Abstract
Bone metastases are a serious problem in patients with advanced cancer disease and their presence usually signifies serious morbidity prior to the patient’s death. In breast cancer patients the incidence of bone metastasis is observed to be very high at 70 %, as seen during post-mortem examination. Bone metastasis is difficult to diagnose, treat or follow clinically without radiological tools. This study was designed to evaluate the utility of a novel bone resorption marker–serum tartrate-resistant acid phosphatase 5b (TRACP5b) and the bone formation marker such as serum total alkaline phosphatase (ALP), in comparison with whole body skeletal scintigraphy with Technetium99m MDP for the diagnosis of bone metastases (BM) in breast cancer (BC) patients. This study is intended to help the clinician to diagnose bone metastasis without resorting to radiological tools, as they are not cost effective and carry the risk of radiation. Experimental design: Four groups of samples were analysed. 1st group consists 52 normal female (cancer free women), 2nd group consists 38 BC patients without bone metastasis, 3rd group consists 27 breast cancer patients with limited bone metastasis (3 or less than 3 skeletal lesions) and 4th group consists 35 breast cancer patients with extensive bone metastasis (4 or more than 4 skeletal lesions), conformed by whole body skeletal scintigraphy with Technetium99m MDP. One way ANOVA was used to compare serum TRACP5b and serum ALP among these groups. Both serum TRACP5b and serum ALP are not markedly elevated in limited bone metastasis but are strongly elevated in extensive bone metastasis (p < 0.0001). As seen in this study the biochemical bone resorption marker, serum TRACP5b, abnormally increased in extensive bone metastasis of breast cancer patients and can be used as a specific marker for bone metastasis in lieu of radiological tools.
Keywords: Breast cancer (BC), Bone metastasis (BM), Limited bone metastasis (Lim.BM), Extensive bone metastasis (Ext.BM), Scintigraphy, Tartrate-resistant acid phosphatase, 5b (TRACP5b)
Introduction
Breast cancer (BC) starts as a local disease but it can metastasize to a distant organ such as bone, in more than 60 % of patients with advanced cancer disease. This bone metastases (BM) is virtually incurable and reduces the quality of life of a patient before bringing death. BM results in terrific bone pain, compression of spinal cord and pathological bone fractures. Bone is a highly specialized and dynamic organ that is being remodelled constantly throughout life by two types of cells. Osteoclasts are responsible for bone resorption and osteoblasts are responsible for laying down new bone matrix. The disruption of the normal coupling between these osteoblasts and osteoclasts are believed to be the complication of BM [1, 2]. As the bone gets heavily eroded by activated osteoclasts and new bone formation by osteoblasts is not efficient enough to keep pace, thereby resulting in net bone loss. Recent studies show that the tartrate-resistant acid phosphatase 5b (TRACP 5b) is identified as a marker of osteoclasts and bone resorption [3, 4]. Osteoclasts secrete tartrate resistant acid phosphatase 5b normally during bone resorption [5, 6]. After menopause, in response to the lack of oestrogen effect on bones, serum TRACP5b activity becomes increased [7, 8]. TRACP 5b has an added advantage than other bone markers as it does not show much dependence either on food intake or diurnal rhythm [9]. Its activity is not affected by kidney or liver function. This is an important point in the case of cancer patients having additional liver metastases [9, 10]. Bone scintigraphy is traditionally used method for initial evaluation to detect BM and later to monitor the same. It is considered as gold standard. But there are some limitations as it is expensive and the fear of radiation due to repeated use on the same set of patients having BM, for continuous evaluation. This study is intended to know (1) Whether the marker, serum TRACP 5b, can be used to detect BM at an early stage (Lim. BM), (2) Whether the activity of the marker consistent with the presence of BM and extent of skeletal involvement (3) Whether this marker can be used to detect BM in lieu of whole body skeletal scintigraphy with Technetium99m MDP.
Materials and Methods
The present study was carried out in the Department of Biochemistry, Gandhi Hospital, Secunderabad and in the Department of Nuclear Medicine, MNJ Institute of Oncology and Regional Cancer Centre, Red Hills, Hyderabad. This study was approved by the Institutional Ethics Committee of MNJ Institute of Oncology and Regional Cancer Centre, Red Hills, Hyderabad. The study population comprised of 152 females and they are classified into four groups.
Group1 comprises 52 normal, cancer free, females aged 18–80.
Groups 2, 3 and 4 comprise female patients with confirmed BC, diagnosed earlier by histo-pathological and radiological studies. They were categorised on the basis of absence or presence of skeletal lesions, and their number detected through their whole body skeletal scintigraphy with Technetium99m MDP.
Group 2 consists of 38 BC patients without BM.
Group 3 consists of 27 BC patients with limited BM (3 or less than 3 skeletal lesions).
Group 4 consists of 35 BC patients with extensive BM (4 or more than 4 skeletal lesions).
Patients and control group were recruited after informed consent was obtained. The venous blood was drawn before conducting bone scintigraphy (for the patients)and allowed to clot at room temperature for 30–60 min and centrifuged for 20 min in a refrigerated centrifuge at 10,000×g at 4 °C and were stored at −70 °C in aliquots, where TRACP 5b activity is stable for years. The TRACP 5b activity was measured within 1 month of blood collection. Samples were thawed to room temperature immediately before estimating TRACP 5b activity. Serum Tartrate-resistant acid phosphatase 5b activity and alkaline phosphatase (ALP) were estimated.
A dose of 20 mCi Technetium99m MDP (methylene diphosphonate) is administered intravenously to the patients. After 2–4 h, whole body bone scintigraphy was conducted on these patients using Duel Headed Gama Camera.
Subjects with fractures, osteoporosis, primary and secondary hyperparathyroidism, and all bone related problems are considered as exclusion criteria for normal group. Patients treated with aromatase inhibitors and bisphosphonates and patients with extensive BM for various times prior to enrolment are the exclusion criteria for carcinoma breast samples in this study.
Tartrate-resistant acid phosphatase 5b activity was estimated spectrophotometrically as described by Lau et al. (1987) with modifications by Yamagishi et al. (2009) according to the following equation:
 |
Reagents:
Substrate solution contains 100 mM pNPP in 200 mM sodium acetate buffer containing 80 mM sodium tartrate, 400 mM potassium chloride and 42 U/ml heparin, adjusted to pH 5.6 by addition of conc. HCl.
1 M NaOH.
The clear serum samples were obtained by centrifugation at 10,000×g for 20 min at 4 °C and stored at −70 °C. Serum samples were diluted tenfold with distilled water before analysing the samples. Diluted samples were incubated for 1 h. at 37 °C. Then, 50 μl of the diluted sample were added to 50 μl of substrate solution in a micro plate. The reaction was carried out for 1 h at 37 °C and quenched by adding 50 μl of 1 M NaOH. (All the chemicals used in this assay are analytical grade chemicals).
A standard calibration curve was constructed using p-nitro phenol (pNP) (AR Grade Chemical) solution of known concentrations (5–25 μg/ml in 0.05 M NaOH) and the absorbance was measured at 405 nm in a micro plate reader. The amount of p-nitro phenol production was calculated through a comparison with standard curve obtained with p-nitro phenol solution. The results were expressed as units per litre. One unit (1 U) of TRACP 5b activity was defined as the amount of enzyme required to hydrolyse 1 micro mole (μmol) of p-nitro phenyl phosphate (pNPP) per minute at 37 °C. The samples were diluted further and reanalysed if the activity of TRACP 5b exceeded the range of the standard curve.
B) ALP is estimated by pNPP-AMP Kinetic Assay method (By a diagnostic kit (Assay recommended by International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)).
Results
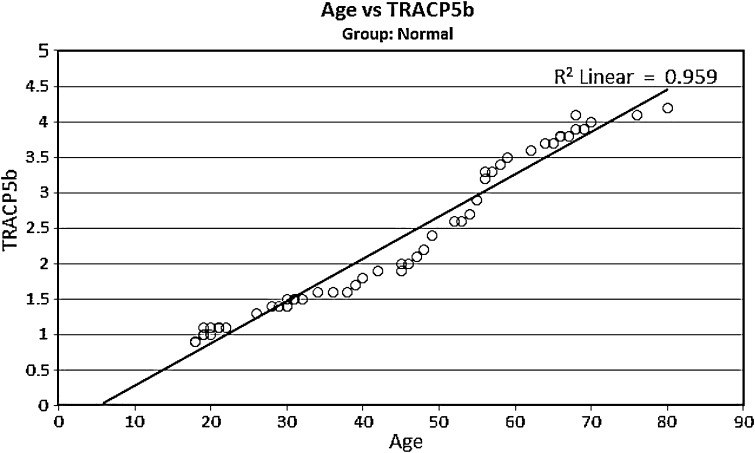
Serum TRACP 5b activity in 52 healthy, cancer free women between 18 and 80 years, was determined. The mean values of the entire group was 2.3 ± 1.1 units/L and are significantly, positively, correlated with age (p < 0.0001). In healthy women age is a significant factor for the increase of serum TRACP 5b activity. This may be due to the increasing physiological bone turnover of women with increasing age and postmenopausal osteoporosis, particularly after the age of 45 years (Fig. 1).
Fig. 1.
The distribution of the serum TRACP 5b activity in 52 normal females of different ages show trends of rising serum TRACP 5b activity as age increases
Mean and SD values of normal and different subgroups of BC patients such as, BC without BM, BC with limited BM and BC with extensive BM are displayed in Table 1. BC with extensive BM shows significantly high mean values when compared with other groups.
Table 1.
Mean and SD values of Age, serum TRACP 5b and serum ALP in normal, BC without BM (Breast cancer without bone metastases), BC with lim. BM(Breast cancer with limited bone metastases), and BC with ext. BM (Breast cancer with extensive bone metastases), groups based on whole body skeletal scintigraphy in breast cancer patients
| GP1 normal n = 52 | GP2 BC without BM n = 38 | GP3 BC with limited BM n = 27 | GP4 BC with Extensive BM n = 35 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 44.1 | 18.1 | 51.5 | 14.3 | 57.4 | 12.4 | 50.2 | 16.2 |
| TRACP5b | 2.3 | 1.1 | 2.6 | 0.8 | 3.0 | 0.7 | 8.9 | 2.6 |
| ALP | 79.6 | 30.6 | 81.8 | 26.2 | 130.9 | 79.7 | 712.8 | 296.2 |
Statistics
One way ANOVA was used to compare serum TRACP 5b and serum ALP among groups and post Hoc tests for multiple comparisons. Serum TRACP 5b levels in BC with extensive BM showed significant values when compared with all the groups (p < 0.0001). BC with limited BM also showed significant levels when compared to normal group (p < 0.039). Serum ALP also showed significant values in BC with extensive BM when compared with all other groups (p < 0.0001).
Within-assay precision is calculated by the intra-assay coefficient of variation, which was determined by simultaneous assay duplicates of fifteen sera ranging from 1.8 to 13.6 units/L was 4.2 % (n = 15). The inter-assay analytical error (% CV) was determined by assay of aliquots of five sera ranging from 2.84 to 9.82 units/L run on four different times. The inter assay CVs were 8.2 % (n = 5).
Sensitivity and Specificity of Serum TRACP 5b Activity:
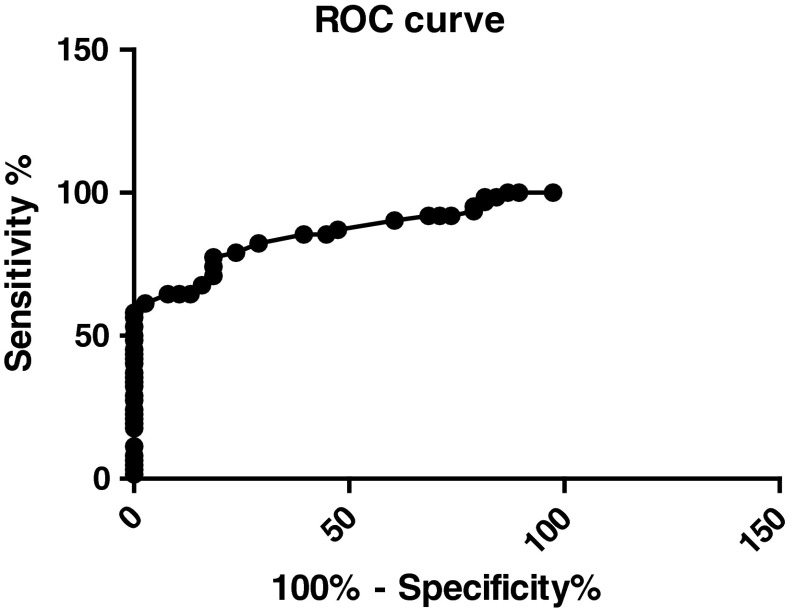
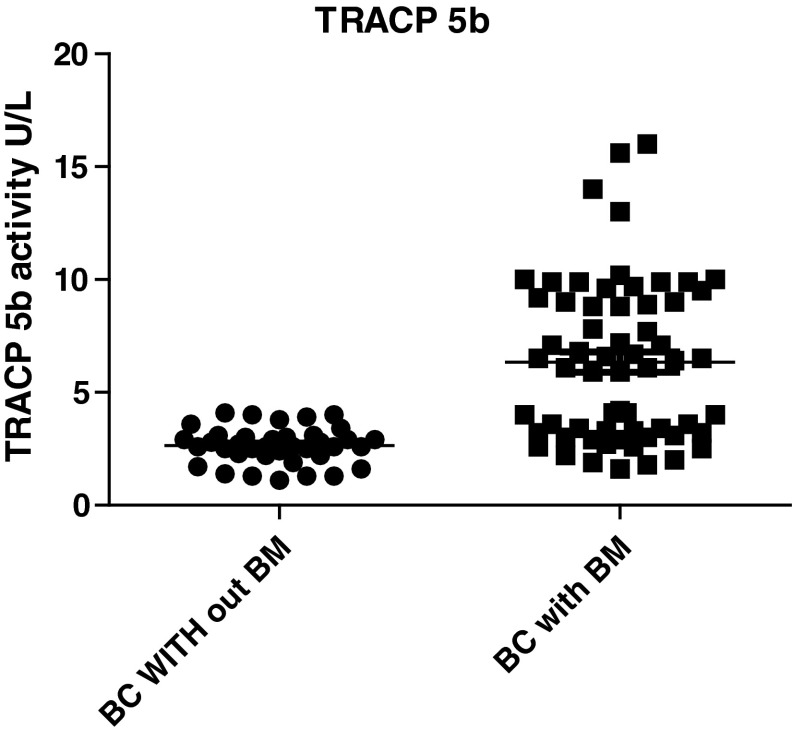
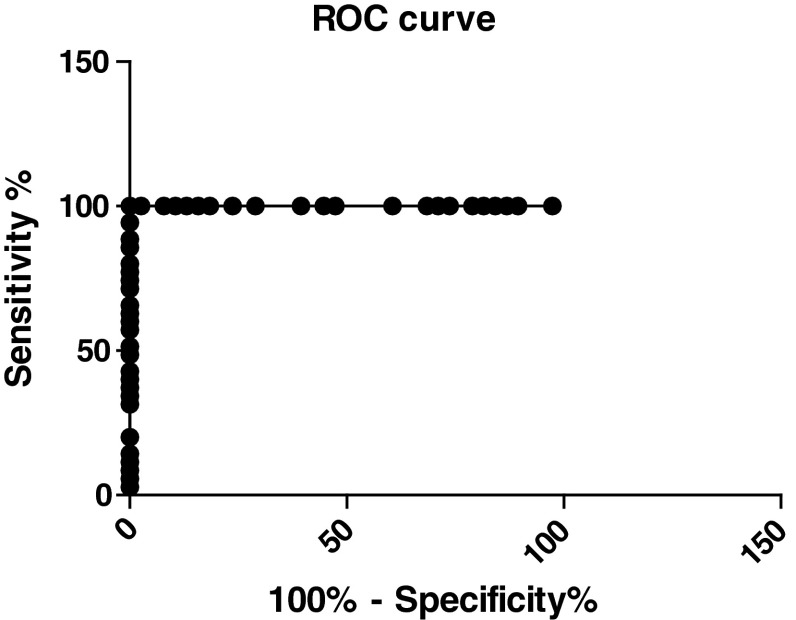
The sensitivity and specificity were estimated by Receiver-Operating Characteristic (ROC) Curves by using Graph Pad Prism statistical software. The ROC curves plotted for TRACP 5b activity in 62 BC patients with BM (Limited and Extensive BM) versus 38 BC patients without BM as control subjects. The area under the curve (AUC) generated from the serum TRACP 5b activity was 0.8525 (95 %CI 0.7800–0.9250) and the p value is <0.0001 (Figs. 2, 3). The area under the curve increased to 1.000 (95 % CI 1.0–1.0) for the 38 patients with extensive metastases versus 38 BC patients without BM as control subjects, and the p value is <0.0001 (Figs. 4, 5). This increase of sensitivity, when only BC with ext. BM is considered, makes it a reliable indicator for BM.
Fig. 2.
The ROC curves generated from the serum TRACP 5b activity in 62 breast cancer(BC) patients with bone metastases(BM) (limited BM and extensive BM) versus 38 BC patients without BM(AUC = 0.8525)
Fig. 3.
Distribution of the serum TRACP 5b activity in 62 breast cancer(BC) patients with bone metastases(BM) (limited BM and extensive BM) versus 38 BC patients without BM
Fig. 4.
The ROC curves generated from the serum TRACP 5b activity in 35 breast cancer(BC) patients with extensive bone metastases(Ext. BM) versus 38 breast cancer(BC) patients without bone metastases(BM). (AUC = 1.000)
Fig. 5.
Distribution of the serum TRACP 5b activity in 35 breast cancer(BC) patients with extensive bone metastases(Ext. BM) versus 38 breast cancer(BC) patients without Bone Metastases(BM)
Discussion
In BC patients the incidence of BM is observed to be very high at 70 %, as seen during post-mortem examination. When treating patients with BC, identification of BM is an important issue. Image studies such as plain radiography, bone scintigraphy, computerized tomography, and magnetic resonance play a major role in detection and follow-up of BM of BC patients. But each image measure has its own limitations like radiation injury and cost of imaging. There is a need for biochemical bone markers present in serum, which facilitates easy sampling and useful to screen BC patients on a routine basis to detect BM at the earliest without resorting to expensive radiological tools. The advantage of biochemical bone markers over image studies are (1) Cost effectiveness (2) No fear of radiation (3) Increased sensitivity (4) Relate to systemic changes rather than local (5) Rapid response to treatment (6) Differentiation of healing lesions from progressive lesions and (7) Provides more information on the mechanisms and cellular dynamics of bone destruction [13–18].
In this study, we have compared a biochemical bone resorption marker, serum TRACP 5b activity along with serum ALP, a bone formation marker, in different groups based on the number of lesions detected through whole body skeletal scintigraphy with Technetium99m MDP, i.e., normal females, BC patients without BM, BC patients with Limited BM and BC patients with extensive BM. Serum TRACP 5b activity and serum ALP are higher in BC patients with extensive BM than normal subjects, BC without BM and BC with Lim.BM. Both these markers are correlated with each other. The elevated levels of serum ALP in BM are difficult to interpret due to the possible presence of additional liver metastases. This can be overcome by identifying those patients, whose increased levels should be due to bone disease and not due to liver disease. Serum TRACP 5b activity in patients with BC without BM were not different from that of normal, healthy women. The trends for rising serum TRACP 5b with age in patients with early BC or BC with limited BM were no different from healthy women, although in some patients there is moderate rise (p < 0.039). Serum TRACP 5b activity increased abnormally in all the patients with extensive BM (4 or more skeletal lesions) (p < 0.0001) and these values kept increasing with extension of BM. This study indicates that the mean TRACP 5b activity is definitely elevated in BC patients with BM, but not significant enough in early limited BM (Fig. 6). TRACP 5b is a very sensitive and specific marker in extensive BM and can be used as marker for early detection of extensive metastases in BC patients.
Fig. 6.
Serum TRACP 5b activity in normal and different subgroups of breast cancer patients, data expressed as mean 1SD (normal, breast cancer without bone metastases, breast cancer with limited bone metastases, breast cancer with extensive bone metastases)
Conclusion
The biochemical bone resorption marker, serum TRACP 5b can alone be used as a reliable marker of BM in BC patients, without resorting to bone scintigraphy which is not cost effective and its inherent risk of radiation exposure. Physicians commonly use serum ALP as a marker to detect or monitor BM in BC patients. However, this study’s results conclude that serum TRACP 5b is a more sensitive and specific marker than serum ALP to detect or monitor BM in BC patients.
References
- 1.Kanis JA, McCloskey EV. Bone turnover and biochemical markers in malignancy. Cancer. 1997;80:1538–1545. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1538::AID-CNCR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80:1546–1556. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1546::AID-CNCR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Halleen JM, Alatalo SL, Suominen H. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15:1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 4.Janckila AJ, Takahashi K, Sun SZ. Tartrate-resistant acid phosphatase isoform 5b as serum marker for osteoclastic activity. Clin Chem. 2001;47:74–80. [PubMed] [Google Scholar]
- 5.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Vaananen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15:1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 6.Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbrock H, Seifert-Klauss V, Kaspar S, Busch R, Luppa PB. Changes of biochemical bone markers during the menopausal transition. Clin Chem Lab Med. 2002;40:143–151. doi: 10.1515/CCLM.2002.025. [DOI] [PubMed] [Google Scholar]
- 8.Halleen JM, Ylipahkala H, Alatalo SL. Serum tartrate resistant acid phosphatase 5b, but not 5a, correlates with other markers of bone turnover and bone mineral density. Calcif Tissue Int. 2002;71:20–25. doi: 10.1007/s00223-001-2122-7. [DOI] [PubMed] [Google Scholar]
- 9.Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell Rand Blumsohn A. Clinical performance of immunoreactive tartrate resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004;34:187–194. doi: 10.1016/j.bone.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Halleen JM, Alatalo SL, Janckila AJ, Woitge HW, Seibel MJ, Vaananen HK. Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. ClinChem. 2001;47:597–600. [PubMed] [Google Scholar]
- 11.Yamagishi N, Takehana K, Kim D, Miura M, Hirata T, Devkota B, et al. Fluorometric Method for Measuring Plasma Tartrate-Resistant Acid Phosphatase Isoform 5b and its application in cattle. J Vet Med Sci. 2009;71(12):1637–1642. doi: 10.1292/jvms.001637. [DOI] [PubMed] [Google Scholar]
- 12.Lee HB, Alam MR, Seol JW, Kim NS. Tartrate-resistant acid phosphatase, matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in early stages of canine osteoarthritis. Vet Med. 2008;53(4):214–220. [Google Scholar]
- 13.Coleman RE. Biochemical markers of malignant bone disease. London: Martin Dunitz Ltd.; 2000. [Google Scholar]
- 14.Galasko CS. The significance of occult skeletal metastases, detected by skeletal scintigraphy, in patients with otherwise apparently ‘‘early’’ mammary carcinoma. Br J Surg. 1975;62:694–696. doi: 10.1002/bjs.1800620906. [DOI] [PubMed] [Google Scholar]
- 15.Sanal SM, Flickinger FW, Caudell MJ. Detection of bone marrow involvement in breast cancer with magnetic resonance imaging. J Clin Oncol. 1994;12:1415–1421. doi: 10.1200/JCO.1994.12.7.1415. [DOI] [PubMed] [Google Scholar]
- 16.Yeh KA, Fortunato L, Ridge JA. Routine bone scanning in patients with T1 and T2 breast cancer: a waste of money. Ann Surg Oncol. 1995;2:319–324. doi: 10.1007/BF02307064. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RE, Mashiter G, Whitaker KB. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29:1354–1359. [PubMed] [Google Scholar]
- 18.Vogel CL, Schoenfelder J, Shemano I. Worsening bone scan in the evaluation of antitumor response during hormonal therapy of breast cancer. J Clin Oncol. 1995;13:1123–1128. doi: 10.1200/JCO.1995.13.5.1123. [DOI] [PubMed] [Google Scholar]