free-flow magnetophoresis: Separation and detection of mixtures of magnetic particles in continuous flow. J. Magn. Magn. Mater. 2006;307:237–244. [Google Scholar]
- Pamme N, Wilhelm C. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip. 2006;6:974–980. doi: 10.1039/b604542a. [DOI] [PubMed] [Google Scholar]
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003;36:R167–R181. [Google Scholar]
- Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009;42:224001. [Google Scholar]
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- Park J, An KJ, Hwang YS, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- Pasparakis G, Manouras T, Selimis A, Vamvakaki M, Argitis P. Laser-induced cell detachment and patterning with photodegradable polymer substrates. Angew. Chem. Int. Edit. 2011;50:4142–4145. doi: 10.1002/anie.201007310. [DOI] [PubMed] [Google Scholar]
- Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Paul BK, Moulik SP. Uses and applications of microemulsions. Curr. Sci. 2001;80:990–1001. [Google Scholar]
- Paul F, Melville D, Roath S, Warhurst DC. A Bench Top Magnetic Separator for Malarial Parasite Concentration. IEEE Trans. Magn. 1981a;17:2822–2824. [Google Scholar]
- Paul F, Roath S, Melville D, Warhurst DC, Osisanya JOS. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet. 1981b;318:70–71. doi: 10.1016/s0140-6736(81)90414-1. [DOI] [PubMed] [Google Scholar]
- Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. U. S. A. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson F, Aberg L, Sward-Nilsson AM, Laurell T. Free flow acoustophoresis: Microfluidic-based mode of particle and cell separation. Anal. Chem. 2007;79:5117–5123. doi: 10.1021/ac070444e. [DOI] [PubMed] [Google Scholar]
- Petersson F, Nilsson A, Holm C, Jonsson H, Laurell T. Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces. Lab Chip. 2005;5:20–22. doi: 10.1039/b405748c. [DOI] [PubMed] [Google Scholar]
- Pethig R. Dielectrophoresis: Using inhomogeneous AC electrical fields to separate and manipulate cells. Crit. Rev. Biotechnol. 1996;16:331–348. [Google Scholar]
- Pethig R. Review Article-Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics. 2010;4:022811. doi: 10.1063/1.3456626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanic Ii TR, Blackwell JD, Shubayev VI, Finones R, Jin S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials. 2007;28:2572–2581. doi: 10.1016/j.biomaterials.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Brown MA, Iyer RK, Radisic M, Murthy SK. Controlled capture and release of cardiac fibroblasts using peptide-functionalized alginate gels in microfluidic channels. Lab Chip. 2009a;9:1507–1510. doi: 10.1039/b823523f. [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Kniazeva T, Mayer JE, Jr., Murthy SK, Sales VL. Development of microfluidics as endothelial progenitor cell capture technology for cardiovascular tissue engineering and diagnostic medicine. FASEBJ. 2009b;23:3309–14. doi: 10.1096/fj.09-130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe BD, Lewis LH, Murthy SK. Computational design optimization for microfluidic magnetophoresis. Biomicrofluidics. 2011a;5:013413. doi: 10.1063/1.3553239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe BD, Mahalanabis M, Lewis LH, Klapperich CM, Murthy SK. Clinically Relevant Microfluidic Magnetophoretic Isolation of Rare-Cell Populations for Diagnostic and Therapeutic Monitoring Applications. Anal. Chem. 2012;84:1336–1344. doi: 10.1021/ac2022844. [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Nagesha DK, Dipietro RS, Sridhar S, Heiman D, Murthy SK, Lewis LH. Thermomagnetic determination of Fe3O4 magnetic nanoparticle diameters for biomedical applications. J. Magn. Magn. Mater. 2011b;323:2310–2317. [Google Scholar]
- Plouffe BD, Njoka DN, Harris J, Liao JH, Horick NK, Radisic M, Murthy SK. Peptide-mediated selective adhesion of smooth muscle and endothelial cells in microfluidic shear flow. Langmuir. 2007;23:5050–5055. doi: 10.1021/la0700220. [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Radisic M, Murthy SK. Microfluidic depletion of endothelial cells, smooth muscle cells, and fibroblasts from heterogeneous suspensions. Lab Chip. 2008;8:462–472. doi: 10.1039/b715707j. [DOI] [PubMed] [Google Scholar]
- Prasongchean W, Ferretti P. Autologous stem cells for personalised medicine. New Biotechnol. 2012;29:641–650. doi: 10.1016/j.nbt.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Pratt ED, Huang C, Hawkins BG, Gleghorn JP, Kirby BJ. Rare cell capture in microfluidic devices. Chem. Eng. Sci. 2011;66:1508–1522. doi: 10.1016/j.ces.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. Biophysical aspects of blood flow in the microvasculature. Cardiovasc. Res. 1996;32:654–667. [PubMed] [Google Scholar]
- Radbruch A, Mechtold B, Thiel A, Miltenyi S, Pfluger E. High-Gradient Magnetic Cell Sorting. Methods in Cell Biology. 1994;42 doi: 10.1016/s0091-679x(08)61086-9. [DOI] [PubMed] [Google Scholar]
- Radisic M, Iyer RK, Murthy SK. Micro- and nanotechnology in cell separation. Int. J. Nanomed. 2006;1:3–14. doi: 10.2147/nano.2006.1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W, Huang P, Belov L, Chrisp JS, Christopherson RI, Stapelberg PM, Warner FJ, George J, Bowen DG, Strasser SI, Koorey D, Sharland AF, Mccaughan GW, Shackel NA. Analysis of human liver disease using a cluster of differentiation (CD) antibody microarray. Liver Internat. 2012;32:1527–1534. doi: 10.1111/j.1478-3231.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- Recktenwald D, Radbruch A. Cell Separation Methods and Applications. Marcel Dekker Inc.; New York: 1997. [Google Scholar]
- Redl FX, Black CT, Papaefthymiou GC, Sandstrom RL, Yin M, Zeng H, Murray CB, O’brien SP. Magnetic, electronic, and structural characterization of nonstoichiometric iron oxides at the nanoscale. J. Am. Chem. Soc. 2004;126:14583–14599. doi: 10.1021/ja046808r. [DOI] [PubMed] [Google Scholar]
- Research B. Life Science Tools and Reagents: Global Markets. 2011. [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, Mcallister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaut C, Berry A, Chevalley S, Reybier K, Morlais I, Parzy D, Nepveu F, Benoit-Vical F, Valentin A. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malaria J. 2008;7:45. doi: 10.1186/1475-2875-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert D, Pamme N, Conjeaud H, Gazeau F, Iles A, Wilhelm C. Cell sorting by endocytotic capacity in a microfluidic magnetophoresis device. Lab Chip. 2011;11:1902–1910. doi: 10.1039/c0lc00656d. [DOI] [PubMed] [Google Scholar]
- Roca AG, Costo R, Rebolledo AF, Veintemillas-Verdaguer S, Tartaj P, Gonzalez-Carreno T, Morales MP, Serna CJ. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2009;42:224002. [Google Scholar]
- Roncalli JG, Tongers J, Renault MA, Losordo DW. Endothelial progenitor cells in regenerative medicine and cancer: a decade of research. Trends Biotechnol. 2008;26:276–83. doi: 10.1016/j.tibtech.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Rusling JF, Kumar CV, Gutkind JS, Patel V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135:2496–2511. doi: 10.1039/c0an00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada T, Fujigaya T, Niidome Y, Nakazawa K, Nakashima N. Near-IR laser-triggered target cell collection using a carbon nanotube-based cell-cultured substrate. ACS Nano. 2011;5:4414–4421. doi: 10.1021/nn2012767. [DOI] [PubMed] [Google Scholar]
- Safarik I, Safarikova M. Use of magnetic techniques for the isolation of cells. J. Chromat. B. 1999;722:33–53. [PubMed] [Google Scholar]
- Sales VL, Engelmayr GC, Jr., Johnson JA, Jr., Gao J, Wang Y, Sacks MS, Mayer JE., Jr. Protein precoating of elastomeric tissue-engineering scaffolds increased cellularity, enhanced extracellular matrix protein production, and differentially regulated the phenotypes of circulating endothelial progenitor cells. Circulation. 2007a;116:I-55–63. doi: 10.1161/CIRCULATIONAHA.106.6806637. [DOI] [PubMed] [Google Scholar]
- Sales VL, Mettler BA, Lopez-Ilasaca M, Johnson JA, Mayer JE. Endothelial progenitor and mesenchymal stem cell-derived cells persist in tissue-engineered patch in vivo: Application of green and red fluorescent protein-expressing retroviral vector. Tissue Eng. 2007b;13:525–535. doi: 10.1089/ten.2006.0128. [DOI] [PubMed] [Google Scholar]
- Saud AK, Edward PF. Coupled particle, Aifluid transport and magnetic separation in microfluidic systems with passive magnetic functionality. J. Phys. D Appl. Phys. 2013;46:125002. [Google Scholar]
- Schneider T, Karl S, Moore LR, Chalmers JJ, Williams PS, Zborowski M. Sequential CD34 cell fractionation by magnetophoresis in a magnetic dipole flow sorter. Analyst. 2010;135:62–70. doi: 10.1039/b908210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Moore LR, Jing Y, Haam S, Williams PS, Fleischman AJ, Roy S, Chalmers JJ, Zborowski M. Continuous flow magnetic cell fractionation based on antigen expression level. J. Biochm. Biophy. Methods. 2006;68:1–21. doi: 10.1016/j.jbbm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Schumm M, Lang P, Taylor G, Kuci S, Klingebiel T, Buhring HJ, Geiselhart A, Niethammer D, Handgretinger R. Isolation of highly purified autologous and allogeneic peripheral CD34(+) cells using the CliniMACS device. J. Hematol. 1999;8:209–218. doi: 10.1089/106161299320488. [DOI] [PubMed] [Google Scholar]
- Selvan ST, Patra PK, Ang CY, Ying JY. Synthesis of Silica-Coated Semiconductor and Magnetic Quantum Dots and Their Use in the Imaging of Live Cells. Angew. Chem. Int. Edit. 2007;119:2500–2504. doi: 10.1002/anie.200604245. [DOI] [PubMed] [Google Scholar]
- Seo J, Lean MH, Kole A. Membrane-free microfiltration by asymmetric inertial migration. Appl. Phys. Lett. 2007;91:033901. [Google Scholar]
- Sethu P, Moldawer LL, Mindrinos MN, Scumpia PO, Tannahill CL, Wilhelmy J, Efron PA, Brownstein BH, Tompkins RG, Toner M. Microfluidic isolation of leukocytes from whole blood for phenotype and gene expression analysis. Anal. Chem. 2006;78:5453–5461. doi: 10.1021/ac060140c. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Behzadi S, Laurent S, Laird Forrest M, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. Methods of cell separation. Elsevier Science; Amsterdam: 1988. [Google Scholar]
- Shenkman RM, Chalmers JJ, Hering BJ, Kirchhof N, Papas KK. Quadrupole Magnetic Sorting of Porcine Islets of Langerhans. Tissue Eng. Pt. C Meth. 2009;15:147–156. doi: 10.1089/ten.tec.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevkoplyas SS, Siegel AC, Westervelt RM, Prentiss MG, Whitesides GM. The force acting on a superparamagnetic bead due to an applied magnetic field. Lab Chip. 2007;7:1294–1302. doi: 10.1039/b705045c. [DOI] [PubMed] [Google Scholar]
- Shi JJ, Huang H, Stratton Z, Huang YP, Huang TJ. Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW) Lab Chip. 2009;9:3354–3359. doi: 10.1039/b915113c. [DOI] [PubMed] [Google Scholar]
- Shin DS, Hyun Seo J, Sutcliffe JL, Revzin A. Photolabile micropatterned surfaces for cell capture and release. Chemical Comm. 2011;47:11942–11944. doi: 10.1039/c1cc15046d. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Pisanic Ii TR, Jin S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliver. Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin A, Murthy SK, Revzin A, Tompkins RG, Toner M. Enrichment using antibody-coated microfluidic chambers in shear flow: Model mixtures of human lymphocytes. Biotechnol. Bioeng. 2005;91:816–826. doi: 10.1002/bit.20556. [DOI] [PubMed] [Google Scholar]
- Singh A, Suri S, Lee T, Chilton JM, Cooke MT, Chen W, Fu J, Stice SL, Lu H, Mcdevitt TC, Garcia AJ. Adhesion strength-based, label-free isolation of human pluripotent stem cells. Nat. Meth. 2013;10:438–444. doi: 10.1038/nmeth.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Ganguly R, De AK, Puri IK. Single magnetic particle dynamics in a microchannel. Phys. Fluids. 2007;19:117102. [Google Scholar]
- Sinha A, Ganguly R, Puri IK. Magnetic separation from superparamagnetic particle suspensions. J. Magn. Magn. Mater. 2009;321:2251–2256. [Google Scholar]
- Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, Connelly MC, Terstappen LWMM, O’hara SM. Global Gene Expression Profiling of Circulating Tumor Cells. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- Society AC. Cancer Facts & Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- Soenen SJ, Rivera-Gil P, Montenegro J-MA, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6:446–465. [Google Scholar]
- Soenen SJH, De Cuyper M. Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine. 2010;5:1261–1275. doi: 10.2217/nnm.10.106. [DOI] [PubMed] [Google Scholar]
- Sofla A, Cirkovic B, Hsieh A, Miklas JW, Filipovic N, Radisic M. Enrichment of live unlabelled cardiomyocytes from heterogeneous cell populations using manipulation of cell settling velocity by magnetic field. Biomicrofluidics. 2013;7:014110. doi: 10.1063/1.4791649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating Activated Endothelial Cells in Sickle Cell Anemia. N. Engl. J. Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- Stemberger C, Dreher S, Tschulik C, Piossek C, Bet J, Yamamoto TN, Schiemann M, Neuenhahn M, Martin K, Schlapschy M, Skerra A, Schmidt T, Edinger M, Riddell SR, Germeroth L, Busch DH. Novel Serial Positive Enrichment Technology Enables Clinical Multiparameter Cell Sorting. PLoS ONE. 2012;7:e35798. doi: 10.1371/journal.pone.0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker HS. General, Organic, and Biological Chemistry. Cengage Learning; Stamford, CT: 2011. [Google Scholar]
- Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber DA, Toner M. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U. S. A. 2010a;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. Isolation and Characterization of Circulating Tumor Cells from Patients with Localized and Metastatic Prostate Cancer. Sci. Transl. Med. 2010b;2:25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Lee JSH, Zhang MQ. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliver. Rev. 2008a;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008b;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SH, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li GX. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- Szaniszlo P, Wang N, Sinha M, Reece LM, Van Hook JW, Luxon BA, Leary JF. Getting the right cells to the array: Gene expression microarray analysis of cell mixtures and sorted cells. Cytometry A. 2004;59A:191–202. doi: 10.1002/cyto.a.20055. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tandon V, Zhang BY, Radisic M, Murthy SK. Generation of tissue constructs for cardiovascular regenerative medicine: From cell procurement to scaffold design. Biotechnol. Adv. 2013;31:722–735. doi: 10.1016/j.biotechadv.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaj P, Morales MD, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003;36:R182–R197. [Google Scholar]
- Tartaj P, Morales MP, Gonzalez-Carreno T, Veintemillas-Verdaguer S, Serna CJ. Advances in magnetic nanoparticles for biotechnology applications. J. Magn. Magn. Mater. 2005;290:28–34. [Google Scholar]
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine: NBM. 2010;6:257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Thiel A, Scheffold A, Radbruch A. Immunomagnetic cell sorting – pushing the limits. Immunotechnology. 1998;4:89–96. doi: 10.1016/s1380-2933(98)00010-4. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Punshon G, Kidane A, Hamilton G, Seifalian AM. Magnetic beads (Dynabead™) toxicity to endothelial cells at high bead concentration: Implication for tissue engineering of vascular prosthesis. Cell Bio. Toxicol. 2003;19:265–272. doi: 10.1023/b:cbto.0000004929.37511.ed. [DOI] [PubMed] [Google Scholar]
- Toner M, Irimia D. Blood-on-a-chip. Annu. Rev. Biomed. Eng. 2005;7:77–103. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XD, Xiong Y, Zborowski M, Farag SS, Chalmers JJ. A novel high throughput immunomagnetic cell sorting system for potential clinical scale depletion of T cells for allogeneic stem cell transplantation. Exp. Hema. 2007;35:1613–1622. doi: 10.1016/j.exphem.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli F, Pescucci C. Isolation of fetal cells from the maternal circulation: Prospects for the non-invasive prenatal diagnosis. Clin. Chem. Lab. Med. 2001;39:494–500. doi: 10.1515/CCLM.2001.082. [DOI] [PubMed] [Google Scholar]
- Ugelstad J, Berge A, Ellingsen T, Schmid R, Nilsen TN, Mork PC, Stenstad P, Hornes E, Olsvik O. Preparation and application of new monosized polymer particles. Prog. Polym. Sci. 1992;17:87–161. [Google Scholar]
- Ugelstad J, Kaggerud KH, Hansen FK, Berge A. Absorption of low molecular weight compounds in aqueous dispersions of polymer-oligomer particles, 2. A two step swelling process of polymer particles giving an enormous increase in absorption capacity. Die Makromolekulare Chemie. 1979;180:737–744. [Google Scholar]
- Urbich C, Dimmeler S. Endothelial Progenitor Cells. Circulation Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Van Beem RT, Nur E, Zwaginga JJ, Landburg PP, Van Beers EJ, Duits AJ, Brandjes DP, Lommerse I, De Boer HC, Van Der Schoot CE, Schnog J-JB, Biemond BJ. Elevated endothelial progenitor cells during painful sickle cell crisis. Exp. Hema. 2009;37:1054–1059. doi: 10.1016/j.exphem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Van Craenenbroeck E, Conraads V, Van Bockstaele D, Haine S, Vermeulen K, Van Tendeloo V, Vrints C, Hoymans V. Quantification of circulating endothelial progenitor cells: a methodological comparison of six flow cytometric approaches. J. Immunol. Methods. 2008;332:31–40. doi: 10.1016/j.jim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Vickers DA, Hincapie M, Hancock WS, Murthy SK. Lectin-mediated microfluidic capture and release of leukemic lymphocytes from whole blood. Biomed. Microdevices. 2011;13:565–71. doi: 10.1007/s10544-011-9527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers D. a. L., Kulik M, Hincapie M, Hancock WS, Dalton S, Murthy SK. Lectin-functionalized microchannels for characterizing pluripotent cells and early differentiation. Biomicrofluidics. 2012;6:024122. doi: 10.1063/1.4719979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, Morales M. a. D. P., Miranda R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology. 2009;20:115103. doi: 10.1088/0957-4484/20/11/115103. [DOI] [PubMed] [Google Scholar]
- Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, Fleisher M, Lilja H, Brogi E, Boyd J, Sanchez-Carbayo M, Holland EC, Cordon-Cardo C, Scher HI, Tempst P. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J. Clin. Invest. 2006;116:271–284. doi: 10.1172/JCI26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel SS, Shulman L, Sammons D. Fetal cells in maternal blood. Clin. Genet. 2001;59:74–79. doi: 10.1034/j.1399-0004.2001.590202.x. [DOI] [PubMed] [Google Scholar]
- Wan Y, Liu Y, Allen PB, Asghar W, Mahmood M. a. I., Tan J, Duhon H, Kim Y-T, Ellington AD, Iqbal SM. Capture, isolation and release of cancer cells with aptamer-functionalized glass bead array. Lab Chip. 2012;12:4693–4701. doi: 10.1039/c2lc21251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GN, Su XG. The synthesis and bio-applications of magnetic and fluorescent bifunctional composite nanoparticles. Analyst. 2011;136:1783–1798. doi: 10.1039/c1an15036g. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhuang J, Peng Q, Li YD. A general strategy for nanocrystal synthesis. Nature. 2005;437:121–124. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]
- Weigl B, Domingo G, Labarre P, Gerlach J. Towards non- and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip. 2008;8:1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Eng. J. Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Wigzell H, Andersson B. Cell separation on antigen-coated columns: elimination of high rate antibody-forming cells and immunological memory cells. J. Exp. Med. 1969;129:23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt B, Wirtz D, Searson PC. Triggering cell detachment from patterned electrode arrays by programmed subcellular release. Nat. Protocols. 2010;5:1273–1280. doi: 10.1038/nprot.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DN, Gold KA, Holoman TRP, Ehrman SH, O. C. W. Surface Modification of Magnetic Nanoparticles Using Gum Arabic. J. Nanopart. Res. 2006;8:749753. [Google Scholar]
- Willis AL, Turro NJ, O’brien S. Spectroscopic characterization of the surface of iron oxide nanocrystals. Chem. Mater. 2005;17:5970–5975. [Google Scholar]
- Woelfle U, Breit E, Pantel K. Influence of immunomagnetic enrichment on gene expression of tumor cells. J. Transl. Med. 2005;3:12. doi: 10.1186/1479-5876-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotschadlo J, Liebert T, Heinze T, Wagner K, Schnabelrauch M, Dutz S, Muller R, Steiniger F, Schwalbe M, Kroll TC, Hoffkene K, Buske N, Clement JH. Magnetic nanoparticles coated with carboxymethylated polysaccharide shells-interaction with human cells. J. Magn. Magn. Mater. 2009;321:1469–1473. [Google Scholar]
- Wu YQ, Deighan CJ, Miller BL, Balasubramanian P, Lustberg MB, Zborowski M, Chalmers JJ. Isolation and analysis of rare cells in the blood of cancer patients using a negative depletion methodology. Methods. 2013;64:169–182. doi: 10.1016/j.ymeth.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N, Hunt TP, Mayers BT, Alsberg E, Whitesides GM, Westervelt RM, Ingber DE. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomed. Microdevices. 2006;8:299–308. doi: 10.1007/s10544-006-0033-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Phillips JA, Yan JL, Li QG, Fan ZH, Tan WH. Aptamer-Based Microfluidic Device for Enrichment, Sorting, and Detection of Multiple Cancer Cells. Anal. Chem. 2009;81:7436–7442. doi: 10.1021/ac9012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23:561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- Yang AHJ, Soh HT. Acoustophoretic Sorting of Viable Mammalian Cells in a Microfluidic Device. Anal. Chem. 2012;84:10756–10762. doi: 10.1021/ac3026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Lang JC, Balasubramanian P, Jatana KR, Schuller D, Agrawal A, Zborowski M, Chalmers JJ. Optimization of an Enrichment Process for Circulating Tumor Cells From the Blood of Head and Neck Cancer Patients Through Depletion of Normal Cells. Biotechnol. Bioeng. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo WS, Hodneland CD, Mrksich M. Electroactive monolayer substrates that selectively release adherent cells. Chembiochem. 2001;2:590–593. doi: 10.1002/1439-7633(20010803)2:7/8<590::AID-CBIC590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Yi DK, Selvan ST, Lee SS, Papaefthymiou GC, Kundaliya D, Ying JY. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J. Am. Chem. Soc. 2005;127:4990–4991. doi: 10.1021/ja0428863. [DOI] [PubMed] [Google Scholar]
- Yoder MC. Human Endothelial Progenitor Cells. Cold Spring Harbor Perspect. Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yun H, Kim K, Lee WG. Cell manipulation in microfluidics. Biofabrication. 2013;5:022001. doi: 10.1088/1758-5082/5/2/022001. [DOI] [PubMed] [Google Scholar]
- Yung CW, Fiering J, Mueller AJ, Ingber DE. Micromagnetic-microfluidic blood cleansing device. Lab Chip. 2009;9:1171–1177. doi: 10.1039/b816986a. [DOI] [PubMed] [Google Scholar]
- Zborowski M. Physics of magnetic cell sorting. In: Zborowski M, editor. Scientific and Clinical Applications of Magnetic Carriers. Plenum; New York: 1997. [Google Scholar]
- Zborowski M, Chalmers JJ. Rare Cell Separation and Analysis by Magnetic Sorting. Anal. Chem. 2011;83:8050–8056. doi: 10.1021/ac200550d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN. Red blood cell magnetophoresis. Biophys. J. 2003;84:2638–2645. doi: 10.1016/S0006-3495(03)75069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zborowski M, Sun LP, Moore LR, Williams PS, Chalmers JJ. Continuous cell separation using novel magnetic quadrupole flow sorter. J. Magn. Magn. Mater. 1999;194:224–230. [Google Scholar]
- Zhang B, Green JV, Murthy SK, Radisic M. Label-Free Enrichment of Functional Cardiomyocytes Using Microfluidic Deterministic Lateral Flow Displacement. PLoS ONE. 2012a;7:e37619. doi: 10.1371/journal.pone.0037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kohler N, Zhang MQ. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu J-Y, Ma S, Zhang Y-J, Zhao X, Zhang X-D, Zhang Z-D. Synthesis of PVP-coated ultra-small Fe3O4 nanoparticles as a MRI contrast agent. J. Mater. Sci.: Mater. Med. 2010;21:1205–1210. doi: 10.1007/s10856-009-3881-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun C, Kohler N, Zhang MQ. Self-assembled coatings on individual monodisperse magnetite nanoparticles for efficient intracellular uptake. Biomed. Microdevices. 2004;6:33–40. doi: 10.1023/b:bmmd.0000013363.77466.63. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen N, Li S, Battig MR, Wang Y. Programmable Hydrogels for Controlled Cell Catch and Release Using Hybridized Aptamers and Complementary Sequences. J. Am. Chem. Soc. 2012b;134:15716–15719. doi: 10.1021/ja307717w. [DOI] [PubMed] [Google Scholar]
- Zhao XK, Xu SQ, Fendler JH. Ultrasmall magnetic particles in langmuir-blodgett-films. J. Phys. Chem. 1990;94:2573–2581. [Google Scholar]
- Zheng W, Gao F, Gu H. Magnetic polymer nanospheres with high and uniform magnetite content. J. Magn. Magn. Mater. 2005;288:403–410. [Google Scholar]
- Zhou J, Belov L, Huang PY, Shin JS, Solomon MJ, Chapuis PH, Bokey L, Chan C, Clarke C, Clarke SJ, Christopherson RI. Surface antigen profiling of colorectal cancer using antibody microarrays with fluorescence multiplexing. J. Immunol. Meth. 2010;355:40–51. doi: 10.1016/j.jim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Zhu B, Smith J, Yarmush ML, Nahmias Y, Kirby BJ, Murthy SK. Microfluidic Enrichment of Mouse Epidermal Stem Cells and Validation of Stem Cell Proliferation In Vitro. Tissue Eng. Pt C Methods. 2013;19:765–773. doi: 10.1089/ten.tec.2012.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Yan J, Revzin A. Catch and release cell sorting: Electrochemical desorption of T-cells from antibody-modified microelectrodes. Colloids Surf. B. 2008;64:260–268. doi: 10.1016/j.colsurfb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nguyen T, Pei R, Stojanovic M, Lin Q. Specific capture and temperature-mediated release of cells in an aptamer-based microfluidic device. Lab Chip. 2012;12:3504–3513. doi: 10.1039/c2lc40411g. [DOI] [PMC free article] [PubMed] [Google Scholar]