Abstract
Antisera raised in mammals to murine immunoglobulin (Ig) do not detect surface Ig on thymus-dependent (T) lymphoma cells as assessed by immunofluorescence analysis. In contrast, chicken antibodies, produced against the (Fab)2 fragment of normal mouse IgG and purified by binding to and elution from IgG-Sepharose 4B, give strong indirect fluorescence with murine T cells and cultured T lymphoma cells. The surface Ig caps, is shed, and reappears, indicating that it is of endogenous origin. Nonlymphoid tumor cells of various myeloid types do not bind this reagent, even though they bear avid Fc receptors. The capacity of chicken antibodies to bind to both bone-marrow-dependent and T cell lymphomas was abolished by adsorption with myeloma-derived kappa chains coupled to Sepharose. The kappa antigenic determinant recognized by the chicken antibodies may thus be different from that seen by mammalian antibodies, and the degree of exposure of Ig on the T lymphoma surface might also affect ease of detectability with these reagents. These data provide direct evidence that T lymphocytes and T lymphoma cells express and synthesize a surface Ig containing determinants that at least 'crossreact with bone-marrow-cell-derived kappa chains.
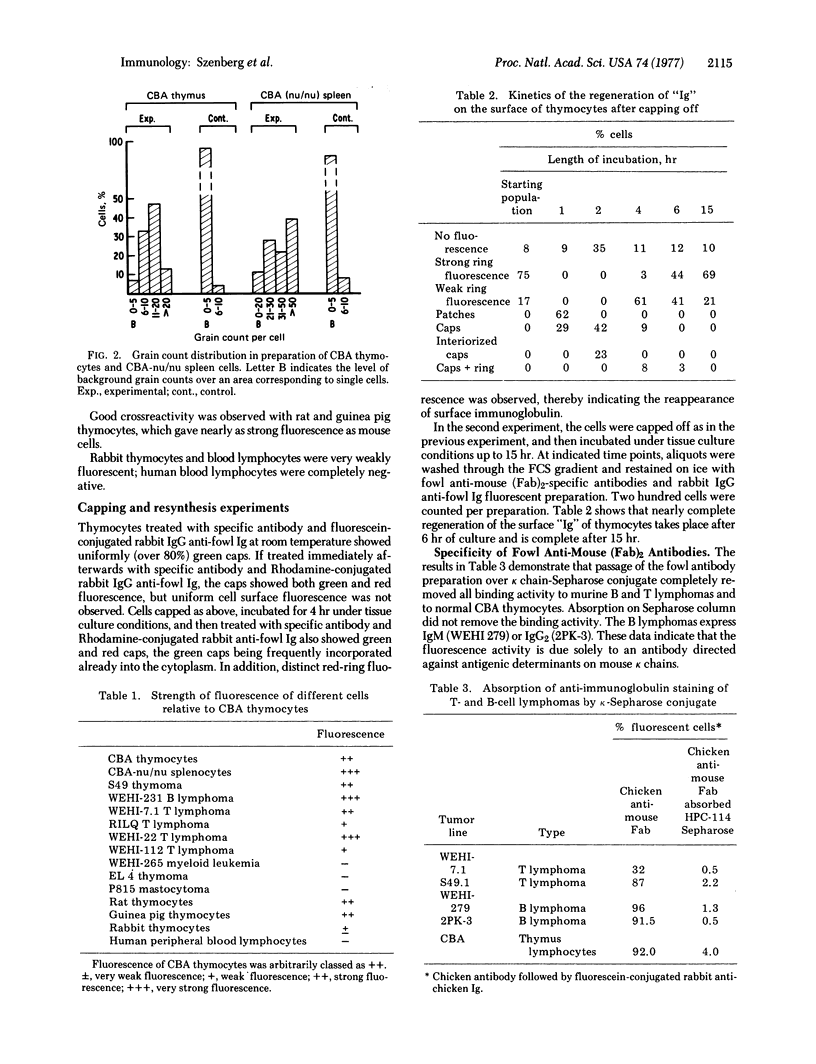
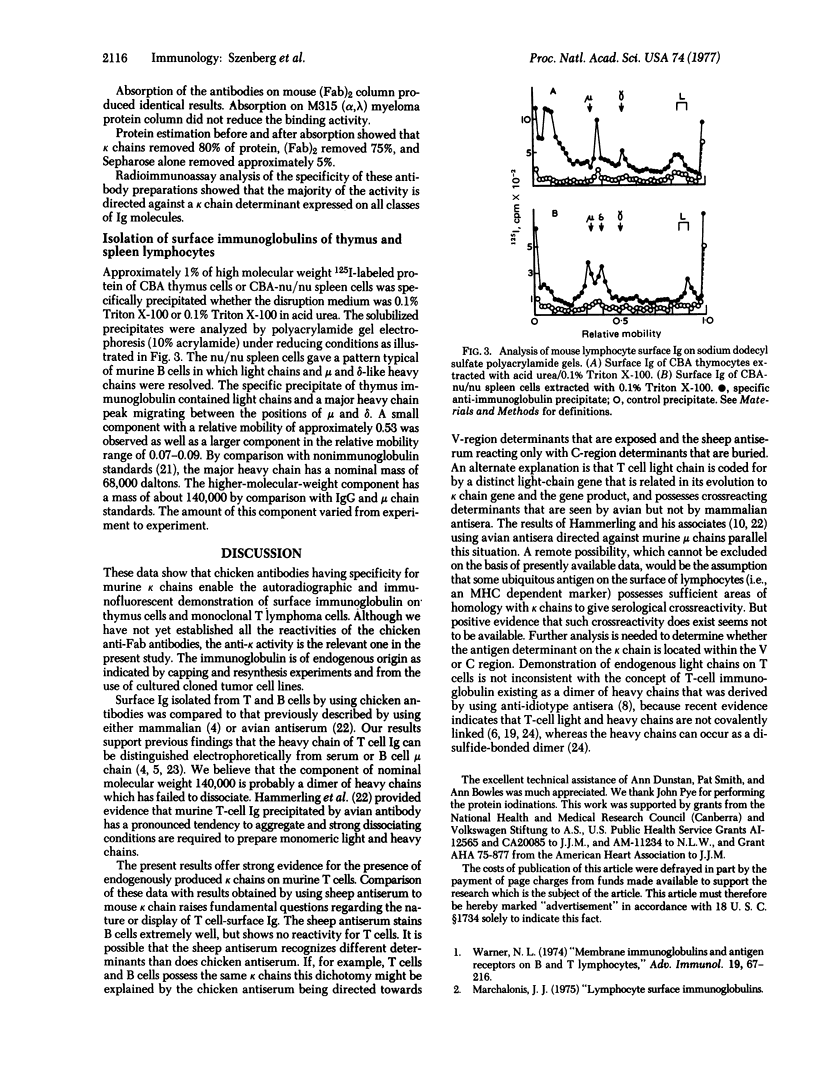
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Wigzell H. Shared idiotypic determinants on B and T lymphocytes reactive against the same antigenic determinants. V. Biochemical and serological characteristics of naturally occurring, soluble antigen-binding T-lymphocyte-derived molecules. Scand J Immunol. 1976;5(5):559–571. doi: 10.1111/j.1365-3083.1976.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Black S. J., Hämmerling G. J., Berek C., Rajewsky K., Eichmann K. Idiotypic analysis of lymphocytes in vitro. I. Specificity and heterogeneity of B and T lymphocytes reactive with anti-idiotypic antibody. J Exp Med. 1976 Apr 1;143(4):846–860. doi: 10.1084/jem.143.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylston A. W., Mowbray J. F. Surface immunoglobulin of a mouse T-cell lymphoma. Immunology. 1974 Nov;27(5):855–861. [PMC free article] [PubMed] [Google Scholar]
- Cone R. E., Brown W. C. Isolation of membrane associated immunoglobulins from T lymphocytes by non-ionic detergents. Immunochemistry. 1976 Jul;13(7):571–579. doi: 10.1016/0019-2791(76)90168-3. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Marchalonis J. J. Surface proteins of thymus-derived lymphocytes and bone-marrow-derived lymphocytes. Selective isolation of immunoglobulin and the theta-antigen by non-ionic detergents. Biochem J. 1974 Jun;140(3):345–354. doi: 10.1042/bj1400345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L., Weiss N., Loor F. Direct evidence for immunoglobulins on the surface of thymus lymphocytes of amphibian larvae. Eur J Immunol. 1972 Aug;2(4):366–370. doi: 10.1002/eji.1830020414. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Haustein D., Goding J. W. Surface immunoglobulin heavy chains of murine splenocytes and thymocytes are different. Biochem Biophys Res Commun. 1975 Jul 22;65(2):483–489. doi: 10.1016/s0006-291x(75)80173-2. [DOI] [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Harris A. W. Immunoglobulin of T lymphoma cells. Biosynthesis, surface representation, and partial characterization. Biochemistry. 1975 May 6;14(9):1826–1834. doi: 10.1021/bi00680a004. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Mack C., Pickel H. G. Immunofluorescence analysis of Ig determinants of mouse thymocytes and T cells. Immunochemistry. 1976 Jun;13(6):525–531. doi: 10.1016/0019-2791(76)90329-3. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Pickel H. G., Mack C., Masters D. Immunochemical study of an immunoglobulin-like molecule of murine thymocytes. Immunochemistry. 1976 Jun;13(6):533–538. doi: 10.1016/0019-2791(76)90330-x. [DOI] [PubMed] [Google Scholar]
- Jones V. E., Graves H. E., Orlans E. The detection of F(ab')2-related surface antigens on the thymocytes of children. Immunology. 1976 Feb;30(2):281–288. [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz C., Lahat N. Surface immunoglobulin of mouse thymus cells and its in vitro biosynthesis. Cell Immunol. 1974 Sep;13(3):397–406. doi: 10.1016/0008-8749(74)90259-7. [DOI] [PubMed] [Google Scholar]
- Ramseier H. Spontaneous release of T-cell receptors for alloantigens. I. Recognition of alloantigens and receptor release dynamics. J Exp Med. 1974 Sep 1;140(3):603–618. doi: 10.1084/jem.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner N. L. Membrane immunoglobulins and antigen receptors on B and T lymphocytes. Adv Immunol. 1974;19(0):67–216. doi: 10.1016/s0065-2776(08)60252-7. [DOI] [PubMed] [Google Scholar]
- Warr G. W., DeLuca D., Marchalonis J. J. Phylogenetic origins of immune recognition: lymphocyte surface immunoglobulins in the goldfish, Carassius auratus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2476–2480. doi: 10.1073/pnas.73.7.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]