Abstract
Prostate carcinoma is the most frequently diagnosed malignancy and the second leading cause of death as a result of cancer in men in the US and other parts of the world. There are conflicting reports on the serum levels of testosterone and 17β-estradiol (E2) in benign prostatic hyperplasia (BPH) and prostate cancer. This study was designed to evaluate the serum concentrations of these hormones in patients with these disorders. Serum levels of prostate specific antigen (PSA), total testosterone and estradiol were determined in 228 subjects comprising of 116 subjects with BPH, 62 subjects with prostate cancer (CaP) and 50 age-matched apparently healthy controls, using ELISA methods. PSA levels were significantly elevated (p < 0.05) in BPH subjects than controls, while there was no significant difference (p > 0.05) in testosterone and estradiol levels of these subjects. PSA and estradiol levels were significantly higher (p < 0.05) in CaP subjects than in controls, while there was no observed significant difference (p > 0.05) in testosterone levels. CaP subjects had significantly raised PSA, testosterone, and estradiol levels than BPH subjects. The mean molar ratio of testosterone: E2 was lowest among CaP patients (134:1) and highest among controls (166:1). Significant positive correlation between PSA and 17β-estradiol was observed in prostate disorders (BPH and CaP patients: r = 0.347; p = 0.000). Significant negative correlations between testosterone and PSA were also observed among BPH patients (r = −0.221, p = 0.049) and control subjects (r = −0.490, p = 0.000). No significant correlation existed between testosterone and PSA in CaP patients (r = 0.051, p = 0.693). Correlations between age and estradiol in both BPH and CaP were not significant (p > 0.05). This study has shown that, there was a significant increase in serum estradiol in CaP subjects, while the testosterone levels in both BPH and CaP subjects were not different from those of controls.
Keywords: Estradiol, Testosterone, PSA, BPH, Molar ratio, Prostate cancer
Introduction
Prostate carcinoma is the most frequently diagnosed malignancy and the second leading cause of death as a result of cancer in men in the US and other parts of the world [1]. Epidemiological surveys have shown that, prostate cancer is typically a disease of men over age 50 years and the incidence tends to increase with advancing age [2]. There are remarkable differences in incidence of prostate cancer among races. Surveys have shown that, it is uncommon among Asians and very common among blacks, with Americans and Europeans occupying the middle level in terms of prevalence [3, 4]. The incidence of prostate cancer in Nigerian is on the increase and has become the number one cancer in men, constituting 11 % of all male cancers [5, 6]. Studies from various parts of Nigeria, including Calabar, indicate an upward trend in the incidence of the disease as well as a rise in the resultant morbidity and mortality [7]. Studies from other parts of Africa such as Yaounde, Cameroon and Kwazulu-Natal region, South Africa have also indicated an upward trend in the incidence of prostate cancer [8, 9].
Very important risk factors thought to be involved in prostate carcinogenesis are the steroid hormones [10]. Androgens are clearly important in the growth, development, maturation and maintenance of the prostate gland and there is no doubt that BPH and CaP are related to the action of androgens. Testosterone is the mediator of prostate growth after it is converted to dihydrotestosterone (DHT) in the prostate itself by the enzyme 5α-reductase [11]. The adult prostate is dependent upon the presence of testicular androgens to maintain secretory activity and to attend a fully differentiated state. Both epithelial and stromal cells undergo apoptosis in response to androgen ablation in the prostate [12]. Generally, serum testosterone concentrations in men decline with age [13]. A potential risk of testosterone therapy is an increased incidence of prostate cancer. Whereas androgen depletion hinders the development and clinical progression of prostate cancer, exogenous testosterone may stimulate growth of metastatic prostate cancer [14, 15]. Testosterone appears to be an independent predictor of disease and enhances the predictive accuracy for BPH [16]. It is currently uncertain whether higher levels of serum testosterone are associated with a higher risk of prostate cancer.
Estrogens, mainly 17-β-estradiol also elicit effects on the prostate, mediated mainly through intraprostatic estrogen receptors—α and β (ER-α, ER-β) [17]. Estrogens are also thought to play a role in the etiology of BPH, perhaps by rendering prostate cells more susceptible to the action of testosterone and DHT [18]. Despite this evidence for androgenic and estrogenic involvements in BPH development, some researchers are of the opinion that since not all patients benefit from hormonal therapy, factors other than hormones are involved and could be more important [19].
Results of studies on the association of prostate cancer and serum levels of testosterone and estradiol have been largely controversial, as these results have indicated either positive or negative associations or no association at all. A nested case control analysis of participants in the USA physicians’ health study, showed a positive association of serum total testosterone and free testosterone index with prostate cancer risk. The associations were strongest among older men [20]. Two recent nested case control studies, however, found no association between serum androgens and prostate cancer [20, 21]. A study by Mearini et al. [16] supports experimental findings that prostate cancer is frequently associated with low testosterone levels.
The above discrepancies on the serum levels of these hormones in BPH and prostate cancer have necessitated this study to actually evaluate the serum concentrations of these hormones and PSA in patients with these disorders and also possibly evaluate the suitability of these hormones as additional serum markers for the diagnosis and prognosis of prostate cancer and benign prostatic hyperplasia (BPH).
Materials and Methods
Subject Selection
The subjects for this study were patients attending the urology out-patient clinic of the University of Uyo Teaching Hospital, Uyo, Akwa Ibom State, Nigeria. The patients were those with confirmed prostate cancer or BPH as judged by biopsy examination report. All subjects were ambulant and attended the clinic on their own. Age-matched control subjects obtained randomly in the city of Uyo from apparently healthy men were also included in this study. Informed consent of the subjects were obtained before blood collection. The selection of subjects and collection of blood samples from these subjects lasted from April 2008 to December, 2008. A total of 228 subjects comprising of 116 subjects with BPH, 62 subjects with prostate cancer (CaP) and 50 age-matched apparently healthy controls, were selected for the study. Sample size was calculated based on prostate cancer prevalence and male population in Uyo, Akwa Ibom State. Approval for this study was obtained from the Research Ethical Committee of the University of Uyo Teaching Hospital. Blood samples were obtained from the subjects.
Sample Collection, Preservation and Analysis
Serum samples were used for the various analyses in this study. The serum obtained were stored at −70 °C until analyses. The assays for serum PSA, testosterone and 17β-estradiol were carried out using the enzyme linked immunosorbent assay (ELISA) with reagents from Fortress Diagnostics Limited, BT41 1QS, United Kingdom. The absorbances and concentrations were read with Rayto RT-2100c microplate reader manufactured by Rayto Life and Analytical Sciences Co. Ltd. China. Assay procedures for all parameters were controlled using commercial control sera provided by the kit manufacturer. Cross-reactivities with other matrix components were less than 1 %.
Statistical Analysis
Data was analysed using SPSS 17.0 software (SPSS. Chicago, IL, USA). Difference between means of the groups were analysed using ANOVA Post hoc and Student’s t test, Pearson’s correlation was performed to study the relationship between parameters. Significant difference was considered at p < 0.050.
Results
This study measured the serum testosterone, 17β-estradiol and PSA of BPH and prostate cancer patients and compared same with those of controls. There was statistically significant difference between the means of PSA, testosterone, and estradiol in controls, BPH and Prostate cancer subjects. Also, the mean molar ratios of testosterone: estradiol for the three groups decreased steadily thus; controls > BPH > CaP (166 > 157 > 134). There was a statistically significant (p < 0.05) difference between serum PSA of controls and BPH subjects, while the differences were not statistically significant (p > 0.05) with testosterone and 17β-estradiol of the same groups (Table 1). As shown in Table 2, there were significant (p < 0.05) differences between PSA of the controls and prostate cancer subjects, 17β-estradiol of the same groups (t = 12.75 and 5.47 respectively) while the difference with total testosterone of the same groups, was not statistically significant (p > 0.05). The serum PSA, testosterone and 17β-estradiol between BPH and CaP subjects were significantly (p < 0.05) different in all three parameters (Table 3) (Fig. 1).
Table 1.
Comparison of serum PSA, testosterone and estradiol between BPH patients and controls
| Parameter | Control | BPH | t Values | p Value | Significance | |
|---|---|---|---|---|---|---|
| n = 50 | n = 116 | t-cal | t-tab | |||
| PSA (ng/ml) | 1.51 ± 0.68 | 8.86 ± 6.82 | 7.50 | 1.96 | 0.00 | S |
| Testosterone (ng/ml) | 4.90 ± 1.83 | 4.38 ± 2.04 | 1.53 | 1.96 | 0.119 | NS |
| Estradiol (pg/ml) | 29.38 ± 7.10 | 30.17 ± 11.78 | 0.44 | 1.96 | 0.594 | NS |
Results presented as mean ± SD
S significant, NS not significant, t-cal calculated t value, t-tab tabulated t value
Table 2.
Comparison of serum PSA, testosterone and estradiol between prostate cancer patients and controls
| Parameter | Control | BPH | t Values | p Value | Significance | |
|---|---|---|---|---|---|---|
| n = 50 | n = 62 | t-cal | t-tab | |||
| PSA (ng/ml) | 1.51 ± 0.68 | 48.03 ± 25.64 | 12.75 | 1.98 | 0.000 | S |
| Testosterone (ng/ml) | 4.90 ± 1.83 | 5.16 ± 2.54 | 1.91 | 1.98 | 0.526 | NS |
| Estradiol (pg/ml) | 29.38 ± 7.10 | 43.39 ± 16.79 | 5.47 | 1.98 | 0.000 | S |
Results presented as mean ± SD
S significant, NS not significant, t-cal calculated t value, t-tab tabulated t value
Table 3.
Comparison of serum PSA, testosterone and estradiol between BPH and prostate cancer patients
| Parameter | BPH | CaP | t Values | p Value | Significance | |
|---|---|---|---|---|---|---|
| n = 116 | n = 62 | t-cal | t-cal | |||
| PSA (ng/ml) | 8.86 ± 6.82 | 48.03 ± 25.64 | 15.3 | 1.96 | 0.000 | S |
| Testosterone (ng/ml) | 4.38 ± 2.04 | 5.16 ± 2.54 | 2.20 | 1.96 | 0.037 | S |
| Estradiol (pg/ml) | 30.17 ± 11.78 | 43.39 ± 16.79 | 6.06 | 1.96 | 0.000 | S |
Results presented as mean ± SD
S significant, NS not significant, t-cal calculated t value, t-tab tabulated t value
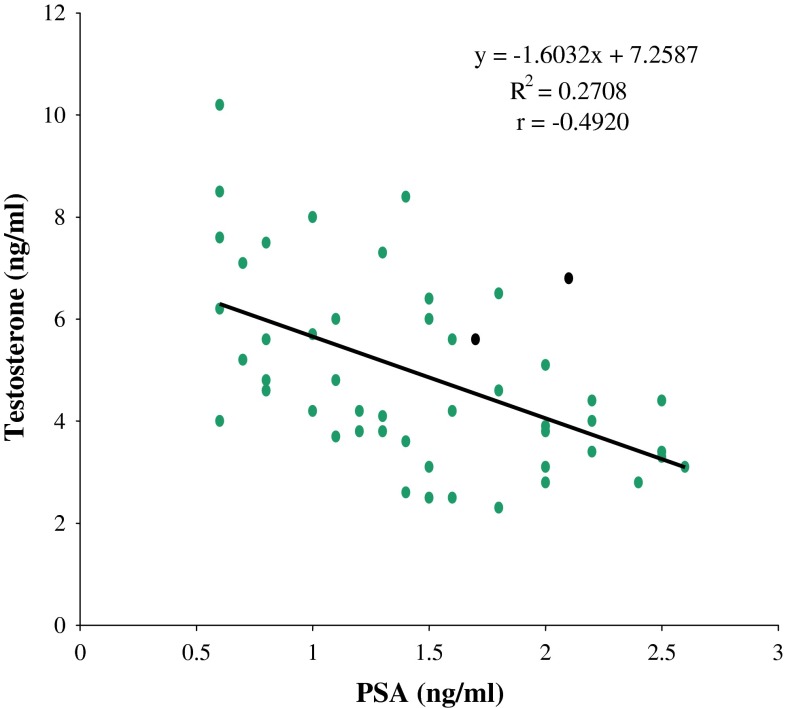
Fig. 1.
Correlation graph of PSA and testosterone in control subjects
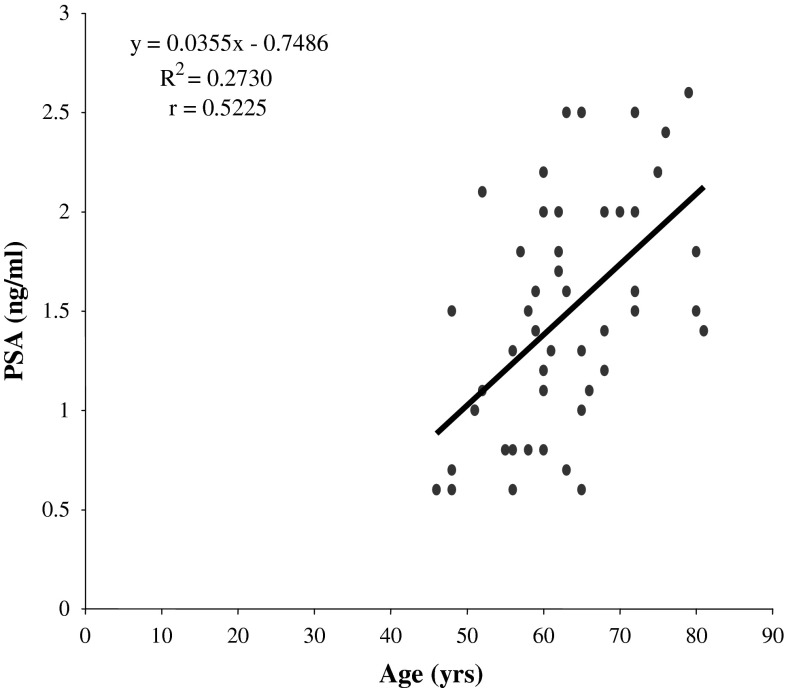
Significant positive correlation between PSA and 17β-estradiol was observed in prostate disorders (BPH and CaP patients: r = 0.347; p = 0.000). Significant negative correlations between testosterone and PSA were also observed among BPH patients (r = −0.221, p = 0.049) and control subjects (r = −0.490, p = 0.000). No significant correlation existed between testosterone and PSA in CaP patients (r = 0.051, p = 0.693). As shown in Fig. 2 and Fig. 3 respectively, there were significant (p < 0.05) positive correlations between age and PSA in the BPH and control groups (r = 0.33 and 0.52 respectively), while it was not significant (p > 0.05) in the CaP group(r = 0.18). We found significant (p < 0.05) negative correlations between age and serum testosterone in the CaP and control groups (r = −0.27 and −0.85 respectively) (Figs. 4, 5), while it was not significant (p > 0.05) in the BPH group (r = 0.08). We found no statistically significant (p > 0.05) correlations between age and 17β-estradiol in control subjects, subjects with BPH and subjects with CaP (r = 0.04, 0.05 and −0.01 respectively). The correlations between serum testosterone and 17-β estradiol in control, BPH and CaP patients, were all not significant (p > 0.05, r = 0.15, 0.12 and 0.13 respectively).
Fig. 2.
Correlation graph of age and PSA levels in control subjects
Fig. 3.
Correlation graph of age and PSA levels in BPH subjects
Fig. 4.
Correlation graph of age and testosterone in control subjects
Fig. 5.
Correlation graph of age and testosterone in prostrate cancer subjects
Discussion
The serum levels of testosterone and 17-β estradiol in patients with BPH and prostate carcinoma (CaP) have largely been a controversy. Gann et al. [20] reported raised serum testosterone levels in these diseases, Mearini et al. [16] reported reduced serum levels. Other studies reported that these hormones are not affected in these disorders [11, 22]. Our findings show that levels of testosterone in CaP did not differ significantly from the level in controls, but E2 was significantly raised compared to levels in controls and BPH. Although actual circulating levels of testosterone is similar in both controls and CaP, the high level of E2 is likely derived from increased transformation of testosterone to E2.
The serum levels of estradiol were not affected by age. There was no correlation between age and estradiol in both controls and the study groups. Also the correlation of testosterone with estradiol in both controls and the disease groups were not significant. The increased serum levels of 17β-estradiol in patients with prostate cancer could be due to increased activities of the enzyme, aromatase which converts testosterone to 17β-estradiol in the prostatic epithelia [18]. It has been reported that, 17β-estradiol and DHT work synergistically to promote prostate carcinogenesis [23]. Estrogen renders the prostatic cells susceptible to the action of DHT [24]. This study showed that, there was no significant difference between the serum levels of 17β-estradiol in patients with BPH when compared with controls. In benign hyperplasia, the cells of the prostatic epithelia and stroma are well differentiated resembling the normal prostatic cells and would probably exhibit control over the process of aromatization [23]. This suggests that, there still remains a good level of regulation in the secretion and activities of the enzyme, aromatase which converts testosterone to 17β-estradiol.
Epidemiological evidence has implicated androgens and estrogens as playing possible roles in the genesis of human prostate cancer [25, 26]. In aggregate, our findings showed that cancer, largely a disease of older men, develops in a hormonal milieu where circulating levels of estrogens predominate over declining levels of androgens. African-American men, who have the highest incidence of prostate cancer in the world, have elevated levels of estrogens at early ages [27] and higher estrogen exposure in utero. In contrast, Japanese males, who have a low incidence of disease, have low serum estradiol-17β (E2) values when compared with age-matched Caucasians [28]. The molar ratio of testosterone to estradiol in the controls (166:1) was higher than that of BPH (157:1) patients which in turn was higher than that of CaP patients (134:1). The molar ratio of the CaP patients was the lowest, probably because of a much higher level of estradiol among the patients. The findings in this study emphasizes that estradiol (E2) appears to play a more important role in the development of BPH and prostate cancer than testosterone. This increased serum level in E2 has been attributed to the action of the enzyme aromatase on testosterone, which converts it to E2. This is probably why testosterone levels in BPH and CaP are not significantly raised.
PSA in this study increased with advancing age and a significant negative correlation between testosterone and PSA was observed among controls. Similar correlation between testosterone and PSA was not significant among BPH and CaP patients. This can be explained by the fact that, serum PSA increases with advancing age due to increased prostate volume, while testosterone decreases with advancing age. This negative correlation is gradually lost in BPH and CaP probably because of the associated increase in PSA levels in these conditions regardless of the age of patients. The expected increase in PSA and decline of testosterone with age observed in control and to a lesser extent in BPH did not occur in CaP patients. This suggests strongly a role for these hormones in BPH and CaP. The direct relationship between age and PSA observed among BPH subjects was weaker than those of control subjects, and did not however exist among the CaP patients. This gradual loss of a positive correlation between age and PSA among BPH and CaP patients is probably because, while PSA levels continue to increase in these diseases, the age remains unchanged. This study has shown that, in healthy individuals, there is a strong negative correlation between testosterone and age. The negative correlation between testosterone and age observed in control subjects was also not observed with CaP patients, probably because, the prostate epithelia in CaP have lost the ability to produce and retain testosterone. The continuous increase in the prostate volume, extensive damage to the prostate cells and increased expression and activities of the aromatase and testosterone-5α-reductase enzymes associated with advanced prostate cancer are probably the cause of the very significant change observed in these parameters between BPH and prostate cancer. It may therefore be possible to use the levels of PSA and estradiol to make a differential diagnosis between BPH and prostate cancer. There was a significant positive correlation between PSA and estradiol in BPH and to a lesser extent in prostate cancer patients. This finding indicates that the level of E2 increases with the concentration of PSA hence relating the activities of aromatase enzyme with prostate cellular proliferation. The action of E2 in prostatic hormonal carcinogenesis is thought to be mediated by estrogen receptor α (ERα) [29]. High level of E2 had been shown to up-regulates telomerase activity in normal prostrate epithelial cells [30] through this receptor and proliferative response to high E2 in the prostate glands of mice had been demonstrated except in those lacking ERα [31]. The molecular mechanism modifying the ERα-mediated action of E2 in hormonal prostrate carcinogenesis could be linked to increase expression of c-fos proto-oncogene which in the presence of c-jun forms transcription factor activated protein 1 (AP-1). AP-1 is involved in the transformation of prostatic cell to a neoplastic phenotype. AP-1 complex has also been implicated in the transformation and progression of cancer via the up-regulation of diverse genes that are involved in cellular proliferation, differentiation, invasion and cellular damage.
Conclusion
This study has been able to show that, the serum level of 17β-estradiol is raised significantly in patients with prostate cancer and to a lesser extent in BPH thus emphasizing the role of 17β-estradiol in prostate carcinogenesis in the milieu of normal testosterone. The study further showed a strong positive correlation between PSA and 17β-estradiol among CaP and BPH patients which supports the relationship between aromatization and prostate proliferative activities. Serum 17β-estradiol can be included as an additional serum marker for prostate cancer diagnosis after due standardization and validation.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics 2005. Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Anand P, Chokkalingam C. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 3.Ekman P. Genetic and environmental factors in prostate cancer genesis: identifying high risk cohorts. Eur Urol. 1999;53:360–362. doi: 10.1159/000019910. [DOI] [PubMed] [Google Scholar]
- 4.Boyle P, Seven G, Giles GG. The epidemiology of prostate cancer. N Am J. 2003;30:207–210. doi: 10.1016/s0094-0143(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 5.Ogunbiyi JO, Shittu OB. Increased incidence of prostate Cancer in Nigerians. J Natl Med Assoc. 1999;90(3):159–164. [PMC free article] [PubMed] [Google Scholar]
- 6.Nkposong EO, Lawani J. Primary carcinoma of the prostate in Ibadan. West Afr Med J. 1973;22:108–111. [PubMed] [Google Scholar]
- 7.Ekwere PD, Egbe SN. The changing pattern of prostate cancer in Nigerians: current status in the south-Eastern states. J Natl Med Assoc. 2002;94(7):619–627. [PMC free article] [PubMed] [Google Scholar]
- 8.Angwafo FF. Migration and prostatic cancer: an international perspective. J Natl Med Assoc. 1998;90:5720–5723. [PMC free article] [PubMed] [Google Scholar]
- 9.Olapade-Olaopa EO, Ahiaku E. ‘The changing face’ of prostatic carcinoma in Black Africans. The view at the, 1995 Pan African Urological Surgeons Association meeting. Cancer Topics. 1995;10:4–7. [Google Scholar]
- 10.Bosland MC. Hormonal factors in carcinogenesis of the prostate and testis in humans and animal models. In: Huff J, Boyd J, Barett JC, editors. Cellular and molecular mechanisms of hormonal carcinogenesis. Environmental influences. New York: Wiley-Liss; 1996. pp. 309–352. [Google Scholar]
- 11.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–235. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- 12.Buttyan R, Ghafar MA, Shabsigh A. The effects of androgen deprivation on the prostate gland: cell death mediated by vascular regression. Curr Opin Urol. 2000;10:415–420. doi: 10.1097/00042307-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study on aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 14.Thompson IM, Pauler DK, Goodman PJ. Prevalence of prostate cancer among men with a prostate specific antigen level < or = 4.0 ng/ml. N Engl J Med. 2004;350:2239–2240. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JE, Jr, Whitemore WF., Jr The response of metastatic adenocarcinomas of the prostate to exogenous testosterone. J Urol. 1981;126:370–372. doi: 10.1016/s0022-5347(17)54531-0. [DOI] [PubMed] [Google Scholar]
- 16.Mearini L, Constantini E, Zurchi A, Mearini E, Bini V, Cottini E, Porena M. Testosterone levels in benign prostatic hyperplasia and prostate cancer. Urol Int. 2008;12:134–140. doi: 10.1159/000112602. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafson JA. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong YC, Wang YZ. Growth factors and epithelial-stromal interactions in prostate cancer development. Int Rev Cytol. 2000;12:199–265. doi: 10.1016/s0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- 19.McConnel JD. The long-term effect of Doxazosin, Finasteride and combination therapy on clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2390. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 20.Gann PH, Hennekens CH, Ma J, Loongcope C, Stampfer MJ. Prospective study of sex hormones levels and risk of prostate cancer. J Am Natl Cancer Inst. 1996;88(16):1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 21.Platz EA, Rimm EB, Willet WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professional. J Am Natl Cancer Inst. 2005;92:2009–2017. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 22.Stattin P, Lumme S, Tenkanen L. Levels of circulating testosterone are not associated with increased prostate cancer risks: a pooled prospective study. Int J Cancer. 2004;108:418–424. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths K. Estrogens and prostatic disease. International Prostate Health Council Study Group. Prostate. 2000;45:87–100. doi: 10.1002/1097-0045(20001001)45:2<87::AID-PROS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Walsh PC, Hutchins GM, Ewing LL. The tissue content of DHT in human prostatic hyperplasia is not supranormal. J Clin Invest. 1983;72:1772–1776. doi: 10.1172/JCI111137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taplin ME, Ho SM. Clinical review 134: the endocrinology of prostate cancer. J Clin Endocrinol Metab. 2001;86:3467–3477. doi: 10.1210/jcem.86.8.7782. [DOI] [PubMed] [Google Scholar]
- 26.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 27.Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986;76:45–48. [PubMed] [Google Scholar]
- 28.de Jong FH, Oishi K, Hayes RB, Bogdanowicz JF, Raatgever JW, van der Maas PJ, Yoshida O, Schroeder FH. Peripheral hormone levels in controls and patients with prostatic cancer or benign prostatic hyperplasia: results from the Dutch-Japanese case–control study. Cancer Res. 1991;51:3445–3450. [PubMed] [Google Scholar]
- 29.Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor α signaling. FASEB J. 2008;22:1512–1520. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 30.Nanni S, Narducci M, Della Pietra L, Moretti F, Grasselli A, De Carli P, Sacchi A, Pontecorvi A, Farsetti A. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest. 2002;110:219–227. doi: 10.1172/JCI0215552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risbridger GP, Wang H, Frydenberg M, Cunha G. The metaplastic effects of estrogen on mouse prostate epithelium: proliferation of cells with basal cell phenotype. Endocrinology. 2001;142:2443–2450. doi: 10.1210/endo.142.6.8171. [DOI] [PubMed] [Google Scholar]