Abstract
Free radicals and other oxidants have gained importance in the field of biology due to their central role in various physiological conditions as well as their implication in a diverse range of diseases. The free radicals, both the reactive oxygen species (ROS) and reactive nitrogen species (RNS), are derived from both endogenous sources (mitochondria, peroxisomes, endoplasmic reticulum, phagocytic cells etc.) and exogenous sources (pollution, alcohol, tobacco smoke, heavy metals, transition metals, industrial solvents, pesticides, certain drugs like halothane, paracetamol, and radiation). Free radicals can adversely affect various important classes of biological molecules such as nucleic acids, lipids, and proteins, thereby altering the normal redox status leading to increased oxidative stress. The free radicals induced oxidative stress has been reported to be involved in several diseased conditions such as diabetes mellitus, neurodegenerative disorders (Parkinson’s disease-PD, Alzheimer’s disease-AD and Multiple sclerosis-MS), cardiovascular diseases (atherosclerosis and hypertension), respiratory diseases (asthma), cataract development, rheumatoid arthritis and in various cancers (colorectal, prostate, breast, lung, bladder cancers). This review deals with chemistry, formation and sources, and molecular targets of free radicals and it provides a brief overview on the pathogenesis of various diseased conditions caused by ROS/RNS.
Keywords: Free radicals, Reactive oxygen species (ROS), Reactive nitrogen species (RNS), Oxidative stress
History
In recent years there is an ever-increasing curiosity in studying the role of free radicals in biology, because of their pivotal role in various physiological conditions as well as their involvement in a diverse range of diseases. For the first time in 1900, Moses Gomberg, Professor of Chemistry at the University of Michigan, speculated the existence of an organic free radical, triphenyl methyl radical (Ph3C•) in the living system [1]. Later in 1954, Gershman proposed “free radical theory of oxygen toxicity”, according to which, the toxicity of oxygen is due to its ability to form free radicals [2]. In the same year, the electron paramagnetic resonance (EPR) studies by Commoner et al. 1954 [3] confirmed the presence of free radicals in biological materials. Soon thereafter Denham Harman, in 1956, proposed the “free radical theory of aging”, which states that free radicals play a central role in the aging process. A second era of free radical research began in 1969 by Mc Cord and Fridovich, who discovered superoxide dismutase, the first enzymatic defense system against superoxide anion [4]. For the first time in 1971, Loschen indicated that the Reactive oxygen species are generated in cellular metabolic respiration [5], which was supported by Nohl and Hegner (1978) [6]. In 1977, Mittal and Murad reported that the hydroxyl radical, OH˙ induces the formation of the second messenger cyclic GMP by activating the enzyme guanylate cyclase [7]. Later in 1989, Hallliwell and Gutteridge reported that reactive oxygen species (ROS) include both free radical and non radical derivatives of oxygen [8]. Since then a massive data has been documented on the role of free radicals in various pathophysiological conditions.
Introduction
Free radicals are the products of normal cellular metabolism. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Because of their high reactivity, they can abstract electrons from other compounds to attain stability. Thus the attacked molecule loses its electron and becomes a free radical itself, beginning a chain reaction cascade which finally damages the living cell [9]. Both ROS and RNS collectively constitute the free radicals and other non radical reactive species [10]. The ROS/RNS play a twofold job as both beneficial and toxic compounds to the living system. At moderate or low levels ROS/RNS have beneficial effects and involve in various physiological functions such as in immune function (i.e. defense against pathogenic microorganisms), in a number of cellular signaling pathways, in mitogenic response and in redox regulation [11, 12]. But at higher concentration, both ROS as well as RNS generate oxidative stress and nitrosative stress, respectively, causing potential damage to the biomolecules. The oxidative stress and nitrosative stress are developed when there is an excess production of ROS/RNS on one side and a deficiency of enzymatic and non enzymatic antioxidants on the other side. Most importantly, the excess ROS can damage the integrity of various biomolecules including lipids [13], proteins [14] and DNA [15] leading to increased oxidative stress in various human diseases such as diabetes mellitus, neurodegenerative diseases, rheumatoid arthritis, cataracts, cardiovascular diseases, respiratory diseases as well as in aging process. This review deals with the chemistry, formation and sources, and molecular targets of free radicals, it highlights the implication of free radicals in various diseased conditions.
Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
In general pro-oxidants/oxidants are termed as ROS/RNS. The most important free radicals produced during metabolic reactions are radicals derived from oxygen, ROS. Both the ROS and RNS can be classified into two groups of compounds namely; radicals and non-radicals (see Table 1). Radicals are the species which contain at least one unpaired electron in the shells around the atomic nucleus and are capable of independent existence. The oxygen molecule itself is a radical, and because of the presence of two unpaired electrons it is referred as biradical. The examples for the radicals include Superoxide (), Oxygen radical (), Hydroxyl (), Alkoxyradical (), Peroxyl radical (), Nitric oxide (nitrogen monoxide) () and nitrogen dioxide () [17]. The high reactivity of these radicals is due to the presence of one unpaired electron which tends to donate it or to obtain another electron to attain stability. The non radical species include hydrogen peroxide (H2O2), hypochlorous acid (HOCl), hypobromous acid (HOBr), ozone (O3), singlet oxygen (1O2), nitrous acid (HNO2), nitrosyl cation (NO+), nitroxyl anion (NO−), dinitrogen trioxide (N2O3), dinitrogen tetraoxide (N2O4), nitronium (nitryl) cation (NO2+), organic peroxides (ROOH), aldehydes (HCOR) and peroxynitrite (ONOOH) [16, 17]. These non radical species are not free radicals but can easily lead to free radical reactions in living organisms [18].
Table 1.
| Free radical | Symbol | Half-life |
|---|---|---|
| Reactive oxygen species-ROS | ||
| Radicals | ||
| Superoxide | O2 •− | 10−6 s |
| Hydroxyl | OH• | 10−10 s |
| Alkoxyl radical | RO• | 10−6 |
| Peroxyl Radical | ROO• | 17 s |
| Non radicals | ||
| Hydrogen peroxide | H2O2 | Stable |
| Singlet oxygen | 1O2 | 10−6 s |
| Ozone | O3 | s |
| Organic peroxide | ROOH | Stable |
| Hypochlorous acid | HOCl | Stable (min) |
| Hypobromous acid | HOBr | Stable (min) |
| Reactive nitrogen species-RNS | ||
| Radicals | ||
| Nitric oxide | NO• | sa |
| Nitrogen dioxide | NO•2 | s |
| Non radicals | ||
| Peroxynitrite | ONOO− | 10−3 s |
| Nitrosyl cation | NO+ | s |
| Nitroxyl anion | NO− | s |
| Dinitrogen trioxide | N2O3 | s |
| Dinitrogen tetraoxide | N2O4 | s |
| Nitrous acid | HNO2 | s |
| Peroxynitrous acid | ONOOH | Fairly stable |
| Nitryl chloride | NO2Cl | s |
a The half life of some radicals depends on the environmental medium, for example the half life of NO• in an air saturated solution may be few minutes. S seconds, min minutes
Properties of Some Free Radicals
Superoxide Ion Radical ()
Superoxide anion radical is the most important widespread ROS formed by the enzymatic process, autooxidation reaction and by a nonenzymatic electron transfer reactions in which an electron is transferred to molecular oxygen [20]. It is mostly produced within the mitochondria and its reactivity with the biomolecules is low. The enzymes that can produce superoxide include xanthine oxidase [21], lipooxygenase, cyclooxygenase [22, 23] and NADPH dependent oxidase. It can exist in two forms such as O•−2 or hydroperoxyl radical (HO2) at low pH [24]. The hydroperoxyl radical is the most important form and can easily enter the phospholipid bilayer than the charged form (O•−2). Under physiological pH the most occurring form is superoxide. It can act as reducing agent and it reduces iron complexes such as cytochrome-c and ferric-ethylene diaminetetraacetic acid (Fe+-EDTA), in which Fe+3 is reduced to Fe+2. It can also act as oxidizing agent and oxidize ascorbic acid and tocopherol.
Superoxide radical react with another superoxide radical in a dismutation reaction (Eq. 1), in which one radical is oxidized to oxygen and other is reduced to hydrogen peroxide [25].
| 1 |
Hydroxyl Radical (OH∙)
Hydroxyl radical is the neutral form of hydroxide ion and is a highly reactive free radical [26]. It can strongly react with both organic and inorganic molecules including DNA, proteins, lipids, and carbohydrates and cause severe damage to the cells than any other ROS can do [27]. It is formed in a Fenton reaction (Eq. 2), in which H2O2 react with metal ions (Fe+2 or Cu+), often bound in complex with different proteins such as ferritin (an intracellular protein that stores iron) and ceruloplasmin (plasma copper carrying protein) or other molecules [28]. Under stress conditions, an excess of O•−2releases free iron from ferritin and the released free iron participates in Fenton reaction to form OH•. It is also formed by the reaction between superoxide radical and H2O2 in a reaction called Haber–Weiss reaction (Eq. 3) [29].
| 2 |
| 3 |
Peroxyl Radical (ROO∙)
It is derived from oxygen in living systems. The simplest form of peroxyl radical is perhydroxyl radical (HOO•) which is formed by the protonation of superoxide [30]. About 0.3 % of the total O•−2 in the cytosol of a typical cell is in the protonated form. It initiates fatty acid peroxidation and also can promote tumor development [31].
Hydrogen Peroxide (H2O2)
Hydrogen peroxide is formed in vivo in a dismutation reaction catalyzed by the enzyme superoxide dismutase (SOD) (Eq. 1). It is not a free radical but it can cause damage to the cell at relatively low concentration (10 μM), but at higher levels, the cellular energy producing enzymes such as glyceraldehhyde-3-phosphate dehydrogenase are inactivated. It can easily penetrate the biological membranes. H2O2 has no direct effect on DNA but can damage DNA by producing hydroxyl radical (OH−) in the presence of transition metal ions [32]. The major antioxidant enzymes that can eliminate the H2O2 include catalase, glutathione peroxidase and peroxiredoxins [33, 34].
Singlet Oxygen (1O2)
It is an electronically high excited, meta-stable state of molecular oxygen and is a highly reactive toxic reactive oxygen species [35]. Upon activation, the molecular oxygen is excited to first state 1Δg and then to next higher excited singlet state, 1εg. The first excited state, 1Δg, has two electrons with opposite spins in the same π* orbital whereas, the second excited state, 1εg, has one electron in each degenerated π* orbital with opposite spins [36]. The 1Δg state is extremely reactive, and compared to the other electronically excited states [36]. It is produced in vivo by the activation of neutrophils (Eq. 4) [37] and eosinophils [38]. It is also formed by some of the enzymatic reactions catalyzed by enzymes such as lipoxygenases [39], dioxygenases [40], and lactoperoxidase [41]. It is a highly potent oxidizing agent that can cause DNA damage [42] and tissue damage [38].
| 4 |
Ozone (O3)
Ozone is a powerful oxidant may be produced in vivo by antibody catalyzed water oxidation pathway which plays an important role in inflammation [43]. It can form free radicals and other reactive intermediates by oxidizing the biological molecules. It can cause lipid peroxidation [44] and oxidize different functional groups, for example, amine, alcohol, aldehyde and sulphydryl, present in proteins [45, 46] and nucleic acids [47]. It can also cause chromosomal aberrations which may be due to direct attack by O3 or by the free radicals generated by it [48].
Hypochlorous Acid (HOCl)
It is a major oxidant produced by the activated neutrophils at the site of inflammation from hydrogen peroxide and chloride in a reaction catalyzed by the enzyme myeloperoxidase [49].
HOCl is a strong reactive species involved in oxidation and chlorination reactions. It can oxidize thiols and other biological molecules including, ascorbate, urate, pyridine nucleotides, and tryptophan [50, 51]. HOCl chlorinates several compounds such as amines to give chloramines; tyrosyl residues to give ring chlorinated products, cholesterol and unsaturated lipids to give chlorohydrins, and it can also chlorinate DNA [52].
Nitric Oxide or Nitrogen Monoxide (NO•)
It is a small molecule generated in tissues by different nitric oxide synthases (NOS)s which convert L-arginine to L-citrulline [53]. In this reaction one of the terminal guanido nitrogen atoms undergo oxidation and produces NO.. Three types of isoforms of NOS such as neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) are involved in the formation of the NO radical.
It is both aqueous and lipid soluble and therefore it readily diffuses through cytoplasm and plasma membrane [54]. The NO• is an important intracellular second messenger stimulates guanylate cyclase and protein kinases and helps in smooth muscle relaxation in blood vessels. It is identical to endothelium derived relaxing factor (EDRF) produced by vascular endothelial cells which is an important mediator of vascular responses [55]. It can also act as an important cellular redox regulator [56] and regulate enzymatic activity by nitrosylating the proteins [57]. Since it is involved in many biological activities like blood pressure regulation, smooth muscle relaxation, neurotransmission, defensive mechanisms and immune regulation, this molecule was regarded as molecule of the year 1992 [58].
Peroxynitrite (OONO−) and Other Reactive Nitrogen Species
Peroxynitrite (OONO−) is formed by the reaction between and . It is highly toxic [59] and can directly react with CO2 to form other highly reactive nitroso peroxo carboxylate (ONOOCO2−) or peroxynitrous acid (ONOOH). The ONOOH further undergo homolysis to form both OH• and NO2 or rearrange to form NO3. OONO- can oxidize lipids, oxidize methionine and tyrosine residues in proteins and oxidizes DNA to form nitroguanine [60]. The nitrotyrosine residues are considered as marker of peroxynitrite induced cellular damage [61].
NO reacts with O2 and water to form nitrate and nitrite ions. One electron oxidation of NO• results in nitrosonium cation () while on electron reduction results in nitroxyl anion (NO−). These two ions can react with NO and form N2O and . can react with a variety of radicals such as H2O2 and HOCl to form N2O3, NO2− and NO3− [62].
Sources of Free Radicals
The ROS can be produced from either endogenous or exogenous sources. The endogenous sources of ROS include different cellular organs such as mitochondria, peroxisomes and endoplasmic reticulum, where the oxygen consumption is high.
Mitochondria
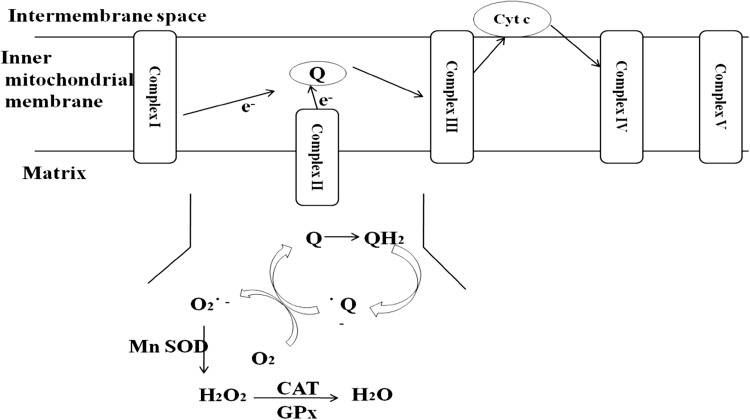
Most of the intracellular ROS are derived from mitochondria (Fig. 1). The superoxide radicals are produced at two major sites in the electron transport chain, namely complex I (NADH dehydrogenase) and complex III (ubiquinone cytochrome c reductase). The transfer of electrons from complex I or II to coenzyme Q or ubiquinone (Q) results in the formation of reduced form of coenzyme Q (QH2). The reduced form QH2 regenerates coenzyme Q via an unstable intermediate semiquinone anion in the Q-cycle. The formed immediately transfers electrons to molecular oxygen leading to the formation of superoxide radical. The generation of superoxide is non-enzymatic and therefore higher the metabolic rate, the greater is the production of the ROS [63].
Fig. 1.
Mitochondrial ROS production
The superoxide anion is converted to hydrogen peroxide by the action of mitochondrial superoxide dismutase (MnSOD). H2O2 can be detoxified by the Catalase (CAT) and glutathione peroxidase (GPx).
The other mitochondrial components which contribute to the formation of ROS include monoamino oxidase, α-ketoglutarae dehydrogenase, glycerol phosphate dehydrogenase and p66shc [64].
The p66Shc is an important member of the ShcA protein family, which contains another two more proteins, p46Shc and p52 Shc. The mammalian p66Shc is a 66-kDa isoform of the growth factor adaptor protein involved in apoptosis. It mediates the production of ROS in mitochondria. Most of the p66Shc is located in cytoplasm with a small fraction localized in the mitochondrial intermembrane space. Upon oxidative stress, p66Shc translocates to mitochondrial intermembrane space, where it associates with cytochrome-c, thus inducing ROS generation [65].
Peroxisomes
In peroxisomes the respiratory pathway involves the transfer of electrons from various metabolites to the oxygen leads to H2O2 formation [66], but is not coupled to oxidative phosphorylation to produce ATP instead free energy is released in the form of heat. The other free radicals produced in peroxisomes include H2O2, O•−2 OH• and NO•. The β-oxidation of fatty acids is the major metabolic process producing H2O2 in the peroxisomes. As reviewed elsewhere, the different peroxisomal enzymes such as acyl CoA oxidases, D-amino acid oxidase, L-α-hydroxy oxidase, urate oxidase, xanthine oxidase, D-aspartate oxidase have been shown to produce different ROS [67] [see Table 2].
Table 2.
ROS producing enzymes in peroxisomes
| Enzyme | Substrate | ROS |
|---|---|---|
| Acyl CoA-oxidases (enzymes of β-oxidation) | Fatty acids | H2O2 |
| D-amino acid oxidase | d-proline | H2O2 |
| L-α-hydroxy oxidase | Glycolate | H2O2 |
| Urate oxidase | Uric acid | H2O2 |
| D-aspartate oxidase | D-aspartate | H2O2 |
| Xanthine oxidase | Xanthine | O•−2, H2O2 |
Endoplasmic Reticulum
The enzymes of endoplasmic reticulum such as cytochrome p-450 and b5 enzymes and diamine oxidase contribute to the formation of ROS [68]. Another important thiol oxidase enzyme, Erop1p catalyses the transfer of electrons from dithiols to molecular oxygen results in the formation of H2O2 [69].
The other endogenous sources of ROS include prostaglandin synthesis, auto-oxidation of adrenalin, phagocytic cells, reduced riboflavin, FMNH2, FADH2, cytochrome P 450, immune cell activation, inflammation, mental stress, excessive exercise, infection, cancer, aging, ischemia etc. [68].
On the other hand, ROS are also produced in the biological systems by various exogenous sources shown in Table 3 [10].
Table 3.
ROS generated from exogenous sources
| Air & water pollution | Ultraviolet light |
| Alcohol | Cooking (smoked meat, used oil, fat) Drugs such as Halothene, Paracetamol, Bleomycine, Doxorubicin, Metrenidazole, Ethanol. CCl4 |
| Tobacco smoke | |
| Transition metals- Cd, Hg, Pb, As | |
| Heavy metals- Fe, Cu, Co, Cr | |
| Industrial solvents | |
| Pesticides | |
| High temperature |
Molecular targets of free radicals
When there is an imbalance between the free radical production (ROS/RNS) and antioxidant defenses, the former will be produced in higher concentrations leading to oxidative stress and nitrosative stress. Since these free radicals are highly reactive, they can damage all the three important classes of biological molecules including nucleic acids, proteins, and lipids [70].
Deoxyribonucleic Acid (DNA)
Both ROS/RNS can oxidatively damage the nucleic acids. The mitochondrial DNA is more vulnerable to the ROS attack than the nuclear DNA, because it is located in close proximity to the ROS generated place. ROS, most importantly, the OH• radical directly reacts with all components of DNA such as purine and pyrimidine bases, deoxyribose sugar backbone [71] and causes a number of alternations including single and double stranded breaks in DNA. The OH∙ radical abstracts hydrogen atoms to produce a number of modified puine as well as pyrimidine base by-products and DNA- protein cross links. The pyrimidine attack by OH• produces different pyrimidine adducts like thymine glycol, uracil glycol, 5-hydroxydeoxy uridine, 5-hydroxy deoxycytidine, hydantoin and others. The purine adducts formed by hydroxyl radical attack include, 8-hydroxydeoxy guanosine, 8-hydroxy deoxy adenosine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine. The other free radical induced adducts of DNA bases include, 5-formyl uracil, cytosine glycol, 5,6-dihydrothyronine, 5-hydroxy-6-hydro-cytosine, 5-hydroxy-6-hydro uracil, uracil glycol, and alloxan [72]. The major free radical induced adducts of the sugar moiety in DNA include glycolic acid, 2-deoxytetrodialdose, erythrose, 2-deoxypentonic acid lactone, 2-deoxypentose-4-ulose [72]. 8-hydroxy deoxyguanosine is considered as the biomarker of oxidative DNA damage and is involved in mutagenesis, carcinogenesis and ageing. The levels of 8-OHdG are higher in mitochondrial DNA than in nuclear DNA [73].
On the other hand, the RNS, most importantly, peroxynitrite (OONO−) interacts with guanine to produce nitrative and oxidative DNA lesions such as 8-nitroguanine and 8-oxodeoxyguanosine respectively [74]. 8-nitroguanine formed is unstable and can be spontaneously removed, resulting in the formation of an apurininc site [75]. Conversely adenine can be paired with 8-nitroguanine during DNA synthesis resulting in a G-T transversions [76]. Accordingly 8-nitroguanine is a mutagenic DNA lesion involved in carcinogenesis.
Ribonucleic acid (RNA)
ROS can attack different RNAs produced in the body. The RNA is more prone to oxidative damage than DNA, due to its single stranded nature, lack of an active repair mechanism for oxidized RNA, less protection by proteins than DNA and moreover these cytoplasmic RNAs are located in close proximity to the mitochondria where loads of ROS are produced. Indeed, RNA is subjected to more oxidative damage than DNA in humans [77]. 7, 8-dihydro-8-oxo-guanosine (8-oxoG) is the most extensively studied RNA damage product and its levels are raised in various pathological conditions like Alzheimer’s disease [78], Parkinson’s disease [79], atherosclerosis [80], hemochromastosis [81] and myopathies [82].
Lipids
The membrane lipids, especially the polyunsaturated fatty acid residues of phospholipids are more susceptible to oxidation by free radicals [83]. The lipid peroxidation is very important in vivo because of its involvement in various pathological conditions. The lipid peroxidation results in the loss of membrane functioning, for example, decreased fluidity, inactivation of membrane bound enzymes and receptors [84]. The lipid peroxidation is initiated, when any free radical attacks and abstracts hydrogen from a methylene groups (CH2) in a fatty acid (LH) which results in the formation of a carbon centered lipid radical (L•). The lipid radical can react with molecular oxygen to form a lipid peroxyl radical (LOO•). The resultant lipid peroxyl radical (LOO•) undergo rearrangement via a cyclisation reaction to form endoperoxides, which finally form malondialdehyde (MDA) and 4-hydroxyl nonenal (4-HNA), the toxic end products of lipid peroxidation that cause damage to the DNA and proteins [85]. These lipid peroxyl radicals can further propagate the peroxidation process by abstracting hydrogen atoms from the other lipid molecules. Isoprostanes (prostaglandin like substances produced by in the body by the esterfication of arachidonic acid) constitute the important product of lipid peroxidation of arachidonic acid and are considered as the makers of the oxidative lipid damage [86].
Proteins
The protein oxidation can be induced by radical species such as O•−2, OH•, peroxyl, alkoxyl, hydroperoxyl as well as by the non radical species such as H2O2, O3, HOCl, singlet oxygen, OONO- [87]. ROS oxidize different amino acids present in the proteins, causing formation of protein–protein cross linkages, results in the denaturing and loss of functioning of proteins, loss of enzyme activity, loss of function of receptors and transport proteins [88]. The sulphur containing amino acids such as methionine and cysteine are more susceptible to oxidation by ROS and are converted to disulphides and methionine sulphoxide [89, 90] respectively. However in biological systems, only these two oxidized forms of proteins can be converted back to their native form by two different enzymes namely disulfide reductases and methionine sulfoxide reductases respectively [91–94].
The ROS mediated attack of different amino acids results in the formation of different oxidation products such as, tryptophan forms nitrotryptophan, kynurenine, formylkynurinine; Phenylalanine forms 2,3-Dihydroxyphenylalanine, 2-, 3-, and 4-hydroxyphenylalanine; Tyrosine forms 3,4-Dihydroxyphenylalanine, tyrosine–tyrosine cross-linkages, Tyr-O-Tyr, cross-linked nitrotyrosine; Histidine forms 2-Oxohistidine, asparagine, aspartic acid; Arginine forms glutamic semialdehyde; Lysine forms a-Aminoadipic semialdehyde; Proline forms 2-Pyrrolidone, 4- and 5-hydroxyproline pyroglutamic acid, glutamic semialdehyde; threonine forms 2-Amino-3-ketobutyric acid; leucine and valine residues form hydroxyl residues [91].
The ROS induced oxidative damage of amino acid residues such as lysine, proline, threonine and arginine yields carbonyl derivatives. The presence of carbonyl groups in proteins has been considered as the marker of ROS mediated protein oxidation [95]. The other specific markers of protein oxidation are O-tyrosine (a marker for hydroxyl radical) and 3-nitrotyrosine (a marker for RNS). An increase in the levels of protein carbonyls is observed in a number of pathological conditions such as, Alzheimer’s disease [96], parkinson’s disease [97], muscular dystrophy [98], cataractogenesis [99], Rheumatoid Arthritis [100], diabetes [101], progeria, atherosclerosis, respiratory dystrous syndrome, Werner’s syndrome [91], and ageing [96, 102].
Free Radicals and Diseases
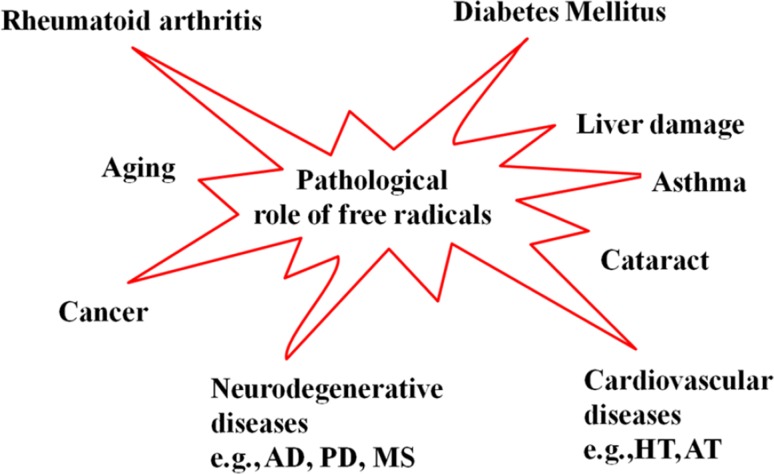
Free radicals are involved in many pathological conditions such as many types of diabetes, neurodegenerative diseases, cardiovascular diseases (CVDs), cancer, cataracts, asthma, rheumatoid arthritis, inflammation, burns, intestinal tract diseases, progerias and ischemic and post-ischemic pathologies. The role of free radicals in some of the important disease conditions (see Fig. 2) is discussed in this section.
Fig. 2.
Pathological role of free radicals
Diabetes Mellitus
Diabetes mellitus is heterogeneous group of chronic disorders characterized by enhanced blood glucose levels (hyperglycemia) resulting from defective insulin secretion (in type I diabetes), resistance to insulin action (in type II diabetes) or both [103]. The major symptoms are thirst, hunger, emaciation, and weakness, eventually lead to coma. DM is associated with the increased production of free radicals or decreased activity of the antioxidant systems, which leads to development of oxidative stress [104, 105]. The hyperglycemic condition induces increased free radical production via four different routes namely, 1) increased glycolysis, results in increased ratio between the rate of oxidation of G3P to 1, 3-DPG, following increased NADH/NAD+ ratio (redox imbalance) 2) activated sorbitol (or polyol) pathway, causes the accumulation of both sorbitol and fructose, results in decreased reduced GSH and increased NADH/NAD+ ratio. 3) autoxidation of glucose, results in the generation of different free radicals such as H2O2, OH•, O•−2 and ketoaldehydes and 4) non enzymatic protein glycation, results in the formation of AGEs which upon interacting with RAGEs generate oxidative stress [106].
Both mitochondrial and non-mitochondrial derived ROS contribute to oxidative stress during DM. Under normal conditions, the electron transport chain complexes I and III are the key sites of superoxide production [107]. However, the increased glucose levels in DM lead to increased glycolysis resulting in the augmented generation of pyruvate, thus raising the inner mitochondrial membrane potential upwards, followed by mitochondrial dysfunction and increased ROS production at electron transport chain complex II [108].
Neurodegenrative Diseases
The central nervous system (CNS) is particularly susceptible to the oxidants due to the presence of high lipid content, high consumption of oxygen, and low levels of antioxidant enzymes, for example, SOD is localized primarily in neurons, and GSH and GPx are localized in astrocytes [109]. The lipid peroxidation by ROS leads to progressive loss of membrane fluidity, decreases membrane potential, and increases permeability to ions such as Ca2+. The regions of the brain such as hippocampus, substantia nigra, and the striatum are particularly susceptible to attack by free radicals [110, 111]. The oxidative-stress state has been also implicated in several neurodegenerative diseases such as Alzheimer’s [112], Parkinson’s [113], Huntington’s, lateral amyotrophic sclerosis [114], and multiple Sclerosis [115].
Parkinson’s Disease (PD)
Parkinson’s disease (PD) is characterized by the loss of dopaminergic neurons (involve in learning, memory and motor control), especially in the midbrain area called the substantia nigra, accompanied by deposition of inclusion bodies (Lewy bodies) of α-synuclein. The redox imbalance causes oxidative damage to these neurons and begins to alter the synthesis and metabolic pathway of dopamine leads to a further increase in oxidative stress because of quinine formation [111]. The characteristic clinical symptoms of PD include, jerky movements, trembling of the hands and lips, and tremors [116]. Dopamine, a neurotransmitter, can also act as a metal chelator, has the ability to generate H2O2 via Fenton reaction. Ceruloplasmin (an extracellular ferroxidase required for regulating cellular iron load and transport) oxidation results in the decreased ferroxidase activity followed by the accumulation of intracellular iron in neurons in PD. The increased levels of Fe+3 mediate the production of hydroxyl radicals, results in the damage of dopaminergic neurons in PD [117].
Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) is characterized by the accumulation of amyloid protein plaques (formed from the improper folding and processing of amyloid β precursor protein-ABPP) [168] and intracellular neurofibrillary tangles made up of abnormal and hyperphosphorylated tau protein [118]. The hyperphosphorylated tau protein aggregates binds to Fe3+, results in the production of neurofibrillary tangles [119]. The Amyloid-β peptide (Aβ) can chelate with transition metal ions (Cu2+, Zn2+ and Fe3+), and produce H2O2 via transition metal mediated catlysis and finally gives toxic OH˙ radical [120]. The lipid peroxidation is also extensive in AD patients, which can induce neuronal death by multiple mechanisms such as impairment of function of ion pumps (both Na+/K+-ATPase and Ca+2-ATPase), glucose transporters and glutamate transporters. The other oxidative markers of protein damage such as protein carbonyls and 3-nitrotyrosine have been also observed in AD patients [118].
Multiple Sclerosis (MS)
MS is an autoimmune neuronal disorder characterized by impaired nerve conduction due to demylination of central nervous system (CNS). The activated microglia/macrophages initiate the MS by the generation of ROS [121] that can induce lipid peroxidation, results in the demylination and damage of neurons. Elevated TBARS levels and reduced protein SH groups, the representatives of protein oxidation and slightly reduced SOD was reported in MS patients [122]. Apart from ROS generation, an impaired iron metabolism has been also considered to play a major role in pathogenesis of disease.
Cancer
It is one of the leading causes of death in humans. Free radicals cause different types of chemical changes in DNA, thus they could be mutagenic and involved in the etiology of cancer [123, 124]. Cancer cells in particular, in comparison to normal cells, have higher levels of ROS and are more susceptible to mitochondrial dysfunction due to their higher metabolic rate [125]. Cancer cells display elevated levels of oxidative stress due to activation of oncogenes and loss of tumor suppressors [126]. ROS by altering the growth signals and gene expression cause continuous proliferation of cancer cells [127]. ROS can damage DNA by inducing base modifications, deletions, strand breakage, chromosomal rearrangements and hyper- and hypo-methylation of DNA [128].
Colorectal Cancer
Colorectal cancer (CRC) is the third most common cancer worldwide, accounting for 608,000 deaths per year [129]. The gastrointestinal tract, particularly the colon and rectum, is continuously exposed to ROS originating from both endogenous and exogenous sources [130]. Colon cancer originates from the epithelial cells that line the bowel. These cells divide rapidly and have a high metabolic rate [131]. Since the intestinal mucosa is constantly confronting with diet and bacterial-derived oxidants and carcinogens, an unrestrained production of free radicals, redox imbalance, and DNA damage occurs, finally leads to an altered intestinal metabolic homeostasis with cancer as an endpoint [133]. The human colorectal tumors have increased levels of nitric oxide (NO) [132], 8-oxodG in DNA [133], and lipid peroxides [134]. Suzuki et al. 2004 [135] have reported increased serum levels of oxidized low density lipoprotein in patients with CRC compared to healthy individuals.
Breast Cancer
Damage to the breast epithelium by ROS can lead to fibroblasts proliferation, hyperplasia of epithelium, cellular atypia and breast cancer [136]. In majority of breast carcinomas the oxidative stress can be induced by the over expression of thymidine phosphorylase enzyme which catabolizes thymidine to thymine and 2-deoxy-D-ribose-1-phosphate; the latter is a powerful reducing sugar that rapidly glycates proteins, generating oxygen radicals within the carcinoma cell [137]. Another breast specific mechanism of oxidative stress induction involves a mammary gland specific lactoperoxidase enzyme catalyzed one electron oxidation of 17-β-oestradiol to a reactive phenoxyl radical [138].
Prostate Cancer
The ROS produced are responsible for the cellular proliferation of prostate cancer cells [139]. Overexpression of NADPH oxidase 1 (Nox1) protein is an early event in the development of prostate cancer. Prostrate Tumors have considerably higher levels of ROS and Nox1 levels [140]. The superoxide produced (by NOX) in prostate cancer cells facilitates cellular immortality through resistance to programmed cell death which results in cancer-promoting effect [141]. Kumar et al. 2008 [142] have reported elevated ROS levels in prostate cancer cells compared with normal cells. Veeramani et al. 2008 [143] have reported that elevated levels of ROS might also be formed by the simultaneous increased levels of p66Shc protein in prostate cancer cells.
Lung Cancer
Lung cancer has been the most commonly diagnosed cancer and is the central cause of cancer death in men worldwide [144]. Lung cancer mortality account 30 % of all cancer related deaths. Oxidative stress plays an important role in lung inflammation and lung cancer [145]. Cigarette Smoking is the most crucial environmental risk factor in lung cancer etiology. It is estimated that smoking accounts for ~80 % of global lung cancer burden in males and 50 % in females [146]. Cigarette smoke particulate matter contains complex mixture of numerous carcinogens and stable ROS with very long half-lives. These ROS can damage the tissues resulting in progressive transformation of cells into the malignant form, which leads to increased frequency of mutations by the oxidative damage to DNA and, eventually leading to lung cancer [147]. Smokers develop lung cancer a 10-fold higher than non-smokers. Lung cancer (LC) and chronic obstructive pulmonary disease (COPD) commonly coexist in smokers, and the presence of COPD increases the risk of developing LC [148].
Bladder Cancer
Bladder cancer is one of the most common cancers across the world, ranking the fourth and tenth in men and women, respectively [149]. The most common risk factors for bladder cancer are cigarette smoking, exposure to industrial carcinogens (aromatic amines), high levels of arsenic intake and diet [150]. Oxidative stress critically contributes to the development of bladder cancer [151]. Various lines of evidence reported an increased oxidative stress in patients with breast cancer [152, 153]. Increased NO levels have been reported in bladder cancer patients [154]. This NO stimulates matrix metalloproteinases (MMPs), especially prolidase activity, which is involved in the terminal step of collagen degradation. Significantly higher serum prolidase activities were reported in patients with bladder cancer than healthy controls [155]. Therefore, increased prolidase activity may, in part, play a role in the pathogenesis of bladder cancer.
Epidemiological studies reveal that low levels of antioxidants are associated with an increased risk of cancer. Significant increase in total oxidant status levels and decrease in total antioxidant status were observed in patients with bladder cancer [156]. Significantly lower levels of plasma protein, total thiol groups and protein-bound thiol groups and elevated levels of Protein carbonyl groups were observed in bladder cancer patients than in healthy controls [157].
Cardiovascular Diseases (CVDs)
Cardiovascular diseases are a class of pathologies involving the heart and blood vessels (arteries, capillaries, and veins). They include cardiac diseases, vascular diseases of the brain and kidney, and peripheral arterial disease. Most of the people are dying due to CVDs compared to other diseases [158].
Atherosclerosis
Atherosclerosis is a condition commonly referred to as hardening of the arteries. Hyperlipidemia is a major risk factor for atherosclerosis. Elevated levels of oxidized low density lipoprotein (LDL), glucose and free fatty acids are found in patients with atherosclerosis, T2D, and obesity [159]. A profound imbalance of oxidants and antioxidants resulting in oxidative stress is observed in atherosclerosis. In the vessel wall, endothelial cells, smooth muscle cells (SMCs) and macrophages are sources of free radicals [160]. Endothelial dysfunction leads to increased endothelial permeability, up regulation of endothelial adhesion molecules, and inflammatory cell infiltration into the arterial wall [160]. A substantial data has been shown that ROS are involved in endothelial injury, dysfunction, and lesion progression [161]. The ROS dependent activation of the MMPs results in the degradation of intimal extracellular matrices and promotes smooth muscle cell migration [162]. Cigarette smoking contain large amount of free radicals and may down-regulate key exogenous and endogenous antioixdants such as vitamin-D, carotenes, GPx and SOD and can lead to the dysfunction of monocytes and vascular smooth muscle cells [163]. The proatherogenic agents such as oxidaised lipids, high glucose and cigarette constituents give rise to increased free radical production.
The endothelial nitric oxide synthase (eNOS) derived NO. plays an important role in maintaining vascular tone and vasoreactivity, vasodialation, platelet aggregation and in maintaining balance between smooth muscle cell growth and differentiation. Decreased NO bioavailabilty is the one of the major feature of the CVDs [249]. During reduced availability of BH4 or L-Arg, eNOS becomes uncoupled from a to a state [164]. The O•−2 formed can interact with NO• to produce ONOO-, a damaging and cytotoxic free radical with potential to disturb cardiovascular function. ONOO- reduces the bioavailability of NO leading to reduced endothelial vascular regulatory capacity and increased vascular dysfunction. ONOO− also inactivates the BH4 cofactor effectively amplifying the damaging effects of eNOS uncoupling the endothelium [164]. Mitochondrial DNA damage is frequently observed in human atherosclerosis in both circulating and vessel wall cells [165]. Oxidative stress mediated damaged mitochondrial DNA that escape autophagy induces a potent inflammatory response in atherosclerosis [166]. Malfunction of DNA repair leads to defects in cell proliferation, apoptosis, and mitochondrial dysfunction, which in turn leads to ketosis, hyperlipidemia, and increased fat storage, promoting atherosclerosis and the metabolic syndrome [167].
Hypertension (HT)
Hypertension (HT) is a major health problem worldwide account 40 % of the total adult population [168]. Persons with hypertension are at an increased risk for stroke, heart disease, kidney failure, and premature mortality. Free radical induced oxidative stress in part contributes to endothelial dysfunction and development of hypertension [169]. Increased ROS generation eliminates NO• by forming ONOO-, thus reducing NO• bioavailability which leads to decreased endothelium-dependent vasodilation resulting in hypertension [170]. A decrease in NO bioavailability and an increase in oxidative stress are present in human hypertension [171]. Oxidation-induced impairment of NO also results in reduced opposition to the vasoconstrictive and hypertensive effects of angiotensin II. Angiotensin II decreases NO bioavailability by promoting oxidative stress [172].
Cataract
It is the most common cause of the visual impairment affecting about 25 million people throughout the world, with the highest incidence occurring in developing countries [173, 174]. It is characterized by opacity of the eye lens that reduces the amount of incoming light and results in visual impairment [173]. Although a number of factors such as genetic factors, diabetes, aging, smoking, drugs, malnutrition, radiation (x-rays and UV rays) and alteration in both endocrine and enzymatic equilibrium have been implicated in cataract formation [175], the free radical induced oxidative stress is considered as one of the major underlying mechanism of cataract disorder [176]. Oxidation of proteins, lipids and DNA is seen in cataract lenses. Proteins lose sulfhydryl (–SH) groups become cross linked by non disulfide bonds, form high molecular aggregates and become insoluble [177]. The oxidative stress induced lipid peroxidation toxic product such as HNE induce the fragmentation of lens proteins contributing towards the opacity of the lens [178]. Oxidative stress has been shown to induce lens opacification both in experimental animal models and cultured lens systems [179].
The cornea absorbs the light in the range of above 300 nm results in the activation of tryptophan, to form N-formyl kynurenine, 3-hydroxy kynurenine and other photoproducts [180, 181]. These photoproducts gradually accumulate in the centre of the lens are capable of generating singlet oxygen which induce protein damage leading to the loss of transparency [181]. Cataract lens have an intracellular ionic imbalance (i.e. altered ionic homeostasis) than normal lens. The ROS induced by UV rays in sunlight alters the ionic homeostasis results in the increased levels of Ca+2 and Na+2, coupled with the decreased levels of Mg+2 and K+2 in the lens [182]. The increased calcium activates calpains, a family of calcium dependent non-lysosomal cysteine proteases [175], which degrade lens proteins such as both α and β crystalline proteins results in opaque lens characteristic of cataract [183]. Elevated levels of H2O2 were observed in cataract lenses than normal lenses [178, 184].
Rheumatoid Arthritis (RA)
RA is chronic multisystem disease of unknown cause which affects approximately 1–2 % of the total world population and women are affected more than men [185]. The disease is characterized by synovial and systemic inflammation with joint swelling, morning stiffness, destruction of articular tissues, joint deformities, fatigue, loss of appetite and weakness [186–189]. It is believed to be a T-lymphocyte driven disease in which a sudden influx of T-cells into the affected joints is followed by an increased number of macrophages and fibroblasts drawn the release of cytokines particularly IL-1 and TNF-alpha. This cytokine release and subsequent migration is thought to be responsible for the chronic inflammation and characteristic changes in RA [188].
Several lines of evidence suggest a role for oxidative stress in the pathogenesis of RA. Both ROS and RNS damage cartilage. Tissue injury in inflammation results in NO. production by articular chondrocytes and synovial fibroblasts and elevated levels of NO. are observed in the serum and synovial fluid of RA patients [190]. The free radicals, particularly and O•−2, inhibit the synthesis of matrix components like proteoglycans by chondrocytes and also damage the extracellular matrix through activation and up regulation of matrix metalloproteinases [191]. The HOCl, produced by myeloperoxidase (MPO) in neutrophils, chlorinate the tyrosine residues to form 3-chlorotyrosine and damage the collagen, thus implicated in arthritogensis. RA patients have increased plasma MPO concentrations [186].
Elevated levels of MDA, NO•, protein carbonyls, oxidized hyaluronic acid and oxidized LDL have been reported in RA patients [192–195]. These oxidized LDL can be ingested in large quantities by monocytes results in the formation of Foam cells that are present in atherosclerotic plaques of vessles and have also been found in RA synovial fluid [196]. Cigarette smoking is also considered as the most established environmental factor for RA. Both particulate and gaseous phases of smoke contain high concentrations of free radicals that can interact with DNA and could cause genetic mutations and activation responsible for the development of RA [197].
Asthma
Free radicals are involved in various respiratory diseases such as respiratory distress syndrome, chronic obstructive pulmonary disease, chronic bronchitis, asthma [198]. Asthma is the most common disorders of the airways of the lungs and is one of the major global health problems [199]. It is characterized by chronic inflammation of the airways involving variable and recurrent airflow obstruction and bronchial hyperreactivity associated with airway remodelling [200, 201]. Airway remodeling is a dynamic process involving mucous hypersecretion, collagen deposition, wall thickening, myocyte hypertrophy and hyperplasia, myofibroblast hyperplasia, vascular proliferation and alterations in airway elastic fibers, all of which culminate in persistent structural alterations of the airway [202]. NO is endogenously produced in mammalian airways by NOS and is known to regulate many aspects of human asthma, including modulation of airway and vascular smooth muscle tone and the inflammation. Increased production of airway NO is a key factor in the development of airway hyperresponsiveness [203].
ROS are produced both intracellularly by lung parenchymal cells and extracellularly by lung macrophages. Increased generation of oxidants have been reported in asthma patients than in healthy individuals [204] which provoked airway inflammation by inducing diverse pro-inflammatory mediators including macrophages, neutrophils and eosinophils [205]. Numerous studies have suggested that oxidative stress is caused by overproduction of various free radicals or by an insufficient antioxidant defense system in asthma and thus it contributes to the tissue damage which is induced by inflammatory cells [206]. Elevated levels of oxidative stress markers such as H2O2, 8-isoprostane, nitric oxide, and carbon monoxide were reported in exhaled air of asthmatic patients [204]. Increased MDA levels, and Protein carbonyls; decreased protein sulfhydryl and antioxidant activity were observed in plasma, bronchoalveolar lavage (BAL) fluid and exhaled air of asthamatic patients [207–209].
Conclusion
Free radicals (ROS/RNS) are produced by normal metabolism and are involved in various physiological and pathological conditions. When there is an imbalance between the antioxidants and oxidants, the fee radicals accumulate leading to vigorous damage to macromolecules such as nucleic acids, proteins and lipids. This leads to tissue damage in various disease conditions such as diabetes mellitus, neurodegenerative diseases, cancer, cardiovascular diseases, cataracts, rheumatoid arthritis, asthma etc. and thus severely hastening the disease progression.
Contributor Information
Alugoju Phaniendra, Email: phaniendra.a@gmail.com.
Dinesh Babu Jestadi, Email: dinesh.jestadi@gamil.com.
Latha Periyasamy, Email: latha.selvamani@yahoo.co.in.
References
- 1.Gomberg M. An Incidence of Trivalent Carbon Trimethylphenyl. J Am Chem Soc. 1900;22:757–771. [Google Scholar]
- 2.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation-A mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 3.Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 4.McCord JM, Fridovich I. Superoxide dismutase an enzymatic function for erythrocuprein (chemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 5.Loschen G, Flohe L, chance B. Respiratory chain linked H O production in pigeon heart mitochondria. FEBS Lett. 1971;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 6.Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 7.Mittal CK, Murad F. Activation of guanylate cyclase by superoxide-dismutase and hydroxyl radical-Physiological regulator of guanosine 3′,5′-monophosphate formation. Proc Natl Acad Sci USA. 1977;74(10):4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 2. Oxford: Clarendon Press; 1989. [Google Scholar]
- 9.Mukherji SM, Singh SP. Reaction mechanism in organic chemistry. Madras: Macmillan IndiaPress; 1986. [Google Scholar]
- 10.Pham-Huy LA, Hua He, Pham-Huy C. Free Radicals, Antioxidants in Disease and Health. Int J Biomed Sci. 2008;4(2):89–96. [PMC free article] [PubMed]
- 11.Valko M, Leibfritz D, Moncola J, Cronin MT, Mazura M, Telser J. Review Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg J, Arner EJ. Reactive oxygen species, antioxidants, and the mammalian Thioredoxin system. Free Radical Biol Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 13.Yla-Herttuala S. Oxidized LDL and atherogenesis. Ann N Y Acad Sci. 1999;874:134–137. doi: 10.1111/j.1749-6632.1999.tb09231.x. [DOI] [PubMed] [Google Scholar]
- 14.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 15.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 16.Kohen R, Nyska A. Invited review Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol Pathol. 2002;30(6):620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Free Radicals and other reactive species in disease. Nature Encyclopedia of life sciences. 2001. p. 1–7.
- 18.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Review Cell Signal. 2007;19(9):1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Mugoni V, Santoro MM. Manipulating redox signaling to block tumor angiogenesis, research directions in tumor angiogenesis, Dr. Jianyuan Chai (Ed.), ISBN: 978-953-51-0963-1, InTech, 2013. doi: 10.5772/54593.
- 20.Michelson AM, McCord JM, Fridovich I. Superoxide and Superoxide Dismutases. London: Academic Press; 1977. p. 320. [Google Scholar]
- 21.Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264(17):9880–9884. [PubMed] [Google Scholar]
- 22.Kontos HA, Wei EP, Ellis EF, Jenkins LW, Povlishock JT, Rowe GT, et al. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ Res. 1985;57(1):142–151. doi: 10.1161/01.res.57.1.142. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in hypertension. Hypertension. 1999;34:539–545. doi: 10.1161/01.hyp.34.4.539. [DOI] [PubMed] [Google Scholar]
- 24.Bielski BHJ, Cabelli DE. Superoxide and hydroxyl radical chemistry in aqueous solution. Active Oxygen in Chemistry. 1996;66–104.
- 25.Bielski BHJ, Cabelli BH, Arudi RL, Ross AB. Reactivity of RO2/O2. Radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1041–1100. [Google Scholar]
- 26.Bedwell S, Dean RT, Jessup W. The action of defined oxygen centred free radicals on human low density lipoprotein. Biochem J. 1989;262(3):707–712. doi: 10.1042/bj2620707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1(5):358–364. [PubMed] [Google Scholar]
- 28.Fenton HJH. Oxidation of tartaric acid in the presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 29.Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc London (A). 1934;147:332–351. [Google Scholar]
- 30.De Grey ADNJ. HO2˙: the forgotten radical. DNA Cell Biol. 2002;21:251–257. doi: 10.1089/104454902753759672. [DOI] [PubMed] [Google Scholar]
- 31.Cerruti PA. Pro-oxidant states and tumor activation. Science. 1985;227:375–381. [Google Scholar]
- 32.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486(1):10–13. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 33.Mates JM, Perez-Gomez C, Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32(8):595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 34.Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999;300:219–226. doi: 10.1016/s0076-6879(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 35.Hojo Y, Okado A, kawazoe S, Mizutani T. In vivo singlet-oxygen generation in blood of chromium(VI)-treated mice an electron spin resonance spin-trapping study. Biol Trace Elem Res. 2000;76(1):85–93. doi: 10.1385/BTER:76:1:85. [DOI] [PubMed] [Google Scholar]
- 36.Agnez-Lima LF, Melo JT, Silva AE, Oliveira AH, Timoteo AR, Lima-Bessa KM, et al. Review DNA damage by singlet oxygen and cellular protective mechanisms. Mutat Res. 2012;751(1):1–14. doi: 10.1016/j.mrrev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 38.Kanovasky JR. Singlet oxygen production by biological systems. Chem Biol Interact. 1989;70(1–2):1–28. doi: 10.1016/0009-2797(89)90059-8. [DOI] [PubMed] [Google Scholar]
- 39.Chan HWS. Singlet oxygen analogs in biological systems: coupled oxygenation of 1,3-dienes by soybean lipoxidase. J Am Chem Soc. 1971;93(9):2357–2358. doi: 10.1021/ja00747a071. [DOI] [PubMed] [Google Scholar]
- 40.Hayaishi O, Nozaki M. Nature and mechanisms of oxygenases. Science. 1969;164:389–396. doi: 10.1126/science.164.3878.389. [DOI] [PubMed] [Google Scholar]
- 41.Kanofsky JR. Singlet oxygen production by lactoperoxidase. J Biol Chem. 1983;258(10):5991–5993. [PubMed] [Google Scholar]
- 42.Sies H, Menck CF. Singlet oxygen induced DNA damage. Mutat Res. 1992;275:367–375. doi: 10.1016/0921-8734(92)90039-r. [DOI] [PubMed] [Google Scholar]
- 43.Lerner RA, Eschenmoser A. Ozone in biology. Proc Natl Acad Sci USA. 2003;100(6):3013–3015. doi: 10.1073/pnas.0730791100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein BD, Lodi C, Collinson C, Balchum OJ. Ozone and lipid peroxidation. Arch Environ Heath. 1969;18:631–635. doi: 10.1080/00039896.1969.10665464. [DOI] [PubMed] [Google Scholar]
- 45.Freeman BA, Mudd JB. Reaction of ozone with sulfhydryls of human erythrocytes. Arch Biochem Biophys. 1981;208(1):212–220. doi: 10.1016/0003-9861(81)90142-9. [DOI] [PubMed] [Google Scholar]
- 46.Mudd JB, Leavitt R, Ongun A, McManus TT. Reaction of ozone with amino acids and proteins. Atmos Environ. 1969;3:669–681. doi: 10.1016/0004-6981(69)90024-9. [DOI] [PubMed] [Google Scholar]
- 47.Mustafa MG. Biochemical Basis of Ozone Toxicity. Free Radical Biol Med. 1990;9:245–265. doi: 10.1016/0891-5849(90)90035-h. [DOI] [PubMed] [Google Scholar]
- 48.Fetner RH. Ozone induced chromosome breakage in human cell culture. Nature. 1962;194:793–794. doi: 10.1038/194793a0. [DOI] [PubMed] [Google Scholar]
- 49.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med. 2000;29(5):403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 50.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci USA. 1981;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840(2):204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 52.Prutz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys. 1996;332(1):110–120. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 53.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 54.Chiueh CC. Neuroprotective properties of nitric oxide. Ann N Y Acad Sci. 1999;890:301–311. doi: 10.1111/j.1749-6632.1999.tb08007.x. [DOI] [PubMed] [Google Scholar]
- 55.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chandhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25(4–5):434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 57.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 58.Koshland DE., Jr The molecule of the year. Science. 1992;258(5090):1861. doi: 10.1126/science.1470903. [DOI] [PubMed] [Google Scholar]
- 59.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 60.Douki H, Cadet J. Peroxynitrite mediated oxidation of purine bases of nucleosides and isolated DNA. Free Rad Res. 1996;24(5):369–380. doi: 10.3109/10715769609088035. [DOI] [PubMed] [Google Scholar]
- 61.Ischiropoulos H, Al-Mehdi AB. Peroxynitrite mediated oxidative protein modifications. FEBS Lett. 1995;364(3):279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 62.Czapski G, Goldstein S. The role of the reactions of NO with superoxide and oxygen in biological systems: a kinetic approach. Free Radic Biol Med. 1995;19(6):785–794. doi: 10.1016/0891-5849(95)00081-8. [DOI] [PubMed] [Google Scholar]
- 63.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 64.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122(2):221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 66.De Duve C, Bauduhuin P. peroxisomes (microbodies and related particles) Physiol Rev. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 67.Schrader M, Fahimi HD. Review Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763(12):1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49(3):481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 69.Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, et al. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Nat Acad Sci USA. 2006;103(2):299–304. doi: 10.1073/pnas.0506448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Droge W. Review Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 71.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Midsomer Norton: Oxford University Press; 1999. [Google Scholar]
- 72.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radical Biol Med. 2002;32(11):1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 73.Barja G. The flux of free radical attack through mitochondrial DNA is related to aging rate. Aging (Milano). 2000;12(5):342–355. doi: 10.1007/BF03339859. [DOI] [PubMed] [Google Scholar]
- 74.Hiraku Y, Kawanishi S, Ichinose T, Murata M. The role of iNOS-mediated DNA damage in infection- and asbestos-induced carcinogenesis. Ann NY Acad Sci. 2010;1203:15–22. doi: 10.1111/j.1749-6632.2010.05602.x. [DOI] [PubMed] [Google Scholar]
- 75.Yermilov V, Rubio J, Ohshima H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376(3):207–210. doi: 10.1016/0014-5793(95)01281-6. [DOI] [PubMed] [Google Scholar]
- 76.Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 77.Hofer T, Badouard C, Bajak E, Ravanat JL, Mattsson A, Cotgreave IA. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol Chem. 2005;386(4):333–337. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- 78.Abe T, Tohgi H, Isobe C, Murata T, Sato C. Remarkable increase in the concentration of 8-hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer’s disease. J Neurosci Res. 2002;70(3):447–450. doi: 10.1002/jnr.10349. [DOI] [PubMed] [Google Scholar]
- 79.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis. 2002;9(2):244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 80.Martinet W, de Meyer GR, Herman AG, Kockx MM. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur J Clin Invest. 2004;34(5):323–327. doi: 10.1111/j.1365-2362.2004.01343.x. [DOI] [PubMed] [Google Scholar]
- 81.Broedbaek K, Poulsen HE, Weimann A, Kom GD, Schwedhelm E, Nielsen P, et al. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radical Biol Med. 2009;47(8):1230–1233. doi: 10.1016/j.freeradbiomed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Tateyama M, Takeda A, Onodera Y, Matsuzaki M, Hasegawa T, Nunomura A, et al. Oxidative stress and predominant Abeta 42 (43) deposition in myopathies with rimmed vacuoles. Acta Neuropathol. 2003;105(6):581–585. doi: 10.1007/s00401-003-0685-2. [DOI] [PubMed] [Google Scholar]
- 83.Siems WG, Grune T, Esterbauer H. 4-Hydroxynonenal formation during ischemia and reperfusion of rat small-intestine. Life Sci. 1995;57(8):785–789. doi: 10.1016/0024-3205(95)02006-5. [DOI] [PubMed] [Google Scholar]
- 84.Bast A. Oxidative stress and calcium homeostasis. In: Halliwell B, Aruoma OI, editors. DNA and free radicals. London: Ellis Horwood; 1993. pp. 95–108. [Google Scholar]
- 85.Marnett LJ. Lipid peroxidation—DNA damage by malondialdehyde. Mutat Res. 1999;424(1–2):83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 86.Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil chem Soc. 1998;75(2):199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butterfield DA, Koppal T, Howard B, Subramaniam R, Hall N, Hensley K, et al. Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann N Y Acad Sci. 1998;854:448–462. doi: 10.1111/j.1749-6632.1998.tb09924.x. [DOI] [PubMed] [Google Scholar]
- 89.Brodie E, Reed DJ. Cellular recovery of glyceraldehyde-3-phosphate dehydrogenase activity and thiol status after exposure to hydroperoxide. Arch Biochem Biophys. 1990;276(1):210–212. doi: 10.1016/0003-9861(90)90028-w. [DOI] [PubMed] [Google Scholar]
- 90.Pryor WA, Jin X, Squadrito GL. One- and two-electron oxidations of methionine by peroxynitrite. Proc Natl Acad Sci USA. 1994;91(23):11173–11177. doi: 10.1073/pnas.91.23.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berlett BS, Stadtman E. Protein oxidation in aging, disease, and oxidative stress. J Bio Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 92.Kikugawa K, Kato T, Okamoto Y. Damage of amino acids and proteins induced by nitrogen dioxide, a free radical toxin, in air. Free Rad Biol Med. 1994;16(3):373–382. doi: 10.1016/0891-5849(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 93.Uchida K, Kawakishi S. 2-oxohistidine as a novel biological marker for oxidatively modified proteins. FEBS Lett. 1993;332(3):208–210. doi: 10.1016/0014-5793(93)80632-5. [DOI] [PubMed] [Google Scholar]
- 94.Garrison WM. Reaction mechanisms in radiolysis of peptides, polypeptides, and proteins. Chem Rev. 1987;8792:381–398. [Google Scholar]
- 95.Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000;33:S99–S108. [PubMed] [Google Scholar]
- 96.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;7091:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 98.Murphy ME, Kehrer JP. Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem J. 1989;260(2):359–364. doi: 10.1042/bj2600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garland D, Russell P, Zigler JS. Oxidative modification of lens proteins. Basic Life Sci. 1988;49:347–353. doi: 10.1007/978-1-4684-5568-7_52. [DOI] [PubMed] [Google Scholar]
- 100.Chapman ML, Rubin BR, Gracy RW. Increased carbonyl content of proteins in synovial fluid from patients with rhematoid arthritis. J Rheumatol. 1989;16(1):15–18. [PubMed] [Google Scholar]
- 101.Jones RH, Hothersall JS. The effect of diabetes and dietary ascorbate supplementation on the oxidative modification of rat lens beta L crystallin. Biochem Med Metab Biol. 1993;50(2):197–209. doi: 10.1006/bmmb.1993.1062. [DOI] [PubMed] [Google Scholar]
- 102.Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987;262(12):5488–5491. [PubMed] [Google Scholar]
- 103.Gavin JR, Alberti KGMM, Davidson MB, DeFronzo RA, Drash A, Gabbe SG, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 104.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5(2):113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 105.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 106.Ahmed RG. The physiological and biochemical Effects of diabetes on the balance between oxidative stress and Antioxidant defense system. Med J Islam World Acad Sci. 2005;15(1):31–42. [Google Scholar]
- 107.Kwong LK, Sohal RS. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch Biochem Biophys. 1998;350(1):118–126. doi: 10.1006/abbi.1997.0489. [DOI] [PubMed] [Google Scholar]
- 108.Bajaj S, Khan A. Antioxidants and diabetes. Indian J Endocrinol Metab. 2012;2:S267–S271. doi: 10.4103/2230-8210.104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pollack M, Leeuwenburgh C. Molecular mechanisms of oxidative stress in aging: free radicals, aging, antioxidants and disease. Elsevier Science B.V. Handbook of Oxidants and Antioxidants in Exercise. 1999;881–923.
- 110.Rivas-Arancibia S, Guevara-Guzmán R, López-Vidal Y, Rodríguez-Martínez E, Zanardo-Gomes M, Angoa-Pérez M, et al. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol Sci. 2010;113(1):187–197. doi: 10.1093/toxsci/kfp252. [DOI] [PubMed] [Google Scholar]
- 111.Santiago-López JA, Bautista-Martínez CI, Reyes-Hernandez M, Aguilar-Martínez S, Rivas- Arancibia S. Oxidative stress, progressive damage in the substantia nigra and plasma dopamine oxidation, in rats chronically exposed to ozone. Toxicol Lett. 2010;197(3):193–200. doi: 10.1016/j.toxlet.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 112.Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP, Chen XC. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer’s disease. Mol Neurodegener. 2011;6(45):1–17. doi: 10.1186/1750-1326-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sevcsik E, Trexler AJ, Dunn JM, Rhoades E. Allostery in a disordered protein: oxidative modifications to α-synuclein act distally to regulate membrane binding. J Am Chem Soc. 2011;133(18):7152–7158. doi: 10.1021/ja2009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao W, Varghese M, Yemul S, Pan Y, Cheng A, Marano P, et al. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1α) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6(1):1–8. doi: 10.1186/1750-1326-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Witherick J, Wilkins A, Scolding N, Kemp K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2010;1–11. [DOI] [PMC free article] [PubMed]
- 116.Fisher LJ, Gage FH. Radical directions in Parkinson’s disease. Nat Med. 1995;1(3):201–203. doi: 10.1038/nm0395-201. [DOI] [PubMed] [Google Scholar]
- 117.Olivieri S, Conti A, Iannaccone S, Cannistraci CV, Campanella A, Barbariga M, et al. Ceruloplasmin oxidation, a feature of Parkinson’s disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J Neurosci. 2011;31:18568–18577. doi: 10.1523/JNEUROSCI.3768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545(1):39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 119.Chang Y, Kong Q, Shan X, Tian G, Ilieva H, Cleveland DW, et al. Messenger RNA oxidation occurs early in disease pathogenesis and promotes motor neuron degeneration in ALS. PLoS ONE. 2008;3(8):1–19. doi: 10.1371/journal.pone.0002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases. A Review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274(1–2):48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 122.Mitosek-Szewczyk K, Gordon-Krajcer W, Walendzik P, Stelmasiak Z. Free radical peroxidation products in cerebrospinal fluid and serum of patients with multiple sclerosis after glucocorticoid therapy. Folia Neuropathol. 2010;48(2):116–122. [PubMed] [Google Scholar]
- 123.Goldstein BD, Witz G. Free radicals and carcinogenesis. Free Radic Res Commum. 1990;11(1–3):3–10. doi: 10.3109/10715769009109662. [DOI] [PubMed] [Google Scholar]
- 124.Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32A(1):30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 125.Acuna UM, Wittwer J, Ayers S, Pearce CJ, Oberlies NH, De Blanco EJ. Effects of (5Z)-7-Oxozeaenol on the Oxidative pathway of cancer cells. Anticancer Res. 2012;32(7):2665–2671. [PMC free article] [PubMed] [Google Scholar]
- 126.Cairns RA, Harris I, McCracken S, Mak TW. Cancer cell metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:299–311. doi: 10.1101/sqb.2011.76.012856. [DOI] [PubMed] [Google Scholar]
- 127.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal hitological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 128.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266(1–2):37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 129.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 130.Blau S, Rubinstein A, Bass P, Singaram C, Kohen R. Differences in the reducing power along the rat GI tract: lower antioxidant capacity of the colon. Mol Cell Biochem. 1999;194(1–2):185–191. doi: 10.1023/a:1006994800272. [DOI] [PubMed] [Google Scholar]
- 131.Foksinski M, Rozalski R, Guz J, Ruszkowska B, Sztukowska P, Piwowarski M, et al. Urinary excretion of DNA repair products correlates with metabolic rates as well as with maximum life spans of different mammalian species. Free Radic Biol Med. 2004;37(9):1449–1454. doi: 10.1016/j.freeradbiomed.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 132.Haklar G, Sayin-Ozveri E, Yuksel M, Aktan AO, Yalcin AS. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001;165(2):219–224. doi: 10.1016/s0304-3835(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 133.Guz J, Foksinski M, Siomek A, Gackowski D, Rozalski R, Dziaman T, et al. The relationship between 8-oxo-7,8-dihydro-2-deoxyguanosine level and extent of cytosine methylation in leukocytes DNA of healthy subjects and in patients with colon adenomas and carcinomas. Mutat Res. 2008;640(1–2):170–173. doi: 10.1016/j.mrfmmm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 134.Rainis T, Maor I, Lanir A, Shnizer S, Lavy A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Dig Dis Sci. 2007;52(2):526–530. doi: 10.1007/s10620-006-9177-2. [DOI] [PubMed] [Google Scholar]
- 135.Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Toyoshima H, et al. Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: a case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol Biomark Prev. 2004;13(11):1781–1787. [PubMed] [Google Scholar]
- 136.Murrell TG. Epidemiological and biochemical support for a theory on the cause and prevention of breast cancer. Med Hypotheses. 1991;36(4):389–396. doi: 10.1016/0306-9877(91)90018-t. [DOI] [PubMed] [Google Scholar]
- 137.Brown NS, Jones A, Fujiyama C, Harris AL, Bicknell R. Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res. 2000;60(22):6298–6302. [PubMed] [Google Scholar]
- 138.Sipe HJ, Jr, Jordan SJ, Hanna PM, Mason RP. The metabolism of 17 beta-estradiol by lactoperoxidase: a possible source of oxidative stress in breast cancer. Carcinogenesis. 1994;15(11):2637–2643. doi: 10.1093/carcin/15.11.2637. [DOI] [PubMed] [Google Scholar]
- 139.Arnold RS, He J, Remo A, Ritsick D, Yin-Goen Q, Lambeth JD, et al. Nox1 expression determines cellular reactive oxygen and modulates c-fos-induced growth factor, interleukin-8, and Cav-1. Am J Pathol. 2007;171(6):2021–2032. doi: 10.2353/ajpath.2007.061144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62(2):200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 141.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285(2):C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 142.Kumar B, Koul S, Khandrika L, Measchan RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 143.Veeramani S, Yuan TC, Lin FF, Lin MF. Mitochondrial redox signaling by p66Shc in involved in regulating androgenic growth stimulation of human prostate cancer cell. Oncogene. 2008;27(37):5057–5068. doi: 10.1038/onc.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.WHO World cancer Report 2008. In: Boyle P, Levin B, editors. Lung cancer, 12. Chapter 5.10.
- 145.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health. 2008;11(1):1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 146.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 147.Hoagland LF 4th, Campa MJ, Gottlin EB, Herndon 2nd JE, Patz Jr EF. Haptoglobin and posttranslational glycan-modified derivatives as serum biomarkers for the diagnosis of nonsmall cell lung cancer. Cancer. 2007;110(10):2260–2268. [DOI] [PubMed]
- 148.Pastora MD, Nogala A, Molina-Pineloa S, Meléndeza R, Salinasa A, González De la Penaa M, et al. Identification of proteomic signatures associated with lung cancer and COPD. J Proteomics. 2013;89:227–237. doi: 10.1016/j.jprot.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 149.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–46. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 150.Wynder EL, Goldsmith R. The epidemiology of bladder cancer: a second look. Cancer. 1977;40:1246–1268. doi: 10.1002/1097-0142(197709)40:3<1246::aid-cncr2820400340>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 151.Opanuraks J, Boonla C, Saelim C, Kittikowit W, Sumpatanukul P, Honglertsakula C, et al. Elevated urinary total sialic acid and increased oxidative stress in patients with bladder cancer. Asian Biomedicine. 2010;4(5):703–710. [Google Scholar]
- 152.Opanuraks J, Boonla C, Saelim C, Kittikowit W, Sumpatanukul P, Honglertsakula C, Tosukhowong P. Elevated urinary total sialic acid and increased oxidative stress in patients with bladder cancer. Asian Biomedicine. 2010;4(5):703–710. [Google Scholar]
- 153.Soini Y, Haapasaari KM, Vaarala MH, Turpeenniemi-Hujanen T, Karja V, Karihtala P. 8-hydroxydeguanosine and nitrotyrosine are prognostic factors in urinary bladder carcinoma. Int J Clin Exp Pathol. 2011;4(3):267–275. [PMC free article] [PubMed] [Google Scholar]
- 154.Eijan AM, Piccardo I, Riveros MD, Sandes EO, Porcella H, Jasnis MA, et al. Nitric oxide in patients with transitional bladder cancer. J Surg Oncol. 2002;81:203–208. doi: 10.1002/jso.10170. [DOI] [PubMed] [Google Scholar]
- 155.Gecit I, Aslan M, Gunes M, Pirincci N, Esen R, Demir H, et al. Serum prolidase activity, oxidative stress, and nitric oxide levels in patients with bladder cancer. J Cancer Res Clin Oncol. 2012;138(5):739–743. doi: 10.1007/s00432-011-1136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ellidag HY, Eren E, Aydın O, Akgol E, Yalcınkaya S, Sezer C, et al. Ischemia Modified Albumin Levels and Oxidative Stress in Patients with Bladder Cancer. Asian Pacific J Cancer Prev. 2013;14(5):2759–2763. doi: 10.7314/apjcp.2013.14.5.2759. [DOI] [PubMed] [Google Scholar]
- 157.Yılmaz IA, Akçay T, Çakatay U, Telci A, Ataus S, Yalcin V. Relation between bladder cancer and protein oxidation. ˙. Int Urol Nephrol. 2003;35(3):345–350. doi: 10.1023/b:urol.0000022920.93994.ba. [DOI] [PubMed] [Google Scholar]
- 158.DeMarchi E, B Faldassari, Bononi A, Wieckowski MR, Pinton P. Oxidative Stress in Cardiovascular Diseases and Obesity: Role of p66Shc and Protein Kinase C Oxidative Medicine and Cellular Longevity. 2013;1-11. Review. [DOI] [PMC free article] [PubMed]
- 159.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, et al. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci. 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 161.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17(2):48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98(11):2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 164.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4(1):53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Botto N, Rizza A, Colombo MG, Mazzone AM, Manfredi S, Masetti S, et al. Evidence for DNA damage in patients with coronary artery disease. Mutat Res. 2001;493(1–2):23–30. doi: 10.1016/s1383-5718(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 166.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1–6. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mercer JR, Cheng KK, Figg N, Gorenne I, Mahmoudi M, Griffin J, et al. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome novelty and significance. Circ Res. 2010;107:1021–1031. doi: 10.1161/CIRCRESAHA.110.218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.A global brief on hypertension. World health day 2013. WHO.
- 169.Zalba G, Jose GS, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ, et al. Oxidative stress in arterial hypertension role of NAD(P)H oxidase. Hypertension. 2001;38(6):1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- 170.Dzau VJ. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 171.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44(3):248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 172.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells: implications in cardiovascular disease. Braz J Med Biol Res. 2004;37(8):1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 173.Hashim Z, Zarina S. Osmotic stress induced oxidative damage: Possible mechanism of cataract formation in diabetes. J Diabetes Complicat. 2012;26(4):275–279. doi: 10.1016/j.jdiacomp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- 175.Nagai N, Fukuhata T, Ito Y. Effect of magnesium deficiency on intracellular ATP Levels in human lens epithelial cells. Biol Pharm Bull. 2007;30(1):6–10. doi: 10.1248/bpb.30.6. [DOI] [PubMed] [Google Scholar]
- 176.Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44(3):155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Berthoud VM, Beyer EC. Forum review article oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11(2):339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci. 1986;38(16):1463–1471. doi: 10.1016/0024-3205(86)90559-x. [DOI] [PubMed] [Google Scholar]
- 179.Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N, Verma SD. Lycopene attenuates oxidative stress induced experimental cataract development: an in vitro and in vivo study. Nutrition. 2003;19(9):794–799. doi: 10.1016/s0899-9007(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 180.Boettner EH, Walter JR. Transmission of the ocular media. GPO Invest Ophthalmol Vis Sci. 1962;1:776–783. [Google Scholar]
- 181.Krishna CM, Uppuluri S, Riesz P, Zigler JS, Jr, Balasubramian D. A study of the photodynamic efficiencies of some eye lens constituents. Photochem Photobiol. 1991;54(1):51–58. doi: 10.1111/j.1751-1097.1991.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 182.Dilsiz N, Olcucu A, Atas M. Determination of calcium, sodium, potassium and magnesium concentrations in human senile cataractous lenses. Cell Biochem Funct. 2000;18(4):259–262. doi: 10.1002/1099-0844(200012)18:4<259::AID-CBF881>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 183.David LL, Azuma M, Shearer TR. Cataract and the acceleration of calpain-induced beta-crystallin insolubilization occurring during normal maturation of rat lens. Invest Ophthalmol Vis Sci. 1994;35(3):785–793. [PubMed] [Google Scholar]
- 184.Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33(6):673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 185.Hapeta B, Koczy B, Fitowska A, Dobrakowski M, Kasperczyk A, Ostałowska A, et al. Metabolism and protein transformations in synovial membrane of a knee joint in the course of rheumatoid arthritis and degenerative arthritis. Pol Orthop Traumatol. 2012;77:53–58. [PubMed] [Google Scholar]
- 186.Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, et al. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (Oxford) 2012;51(10):1796–1803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 187.Desai PB, Manjunath S, Kadi S, Chetana K, Vanishree J. Oxidative stress and enzymatic antioxidant status in rheumatoid arthritis: a case control study. Eur Rev Med Pharmacol Sci. 2010;14(11):959–967. [PubMed] [Google Scholar]
- 188.Grover HS, Gaba N, Gupta A, Marya CM. Rheumatoid arthritis: a review and dental care considerations. Nepal Med Coll J. 2011;13(2):74–76. [PubMed] [Google Scholar]
- 189.De Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35(1):70–76. [PubMed] [Google Scholar]
- 190.Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis. 2009;12(1):29–33. doi: 10.1111/j.1756-185X.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 191.Hitchon CA, El-Gabalawy HS. Review Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6(6):265–278. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grootveld M, Henderson EB, Farell A, Blake DR, Parkes HG, Haycock P. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal lowmolecular-mass metabolites by proton-n.m.r. spectroscopy. Biochem J. 1991;273:459–467. doi: 10.1042/bj2730459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rowley D, Gutteridge JM, Blake D, Farr M, Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (London). 1984;66(6):691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- 194.Dai L, Lamb DJ, Leake DS. Evidence for oxidized low density lipoprotein in synovial fluid from rheumatoid arthritis patients. Free Radic Res. 2000;32(6):479–486. doi: 10.1080/10715760000300481. [DOI] [PubMed] [Google Scholar]
- 195.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 196.Dai L, Lamb DJ, Leake DS, Kus ML, Jones HW, Morris CJ, et al. Evidence for oxidised low density lipoprotein in synovial fluid from rheumatoid arthritis patients. Free Radic Res. 2000;32(6):479–486. doi: 10.1080/10715760000300481. [DOI] [PubMed] [Google Scholar]
- 197.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 198.Kottova M, Pourova J, Voprsalova M. Oxidative stress and its role in respiratory diseases. Ceska Slov Farm. 2007;56(5):215–219. [PubMed] [Google Scholar]
- 199.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 200.Chung KF. Role of inflammation in the hyper reactivity of the airways in asthma. Thorax. 1986;41:657–662. doi: 10.1136/thx.41.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med. 1990;9(3):235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- 202.Xiao M, Zhu T, Wang T, Wen FQ. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-κB in a murine model of asthma. Eur Rev Med Pharmacol Sci. 2013;17(8):1033–1043. [PubMed] [Google Scholar]
- 203.Tohyama Y, Kanazawa H, Fujiwara F, Hirata K, Fujimoto S, Yoshikawa J. Role of nitric oxide on airway microvascular permeability in patients with asthma. Osaka City Med J. 2005;51(1):1–9. [PubMed] [Google Scholar]
- 204.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533(1–3):222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 205.Terada LS. Specificity in reactive oxidant signaling: Think globally, act locally. J Cell Biol. 2006;174(5):615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fujisawa T. Role of oxygen radicals on bronchial asthma. Curr Drug Targets Inflamm Allergy. 2005;4(4):505–509. doi: 10.2174/1568010054526304. [DOI] [PubMed] [Google Scholar]
- 207.Ozaras R, Tahan V, Turkmen S, Talay F, Besirli K, Aydin S, et al. Changes in malondialdehyde levels in bronchoalveolar fluid and serum by the treatment of asthma with inhaled steroid and beta2-agonist. Respirology. 2000;5(3):289–292. doi: 10.1046/j.1440-1843.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 208.Ahmad A, Shameem M, Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann Thorac Med. 2012;7(4):226–232. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pobed’onna HP. Antioxidant protection, metabolites of nitrogen oxide on the forming of oxidative stress in patients with bronchial asthma. Lik Sprava. 2005;(5–6):36–40. [PubMed]