Abstract
Several studies have postulated that psychoacoustic measures of auditory perception are influenced by efferent-induced changes in cochlear responses, but these postulations have generally remained untested. This study measured the effect of stimulus phase curvature and temporal envelope modulation on the medial olivocochlear reflex (MOCR) and on the middle-ear muscle reflex (MEMR). The role of the MOCR was tested by measuring changes in the ear-canal pressure at 6 kHz in the presence and absence of a band-limited harmonic complex tone with various phase curvatures, centered either at (on-frequency) or well below (off-frequency) the 6-kHz probe frequency. The influence of possible MEMR effects was examined by measuring phase-gradient functions for the elicitor effects and by measuring changes in the ear-canal pressure with a continuous suppressor of the 6-kHz probe. Both on- and off-frequency complex tone elicitors produced significant changes in ear canal sound pressure. However, the pattern of results was not consistent with the earlier hypotheses postulating that efferent effects produce the psychoacoustic dependence of forward-masked thresholds on masker phase curvature. The results also reveal unexpectedly long time constants associated with some efferent effects, the source of which remains unknown.
Keywords: medial olivocochlear reflex, middle-ear muscle reflex, forward masking, Schroeder-phase complexes
INTRODUCTION
The mechanical responses to sound in the cochlea have been generally described as time-invariant, with the time constants associated with nonlinear aspects, such as suppression, being generally considered negligible from a perceptual perspective (e.g., Ruggero and Temchin 2007). Consequently, the synapse between the inner hair cell and the spiral ganglion is thought to be the first stage where perceptually relevant changes in responses over time occur in the form of neural adaptation (Smith 1977; Abbas 1979; Smith 1979; Smith and Brachman 1982). On the other hand, physiological evidence has also shown that cochlear gain and consequently cochlear responses can decrease over time due to the activation of efferent fibers that project from the medial olivary complex (MOC) and synapse with the outer hair cells (OHCs) in the organ of Corti (for a review, see Guinan 2006). The time course of the effect of efferent activation is relatively slow, with a 25- to 30-ms latency, followed by a buildup over about 70 ms to a nearly asymptotic level. After the offset of the eliciting stimulus, the effect of efferent activation remains constant for about 25–30 ms and then decays over a 160–200-ms interval (Backus and Guinan 2006).
Despite robust physiological evidence showing decreased peripheral responses due to the stimulation of MOC efferents by electric shocks in cats (Gifford and Guinan 1987; Liberman 1989; Warren and Liberman 1989) and reduced magnitudes of evoked otoacoustic emissions in the presence of acoustic elicitors of the medial olivocochlear reflex (MOCR) in humans (Collet et al. 1990; Veuillet et al. 1991; Collet et al. 1992; Norman and Thornton 1993; Maison et al. 2000; Guinan et al. 2003; Backus and Guinan 2006; Lilaonitkul and Guinan 2009a, 2012), the functional role of the reflex remains poorly understood. There is evidence from physiological animal studies that MOC efferents play a role in protecting the cochlea and the synaptic connections with afferent auditory nerve fibers from the effects of aging (Liberman et al. 2014) and from noise-induced damage (Kujawa and Liberman 1997; Maison et al. 2013). It is likely that they have a similar role in the human auditory system. However, the effects of efferent activation on performance in perceptual tasks remain elusive. Scharf et al. (1994, 1997) measured performance by listeners with Ménière’s disease in a series of basic psychophysical tasks before and after sectioning of the olivocochlear bundle. He found no effect of the sectioning on the detection of tones in quiet and in noise, no effect on tuning measured with a notched noise masker, and no effect on intensity and frequency discrimination. The only significant effect was an improvement in the detection of tones with unexpected frequencies embedded in noise maskers after the sectioning of efferents, which led the authors to conclude that efferent activation may facilitate detection under selective attention. However, Scharf et al. tempered their conclusions by noting that the vestibular neurotomy performed on their patients may not have resulted in a complete elimination of MOC efferent connections to the cochlea.
Recently, a growing number of studies have implicated efferent activation as a factor contributing to various psychophysical effects that appeared consistent with relatively slow changes in cochlear responses over time. Examples of such effects include a so-called temporal effect or overshoot (McFadden and Champlin 1990; von Klitzing and Kohlrausch 1994; Strickland 2004; Strickland and Krishnan 2005; Strickland 2008), the effect of a precursor on cochlear gain and compression, as estimated from the growth of psychophysical forward masking (Krull and Strickland 2008; Jennings et al. 2009; Roverud and Strickland 2010, 2014), changes in frequency selectivity during the course of acoustic stimulation (Jennings et al. 2009; Jennings and Strickland 2012), changes in frequency selectivity due to contralateral noise (Aguilar et al. 2013), changes in the rate of recovery from forward masking for high masker levels (Wojtczak and Oxenham 2009a), and masker phase effects in forward masking by harmonic complexes (Wojtczak and Oxenham 2009b).
All these studies used psychophysical methods and provided no independent estimate or measure of efferent activation. In two recent studies, psychophysical measurements of overshoot were combined with noninvasive physiological measurements of the stimulus frequency otoacoustic emissions (SFOAEs) to verify the MOCR-based explanation of the effect (Keefe et al. 2009; Walsh et al. 2010). While Walsh et al. (2010) found good correspondence between the time course of the psychophysical overshoot and changes in the magnitude of a so-called “nonlinear SFOAE” with a delay from masker onset, Keefe et al. (2009) found no changes in SFOAE threshold with the delay despite very robust (on average 16 dB) psychophysical overshoot in the same listeners. Because of the general lack of consistent independent verification, statements regarding efferent involvement in various psychophysical phenomena remain speculative. The aim of this study was to provide an independent test of the hypothesized role of the MOCR in forward masking by harmonic complexes with identical power spectra and different phase spectra, as used by Wojtczak and Oxenham (2009b). In that study, forward masking produced by Schroeder-phase complexes was measured as a function of their phase curvature, which was defined as
| 1 |
where θ(f) denotes the component starting phase as a function of component frequency f, N is the number of components in the harmonic complex, f0 denotes the fundamental frequency, and C is a constant that was varied between −1 and 1 in steps of 0.25 to obtain maskers with different phase curvatures (Lentz and Leek 2001; Oxenham and Dau 2001a). The study showed that when the probe frequency was 1 or 2 kHz, there was a significant effect of C value on masked thresholds for an on-frequency masker (with components around the probe frequency) but not for an off-frequency masker (with components placed around the frequency an octave below the probe frequency). However, masker phase effects were significant for a 6-kHz probe in both the on- and off-frequency masking conditions.
Two findings of the Wojtczak and Oxenham study were surprising and led them to invoke an additional mechanism to account for their data. The first finding was the effect of masker phase curvature in off-frequency masking of the 6-kHz probe. In earlier studies, masker phase effects had been explained as resulting from the interaction between the phase curvatures of the masker and the cochlear filter tuned to the probe frequency (Smith et al. 1986; Kohlrausch and Sander 1995; Lentz and Leek 2001; Oxenham and Dau 2001a, b) and from compression of the waveform at the output of that filter. The role of compression was evidenced by reduced masker phase effects in listeners with hearing impairment (Summers and Leek 1998; Summers 2000; Oxenham and Dau 2004) and by the presence of the effects in forward masking (Carlyon and Datta 1997; Wojtczak and Oxenham 2009b). In forward masking, listeners cannot use dips in the masker envelope to detect the signal. However, harmonic complexes with fluctuating temporal envelopes have lower rms amplitude after being subjected to compression than the complexes with the same original rms amplitude and spectrum but with flat temporal envelopes. This fact has been used to explain a decreased effectiveness of Schroeder-phase maskers with fluctuating envelopes at the output of the cochlea. Because off-frequency stimuli with frequencies about an octave below the characteristic frequency (CF) of the measurement place are thought to produce a linear response on the basilar membrane (BM; Ruggero et al. 1997), masker phase effects should be observed in forward masking for on-frequency but not for off-frequency maskers.
The second surprising finding in the study by Wojtczak and Oxenham (2009b) was the effect of masker duration: The effect of masker phase curvature was stronger for the 200-ms than for the 30-ms maskers. Because cochlear compression is known to be nearly instantaneous (Ruggero et al. 1997), the duration effect, along with the phase effects in off-frequency masking, suggested that an additional mechanism may be involved. Wojtczak and Oxenham (2009b) suggested that the mechanism may be one (or both) of the two feedback-based mechanisms with relatively long time constants, the MOCR (Backus and Guinan 2006) and the middle-ear muscle reflex (MEMR; Church and Cudahy 1984). Wojtczak and Oxenham (2009b) hypothesized that maskers producing the most modulated envelopes, and thus, the smallest average excitation on the BM (due to the interaction between the masker and cochlear-filter phase curvatures and compression) may be the least effective elicitors of the feedback-based reflexes. On the other hand, maskers producing waveforms with flatter envelopes at the output of the cochlea would result in a greater excitation and therefore would be more effective at eliciting either of the reflexes. As a consequence, maskers with flatter envelopes at the output of the cochlea would produce higher forward-masked thresholds than the maskers with fluctuating envelopes, due to either a greater reduction of cochlear gain at the signal frequency place on the BM (MOCR) or a greater attenuation of the transmission through the middle ear (MEMR). The difference in threshold would be greater for maskers with a longer duration that would allow for a longer buildup time for the effect of the involved reflex. Although either reflex could play a role, the authors favored the explanation in terms of the MOCR because the MEMR has been shown to predominantly affect transmission of low frequencies (<2 kHz) through the middle ear (e.g., Schairer et al. 2007) whereas Wojtczak and Oxenham (2009b) observed the off-frequency masker phase effects only for a 6-kHz probe and not for the 1- and 2-kHz probes.
In the present study, a method for measuring the effect of efferent activation on stimulus frequency otoacoustic emission (SFOAE) developed by Guinan et al. (2003) was implemented to measure changes in the ear-canal sound pressure at the probe frequency due to the on- and off-frequency Schroeder-phase complexes with different phase curvatures. In addition, psychophysical measurements were performed to test an alternative hypothesis that the off-frequency masker phase effects observed by Wojtczak and Oxenham (2009b) were due to residual compression, such that the place along the BM with a CF corresponding to the probe frequency still responded at least somewhat compressively to the off-frequency masker.
EXPERIMENT 1: EFFECTS OF ELICITOR PHASE CURVATURE ON EAR-CANAL SOUND PRESSURE AT THE PROBE FREQUENCY
Rationale
The aim of this experiment was to test the hypothesis that MOC efferent activation is affected by component phase relationships within a harmonic complex tone. The stimuli were the on- and off-frequency Schroeder-phase maskers used by Wojtczak and Oxenham (2009b) with the 6-kHz probe, because unexpected masker phase effects in off-frequency masking conditions were observed for this probe frequency, and because studies have shown that the ipsilaterally activated MEMR should not affect the transmission of a 6-kHz tone through the middle ear (Schairer et al. 2007).
The data from Wojtczak and Oxenham (2009b) showed that for the off-frequency masker of a 6-kHz probe, forward-masked thresholds were the lowest when all the masker components started with the same (0 ° or sine) phase, and they were the highest for maskers with phase curvatures obtained by setting the value of C in Eq. 1 to −1 (Schroeder-phase negative masker) and 1 (Schroeder-phase positive masker). To be consistent with the role of efferent activation, as hypothesized in the study of Wojtczak and Oxenham, the Schroeder-positive and Schroeder-negative complexes should produce a greater reduction in cochlear gain, and consequently a greater reduction in SFOAE magnitude at the probe frequency, than the sine-phase harmonic complex at the same overall intensity.
Listeners
Normal-hearing listeners were used for this study. Their hearing thresholds were below 15 dB HL at audiometric frequencies between 250 and 8,000 Hz, as measured using an ANSI-certified audiometer (Madsen Conera). Three of the recruited listeners were excluded because they showed no significant post-elicitor effects on the ear-canal pressure for the Schroeder-phase elicitors during a 2-h session. Seven listeners (one male, six females), with ages in the range of 21–49 years (median 26 years), provided useable data that were analyzed to test for the effects of the elicitor phase curvature. Prior to data collection, the listeners provided written informed consent and the protocol for this study was approved by the Institutional Review Board of the University of Minnesota.
Stimuli and Procedure
Ear-canal pressure waveforms were recorded during the presentation of a continuous 6-kHz tone and an intermittent Schroeder-phase complex. A schematic illustration of the stimuli in a recording trial is shown in Figure 1. A Schroeder-phase complex consisting of 25 harmonics of a 100-Hz fundamental frequency, presented ipsilaterally with the tonal probe, was used to elicit the MOCR. On-frequency Schroeder-phase complexes (Fig. 1A) consisted of components from 4,800 to 7,200 Hz, and off-frequency complexes (Fig. 1B) consisted of components from 1,600 to 4,000 Hz. These spectral configurations of the on- and off-frequency elicitors were identical to those of the on- and off-frequency forward maskers in the study by Wojtczak and Oxenham (2009b). For each spectral configuration, three phase curvatures of the elicitor, given by C = −1, 0, and 1 in Eq. 1, were used in separate blocks of trials. A trial consisted of eight 8.5-s segments, each comprising 1 s of the probe alone, followed by a 2.5-s interval during which an MOCR elicitor was added to the probe, followed by a 5-s interval containing the probe alone. The elicitor’s polarity was alternated between consecutive segments. This allowed for the cancellation of the physical waveform of the elicitor during the averaging of the recorded waveform across the eight segments while preserving the elicitor’s effect on the ear-canal sound pressure at the probe frequency.
FIG. 1.
Schematic illustration of the spectro-temporal configuration of stimuli used to measure changes in the ear-canal pressure produced by on-frequency (A) and off-frequency (B) harmonic complex elicitors. The plus and minus signs indicate the alternating polarity of the elicitors in consecutive presentations. The blue line represents the continuous probe.
The probe was presented at a level of 50-dB sound pressure level (SPL). This level was 10 dB higher than the probe level used in most SFOAE-based measurements of the effects of MOC efferent activation (Guinan et al. 2003; Backus and Guinan 2006; Lilaonitkul and Guinan 2012). It was necessary to use a higher probe level because the measurements in this study were performed using a 6-kHz probe, and the level of 40 dB SPL was often not sufficiently high to produce a measurable SFOAE at this frequency. It cannot be ruled out that the probe itself activated the MOCR. However, pure tones have been shown to be relatively ineffective elicitors of efferent effects and the on-frequency effects reported in earlier studies usually did not reach significance for elicitor levels below 60–70 dB SPL. In addition, the effects elicited by pure tones have been shown to decrease with increasing frequency of the probe tone (Lilaonitkul and Guinan 2009b, 2012). Walsh et al. (2010) also showed no change in the magnitude of the nonlinear SFOAE measured over the course of 500 ms for a 60-dB SPL 4-kHz probe. It is therefore assumed that any contribution to changes in the ear-canal sound pressure due to a continuous 50-dB SPL 6-kHz probe in this study is negligible. The onset and offset of the probe (at the beginning and end of a trial) were gated with 10-ms raised-cosine ramps. The on-frequency elicitors were presented at 65 dB SPL, and the off-frequency elicitors were presented at 75 dB SPL. The level of the off-frequency elicitors was 10 dB below that used for off-frequency maskers by Wojtczak and Oxenham (2009b) to avoid clipping of the recorded waveform, which occurred for the phase curvature defined by C = 0. All the elicitors were gated with 10-ms raised-cosine ramps.
Prior to testing, it was confirmed for each listener that they had no significant spontaneous emissions within 100 Hz of the probe frequency. Estimates of spontaneous emissions were obtained using the procedure described by Penner et al. (1993). In addition, measurements of the effect of a 60-dB SPL broadband noise elicitor on a tonal probe were performed for probe frequencies within a ±120-Hz range around 6 kHz. The purpose of these measurements was to find a proximal frequency for which the effect of efferent activation was the strongest to ensure that robust effects were observed, as was done in previous studies (e.g., Guinan et al. 2003). Since no appreciable differences were found across the frequencies tested, a 6-kHz probe was used for all the listeners participating in the experiment. In addition, a suppression technique was used to estimate the magnitude of the SFOAE at 6 kHz by intermittently presenting a suppressor tone with a frequency of 5,890 Hz and a level of 70-dB SPL instead of the harmonic complex elicitor during the continuous probe presentation.
Stimuli were generated and recorded on a PC via a 24-bit D/A LynxTwo (LynxStudio) sound card using a sampling rate of 44,100 Hz. The stimuli were delivered to the ear canal via the ear piece of an ER10C system (Etymotic Research). The ear piece contained two sound sources and one microphone. The probe and the elicitor were routed to separate sound sources and presented ipsilaterally to the right ear for all the listeners except S5 for whom stronger effects were found in the left ear. The recorded waveforms were analyzed online for artifact rejection. Only artifact-free recordings contributed to the average waveforms that were used for further analyses. Listeners completed the test with one elicitor configuration (on- or off-frequency chosen at random) with different C values selected in a random order before moving on to the next. For each elicitor, 50 artifact-free 8.5-s segments were recorded. During the recordings, the listeners were seated in a comfortable reclining chair located in a double-walled sound-attenuating booth. They were asked to remain still but awake. The listeners were given breaks as needed during which they could choose to take the ear piece out or remain in the booth with the ear piece in the ear canal. An in-the-ear calibration was performed at the beginning of each session, at the beginning of each elicitor condition, after each break, and any time the probe had to be repositioned, to make sure that the stimuli were presented at the same level throughout the experiment. Typically, recordings for all three C values for one spectral configuration of the elicitor were obtained without a break, before recordings for the second spectral configuration commenced. The measurements of the 6-kHz SFOAE with a suppressor tone were performed after all the measurements with harmonic complex elicitors were completed.
Analysis of the Recorded Waveforms
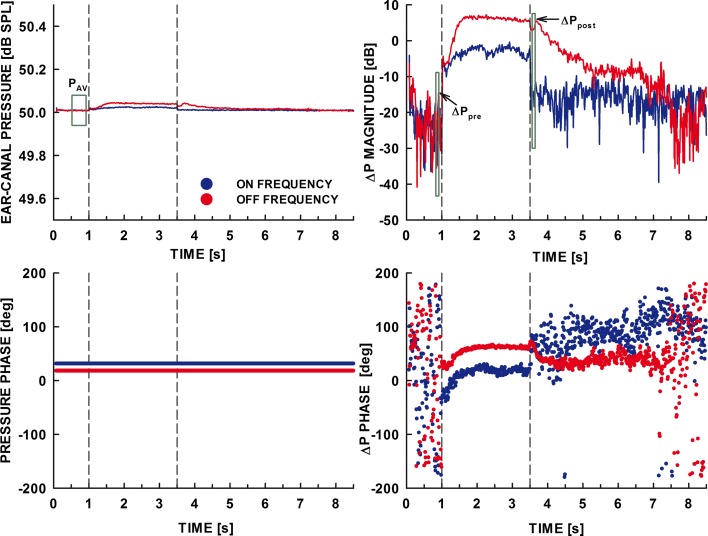
For each condition, recorded segments that passed the online artifact rejection test underwent additional visual screening to remove recordings that showed small but systematic artifacts, such as those resulting from slow shifts in the position of the ear probe during the recorded segment. Discarded segments were replaced by additional data collected to obtain a total of 50 clean segments per subject. The segments were then averaged, resulting in an 8.5-s waveform, and high-pass filtered using an eighth-order Butterworth filter with a cutoff frequency of 400 Hz. The waveform was subsequently heterodyned to obtain a complex-valued ear-canal sound pressure at the probe frequency (Guinan et al. 2003; Backus and Guinan 2006). The heterodyning involved the calculation of the analytic signal from the 8.5-s average waveform, shifting it by the frequency of the probe, and low-pass filtering the resultant complex-valued waveform using a fourth-order Butterworth filter with a cutoff frequency of 50 Hz. The waveform was then downsampled to save storage space. The upper and lower left panels of Figure 2 show the magnitude and phase of the heterodyned ear-canal sound pressure at the probe frequency, respectively, for the on-frequency elicitor (blue line) and the off-frequency elicitor (red line). To extract changes in the ear-canal sound pressure due to the elicitor, the vector average of the complex ear-canal sound pressure (i.e., the mean real and imaginary part) was first calculated within the 500-ms pre-elicitor window. The window extended over the segment of the heterodyned pressure waveform from 550 to 50 ms before the elicitor’s onset (the green rectangle in the top left panel of Fig. 2). Changes in the ear-canal sound pressure were obtained by subtracting the mean real and imaginary parts in the pre-elicitor window from the real and imaginary part of every complex-valued point of the heterodyned pressure waveform, respectively. This vector subtraction resulted in a complex-valued sound pressure representing the noise floor during the time interval when the pressure at the probe frequency was unaffected by an elicitor and in a change in ear-canal sound pressure during the time interval when the elicitor had an effect. The green rectangle positioned at 3.70 s in the top right panel of Figure 2 illustrates the position of the 100-ms post-elicitor window used to estimate the effect of an elicitor on the ear-canal sound pressure at 6 kHz, hereafter referred to as the residual. The residual was calculated using a post-elicitor (rather than during-elicitor) window to avoid effects of (two-tone) suppression of the basilar-membrane response to the probe by the components of the elicitor and to capture the hypothesized dependence of the residual on the elicitor’s phase curvature during the time period over which the elicitor likely produced forward masking. The post-elicitor window was positioned 20 ms after the offset of the elicitor—a delay that did not include the exact temporal position of the probe in the psychophysical forward-masking experiment in the study by Wojtczak and Oxenham (2009b)—to avoid the inclusion of effects of two-tone suppression in the estimate of the residual. A window immediately following the elicitor would include such effects due to the processing performed on the signal (low-pass filtering). The window was also much longer than 10 ms (the duration of the probe in the psychophysical study) to decrease the variability of the estimated residual. However, it is reasonable to assume that the differences in forward masking observed over the first 10 ms of recovery should be present throughout a time period over which forward-masked thresholds are significantly above the threshold in quiet. Thus, it is assumed that an exact match between the temporal position of the probe in the psychophysical task and the position of the post-elicitor window in this study was not necessary for the purpose of testing the working hypothesis. The post-elicitor effect was calculated as 20 log(ΔPpost), where ΔPpost represents the magnitude obtained by averaging the real and imaginary parts of the change in ear-canal sound pressure within the post-elicitor window (top right panel in Fig. 2). The post-elicitor effects were then compared across the three C values of the elicitor.
FIG. 2.
The magnitude of the ear-canal pressure (top left panel), the phase of the ear-canal pressure (bottom left panel), the change in magnitude (top right panel), and the change in phase (bottom right panel) of the ear-canal pressure from averaged recording segments. The blue traces show data obtained for the on-frequency elicitor, and the red traces are for the off-frequency elicitor. The green box in the top left panel illustrates the position of the window over which the vector average of the pressure waveform was calculated. The green boxes in the top right panel illustrate the positions of pre- and post-elicitor windows used to estimate noise floor and the elicitor effect, respectively. The data are for one listener, for the on- and off-frequency elicitors generated with C = −1.
Results and Discussion
Figures 2 and 3 show examples of data from two listeners. Each figure shows data for one arbitrarily selected C value (indicated in the figure captions) to illustrate two general patterns observed in the data. These patterns did not show systematic dependence on the C value or the elicitor condition (on- vs off-frequency) across the listeners, and they are representative of the general trends observed in the data discussed below in detail.
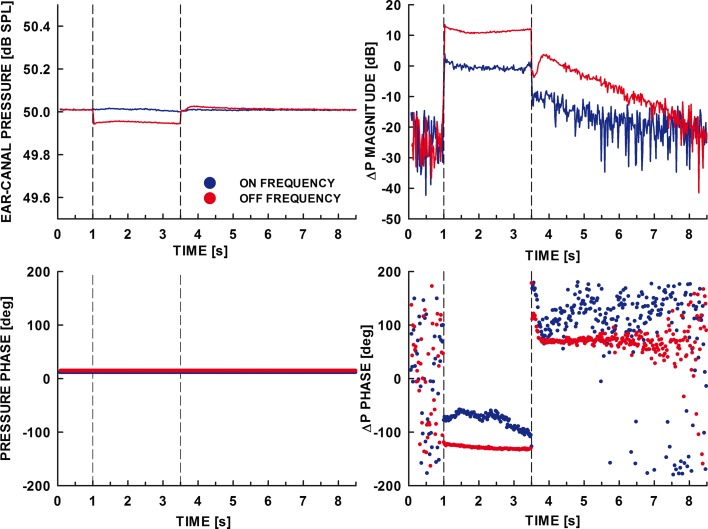
FIG. 3.
As Figure 2 but for a different listener and for the on- and off-frequency elicitors generated with C = 0.
The top and bottom left panels in Figures 2 and 3 show the magnitudes and phases of the averaged 8.5-s heterodyned ear-canal pressure waveforms, respectively, for the on-frequency (blue line) and off-frequency (red line) elicitor conditions. The extracted changes in the magnitude and phase of the ear-canal sound pressure at 6 kHz, ΔP, are shown in the top and bottom right panels, respectively. The vertical dashed lines in all panels mark the elicitor’s onset and offset times.
In both figures, the top right panels show a rapid growth of the ΔP magnitude (expressed in dB) that coincided with the onsets of the on- and off-frequency elicitors. In Figure 2, the fast ΔP onset is followed by a gradual (extending over a few hundred milliseconds) increase in ΔP magnitude to an approximately constant level, whereas in Figure 3 the onset is followed by a small gradual decrease in ΔP magnitude. At first appearance, the effects during the elicitor appear consistent with those reported in the studies of the effect of efferent activation on the SFOAE for broadband and notched noise elicitors (Guinan et al. 2003; Backus and Guinan 2006). The relatively slow buildup in Figure 2 seems consistent with the effect of the MOCR taking over the initial dominant effect produced by two-tone suppression of the response to the 6-kHz probe on the BM. The slow decrease in ΔP after the initial rapid increase in ΔP magnitude in the right top panel of Figure 3 is reminiscent of onset adaptation of the distortion product otoacoustic emissions (DPOAEs) due to the ipsilaterally evoked MOCR in cats shown by Liberman et al. (1996). Liberman et al. argued that the primaries presented at relatively high levels evoke the MOCR which decreases the BM response to the primaries (and thus the amplitude of DPOAE) via efferent feedback. A similar finding in humans was reported by Kim et al. (2001), but the effects were smaller than those in animals. Given these reports, the decrease in ΔP magnitude during the first few hundred milliseconds of the elicitor appears consistent with the effect of the MOCR on the elicitor itself.
However, the above interpretation in terms of the effect of the MOCR on the SFOAE evoked by the 6-kHz probe is complicated by very large ΔP magnitudes during the elicitor, particularly for the off-frequency elicitor. In that condition, the ΔP magnitudes often exceeded the magnitudes of the SFOAEs at 6 kHz measured using the standard suppression technique (e.g., Brass and Kemp 1993; Guinan et al. 2003) by up to 10–15 dB for the subjects whose data are shown in Figures 2 and 3 and for all of the other subjects (data not shown). The large effects are inconsistent with the results from previous studies that used lower probe and elicitor levels to measure the effects of efferent activation on the SFOAE (Guinan et al. 2003; Backus and Guinan 2006; Lilaonitkul and Guinan 2009b, a, 2012). In these studies, the reported ΔP magnitudes were always a fraction of the SFOAE magnitude measured with a single-tone suppressor. Because of this discrepancy and the uncertainty about the mechanism underlying elicitor effects in this study, the ΔP magnitude was not normalized by the SFOAE magnitude estimated using the single-tone suppression technique, as was typically done by Guinan and colleagues (Guinan et al. 2003; Backus and Guinan 2006; Lilaonitkul and Guinan 2009a, 2012). It should be noted that such normalizing would not have affected the relative effects across the three elicitor phase curvatures as it would amount to subtracting the same dB amount from each effect for a given subject.
There are a few possible explanations for why the effect of the elicitor on the ear-canal pressure at the probe frequency was greater than the estimated SFOAE magnitude. One possible explanation is that the intense elicitors used in this study drove the outer hair cell stereocilia at the basal end of the cochlea into their nonlinear region thereby generating an SFOAE-like residual at the probe frequency via local distortion processes, as reported in a study by Guinan (1990). This explanation would imply that the elicitor used as a forward masker in the previous psychophysical study by Wojtczak and Oxenham (2009b) would itself produce an additional source of energy at the probe frequency and the amount of added energy could depend on masker phase curvature, contributing to the observed masker phase effects. Although appealing, this interpretation is weakened by the fact that similarly large effects are present when the harmonic complex elicitor is replaced by a notched noise around 6 kHz and when the elicitor is presented contralaterally to the probe (Walsh and Wojtczak 2014). Another possibility is that since the probe was presented at 50 dB SPL, the SFOAE traveling back through the middle ear to ear canal was generated not only around the place with the CF of 6 kHz but also contained significant contributions from basally distributed generators (Siegel and Badri 2002; Siegel et al. 2003; Siegel et al. 2004; Siegel et al. 2005; Charaziak et al. 2013; Moleti et al. 2013; Sisto et al. 2013). A single-tone suppressor with a frequency 110 Hz below that of the probe may have been insufficient to eliminate an SFOAE generated by all the sources either via two-tone suppression or/and via efferent activation. The highest component of the off-frequency elicitor was 4,200 Hz, so it is unlikely that this elicitor could eliminate the SFOAE at 6 kHz more effectively than the 5,890-Hz tone via two-tone suppression. However, because the off-frequency elicitors had broader spectra, they may have been more effective at suppressing the SFOAE generators via the feedback-based efferent system, thus producing a larger residual, ΔP.
An alternative explanation for the sizeable ΔP magnitude is in terms of the MEMR. The activation of the MEMR would affect the impedance of the middle ear, thereby changing the ear-canal sound pressure in a way unrelated to the inner-ear response and thus the SFOAE. A problem with this explanation is that the effects of the MEMR are known to be quite slow. Even for the most intense activators of the MEMR (i.e., 110 dB or more), the effects have been shown to exhibit at least a 20-ms latency followed by at least a 50–100 ms buildup time (Hung and Dallos 1972). Thus, based on the reported time courses, the MEMR could not account for the rapid change in the ear-canal pressure shown in Figure 3.
After the offset of the elicitor (marked by the vertical dashed line at 3.5 s), the data in both figures show an initial rapid decrease in ΔP magnitude followed by a very slow further decay during which the effect remained significantly above the noise floor for a period of several seconds. The presence of the post-elicitor effect is also evidenced by the relatively narrow spread of the ΔP phase compared to that in the pre-elicitor interval (between 0 and 1 s). As the effect decreased to the level of the noise floor, the ΔP phase became scattered over the range from −180 to 180 °, as expected (e.g., Guinan et al. 2003). For the off-frequency elicitor, data from all the listeners exhibited a nonmonotonicity in the recovery function that followed the fast decrease in ΔP magnitude at the elicitor’s offset. The magnitude of the post-elicitor ΔP was also in most cases larger for the off-frequency elicitor than for the on-frequency elicitor.
Overall, the elicitors used in this study produced substantial changes in the ear-canal pressure that persisted for a long time after their offsets. The recovery times were much longer than those reported for the effects of efferent activation by a 60-dB SPL notched noise on the SFOAE at 1 kHz (Backus and Guinan 2006). The nonmonotonic behavior of the post-elicitor ΔP magnitude suggests that more than one mechanism may have played a role.
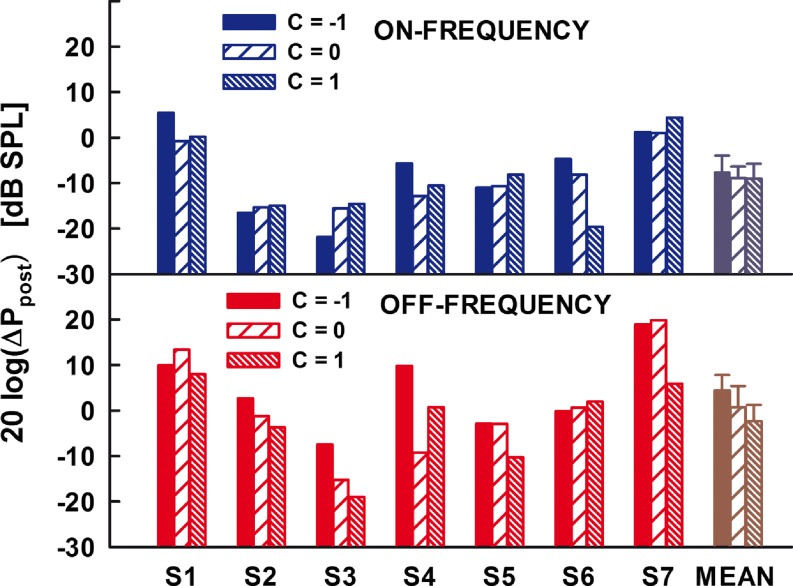
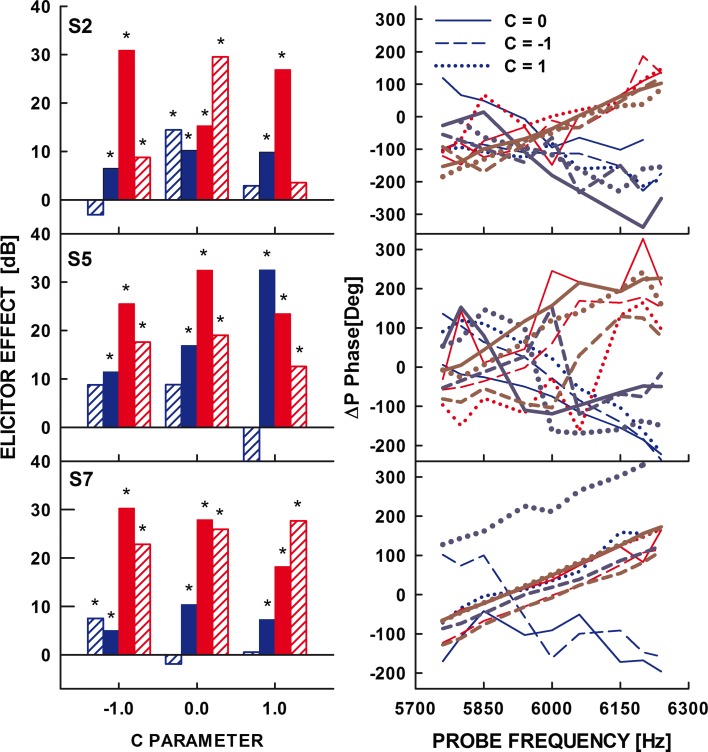
The post-elicitor effects expressed in dB SPL are shown in Figure 4, for the on-frequency elicitor (upper panel) and the off-frequency elicitor (lower panel). In each panel, the three bars plotted for each subject show the effect for the three phase curvatures used, Schroeder-phase negative (filled bar), zero-phase complex (coarse-hatched bar), and Schroeder-phase positive (fine-hatched bar). The effects of the elicitor were considered significant when they exceeded 5 dB (i.e., exceeded two standard deviations from the mean noise floor level calculated from the average pre-elicitor ΔP magnitude obtained by averaging the real and imaginary parts of the complex-valued ΔP within the 100-ms pre-elicitor window positioned at 0.85 s in the top right panel of Fig. 2) and were statistically significant according to the one-tailed Welch’s t test. According to these two criteria, all the effects shown in Figure 4 were significant. The rightmost set of the bars shows the mean effect for the seven subjects tested.
FIG. 4.
Effects of the on-frequency elicitors (upper panel) and off-frequency elicitors (lower panel) for the parameter C values of −1 (filled bars), 0 (coarse-hatched bars), and 1 (fine-hatched bars). The three rightmost bars in both panels represent the mean across the seven listeners tested. The error bars on the rightmost bars represent one standard error of the mean.
To be consistent with the original hypothesis of Wojtczak and Oxenham (2009b), the effects shown in Figure 4 for C = −1 and C = 1 should have been consistently larger or smaller than for C = 0, depending on the mechanism involved. For example, if the effects in Figure 4 were due to the MOCR, and thus due to a reduction of cochlear gain, then a greater reduction in SFOAE magnitude (i.e., taller bars) for C = −1 and 1 than for C = 0 would be consistent with the psychophysical forward-masking data. A similar pattern would be expected if the effects reflected a reduced admittance due to the activation of the MEMR. If, however, the effects were due to an increased admittance at 6 kHz, then a smaller change in the ear-canal pressure (i.e., a smaller increase in admittance) for elicitors with C = −1 and 1 compared with that for the elicitor with C = 0 would be consistent with the psychophysical data. Neither result was consistently observed in the individual or the mean data shown in Figure 4. A repeated-measures two-way ANOVA with the main factors of phase curvature and condition (on- vs off-frequency elicitor) showed that the effect of the phase curvature was statistically significant [F(2,12) = 4.34, p = 0.04]. The post-elicitor effect was significantly larger for off-frequency elicitors than that for on-frequency elicitors [F(1,6) = 35.55, p = 0.001], but there was no significant interaction between the elicitor’s phase curvature and condition [F(2,12) = 0.90, p = 0.43]. Although the effect of the phase curvature was significant, it reflected the tendency for the elicitor effect to be the largest for C = −1 and smallest for C = 1, inconsistent with the working hypothesis proposed to explain psychophysical masking by these harmonic complexes.
In summary, the original hypothesis that masker-phase-dependent changes in forward masking can be explained in terms of efferent effects was not supported. All six elicitors (with three C values in on- and off-frequency conditions) produced significant changes in the ear-canal sound pressure at the probe frequency during and after the elicitor, but the changes were not affected by the phase characteristics of the masker in a way that was consistent with the psychophysical forward-masking data in the study by Wojtczak and Oxenham (2009b). In addition, based on the results shown thus far, it is not possible to determine which of the feedback-based reflexes, the MOCR or MEMR, dominated these pressure changes. In the following experiment, two paradigms were used to gain more insight into the mechanisms producing the changes in ear-canal sound pressure.
EXPERIMENT 2: EXAMINING THE ROLE OF THE MIDDLE-EAR MUSCLE REFLEX
Rationale
Studies examining the influence of the MEMR on measures of reflectance and admittance using wideband probe stimuli have shown no significant effects at frequencies as high as 6 kHz (Feeney and Keefe 2001; Schairer et al. 2007). However, changes in ΔP magnitude shown in Figures 2 and 3 do not appear entirely consistent with the known characteristics of the MOCR either. First, the recovery from the elicitor effect was much longer than that the MOCR time constants estimated by Backus and Guinan (2006). Second, in some subjects, the ΔP magnitude during the elicitor was larger than the magnitude of the SFOAE estimated at 6 kHz using a tonal suppressor (data not shown), which contrasts with the reported MOCR effects that were a fraction of the SFOAE magnitude observed using the suppression paradigm (e.g., Guinan et al. 2003; Backus and Guinan 2006; Lilaonitkul and Guinan 2012).
In this experiment, the role of the MEMR was examined using two techniques that have been implemented in recent SFOAE studies to determine if changes in the ear-canal pressure at the probe frequency were elicited via MOC efferent activation or whether they were due to changes in the acoustic impedance at the tympanic membrane caused by a contraction of the stapedial muscle (Guinan et al. 2003; Lilaonitkul and Guinan 2009b). One technique, described by Guinan et al. (2003), involved measuring the phase-gradient functions for the effects of the elicitor. The technique is based on the assumption that if the effect of the elicitor on the probe originates in the cochlea, as in the case of MOCR activation, then the phase-gradient functions should yield group delays similar to those estimated from SFOAEs in the spectral region around the probe frequency (Shera and Guinan 2003). In contrast, a negligible group delay should be observed if the effect of the elicitor is due to the MEMR. The second technique, described by Lilaonitkul and Guinan (2009b), involved using a continuous suppressor tone and an intermittent elicitor. This technique is based on the assumption that once the SFOAE is eliminated by a continuous suppressor, the effect of efferent activation due to the elicitor should not be observed. Thus, the presence of an elicitor effect with a continuous suppressor tone would suggest that the effect is not generated in the cochlea and therefore is not related to the probe-evoked emission.
Because the cochlear group delay at 6 kHz is short in humans (Shera and Guinan 2003), the variability in the measurements of ΔP phase as a function of frequency could be insufficient to distinguish between the two reflexes (MOCR vs MEMR) with certainty. Similarly, the results obtained with the continuous suppressor would not provide a definitive answer if the continuous suppressor did not completely eliminate the SFOAE generated in the cochlea. By obtaining consistent results from the two techniques, stronger inferences could be made about the reflex mediating the effects shown in experiment 1.
Listeners
Only three of the seven listeners (S2, S5, and S7) were available for testing in this experiment. As in experiment 1, the listeners were asked to remain still but awake during the measurements.
Stimuli and Procedures
Both techniques used probe-elicitor configurations that were identical to those shown in Figure 1, for the on- and off-frequency elicitors. During the test with a continuous suppressor, a 5,890-Hz tone was presented with the 6-kHz probe throughout the trial. The level of the suppressor was set to 70 dB SPL (i.e., 20 dB above the level of the probe). In selected cases where the effects of the elicitor were not eliminated using this suppressor level, a 75-dB SPL suppressor was used to examine if the remaining effect was due to incomplete suppression. For each of the six elicitor conditions, 16 clean (i.e., artifact-free) recordings were obtained and analyzed using the heterodyne technique described above. Based on the data from experiment 1, this number of repetitions was deemed sufficient to obtain reasonable estimates of the effect. Although the suppressor tone was not alternated in polarity during the trial because of its continuous presentation, the component at the suppressor frequency and the emission evoked by it were removed during the analysis of the recorded waveform by low-pass filtering the analytic signal after shifting it by the probe frequency, as described above.
Measurements of the phase-gradient function (ΔP phase as a function of frequency) for each elicitor were performed using nine probe frequencies, from 5,760 to 6,240 Hz in steps of 60 Hz. In all cases, the probes were presented at 50 dB SPL. Sixteen recorded waveforms were averaged to calculate ΔP phase for each probe frequency. The phase estimates were obtained from the ΔP segments starting 50 ms after the onset of the elicitor and ending 50 ms before its offset. Changes in the ear-canal sound pressure at the probe frequency during the elicitor likely reflected a combined effect of BM suppression and one or more of the efferent-based reflexes. In most cases, even for the on-frequency elicitors, changes in the ear-canal sound pressure showed an initial effect with a rapid onset, likely due the BM suppression that was subsequently dominated by an effect with a slower buildup time (see the blue curve in the top right panel of Fig. 2). This pattern suggests that an efferent-based effect dominated changes in the ear-canal sound pressure even during the elicitor, once the effect built up to its asymptotic strength. However, in a few cases, it was not clear which effect was dominant, and the dominating effect sometimes varied across the probe frequencies. For this reason, the phase-gradient functions were also derived for the effects within the 100-ms post-elicitor window, although due to the relatively small number of measurements and the short time interval, these functions are more variable than the ones obtained from the much longer segment during the elicitor.
Results and Discussion
Figure 5 shows the individual data from the three listeners. The left panels show decibel differences between ΔP magnitudes in the 100-ms post- and pre-elicitor windows (). The pre-elicitor window (the green box in the top right panel of Fig. 2) contained a 100-ms sample of the noise floor. This representation was used to illustrate the significance of the elicitor effect or lack thereof. The filled bars show the effects of the on-frequency (blue bars) and off-frequency (red bars) elicitors on ΔP magnitude, calculated based on the effects in dB SPL shown in Figure 4 for the same subjects. The hatched bars show the effects observed with the continuous 70-dB SPL suppressor tone. The asterisks above the bars indicate significant effects of the elicitor according to the 5-dB criterion and the one-tailed Welch’s t test. Increasing the suppressor level from 70 to 75 dB SPL did not result in a decrease of the elicitor effect in any of the selected cases (data not shown), indicating that the significant elicitor effects observed with a continuous suppressor were probably not due to incomplete suppression of the SFOAE by the continuous 5,890-Hz tone. The right panels show phase-gradient functions for the six elicitors used. The blue and red curves show the functions for the on- and off-frequency elicitors, respectively. Different line types represent data for different C values, as indicated in the legend in the top panel. The thicker curves plotted in darker shade were obtained using the effects in the 100-ms post-elicitor window, while the thinner curves in brighter shades were obtained from the segment during the elicitor. Although the data from the post-elicitor window exhibit substantial variability, they show that with two exceptions (S5 for C = −1 and S7 for C = −1 in the on-frequency elicitor condition), the slopes of the functions from the post-elicitor window were similar to those for the during-elicitor segment. The change from a negative slope during the elicitor to a positive slope in the post-elicitor window for S5 suggests that the effect that produced post-elicitor changes in the ear-canal sound pressure was likely dominated by BM suppression during the course of the elicitor. For S7, the results from the post-elicitor window are too noisy to make inferences about the change in the dominant mechanism between the during- and post-elicitor time windows. Linear regression was used to estimate the group delay implied by the phase-frequency function using the less variable during-elicitor estimates. The group delays and the goodness of fits (r2 values provided in the parentheses) are shown in Table 1. Although for some data sets, a straight-line fit poorly represented the shape of the phase-gradient functions (e.g., S5 for the off-frequency elicitor in the middle panel of Fig. 5), it was considered a sufficiently good approximation for estimating the group delay in this study.
FIG. 5.
Left panels show the effects of the on-frequency elicitors (blue bars) and off-frequency elicitors, referenced to the noise floor, in the absence (filled bars) and the presence (hatched bars) of a continuous suppressor of a 6-kHz probe. The asterisks above the bars denote significant elicitor effects. Right panels show ΔP phase as a function of frequency in the region around 6 kHz. The blue curves are for the on-frequency elicitors, and the red curves are for the off-frequency elicitors. Different types of lines are for different phase curvatures of the elicitor, as shown in the legend in the top right panel. Thick curves were obtained using the effects from the 100-ms post-elicitor window. Thinner curves were obtained using changes in the ear-canal pressure during the elicitor.
TABLE 1.
Group delays calculated from straight-line fits to phase-gradient functions for the effects of on- and off-frequency elicitors with phase curvatures given by C = −1, 0, and 1
| Subject | Elicitor | C = −1 (ms) | C = 0 (ms) | C = 1 (ms) |
|---|---|---|---|---|
| S2 | On | 0.66 | 1.3 | 0.61 |
| Off | −1.54 | −1.53 | −1.09 | |
| S5 | On | 2.2 | 1.34 | 2.02 |
| Off | -0.89 | -1.19 | −1.58 | |
| S7 | On | 1.58 | 0.39 | −1.36 |
| Off | −1.38 | −1.19 | −1.35 |
As stated above, two techniques were used to attempt to distinguish between different potential reflexes underlying the effects shown in Figure 4. The results of the two tests would be consistent with the effects of the MOCR activation alone if (1) significant effects disappeared in the presence of a continuous suppressor, and (2) the phase-gradient functions had negative slopes yielding estimates of the group delay consistent with those reported by Shera and Guinan (2003) for humans around 6 kHz (i.e., in the range from about 0.5 to 6 ms). In contrast, the results would be consistent with the effects of MEMR activation if (1) the effects of the elicitors remained unaffected by the presence of the continuous suppressor or became slightly larger due to the increased overall level of the stimulus resulting from the addition of the suppressor, and (2) the phase-gradient functions were flat, indicating a negligible group delay during the elicitor. The results in Figure 5 are not entirely consistent with either set of predictions, making the interpretation difficult.
In seven out of nine cases with the on-frequency elicitor, the presence of the continuous suppressor eliminated the significant effect of the elicitor, as measured by ΔP. In contrast, in eight out of nine cases with the off-frequency elicitor, the presence of the continuous suppressor did not eliminate a significant ΔP. Thus, based only on the effects of the continuous suppressor, it would appear that in general, the effects of the on-frequency elicitor were mediated by the MOCR, whereas the effects of the higher-level off-frequency elicitor were mediated by another mechanism, possibly the MEMR. However, an analysis of the phase-gradient data did not always yield the same conclusions. For instance, for subject S2, the effect of the on-frequency elicitor measured with a continuous suppressor remained significant for the elicitor phase curvature defined by C = 0, but the effect of the same elicitor yielded the longest estimated group delay. Also, most of the off-frequency elicitor data obtained with a continuous suppressor showed a significant residual ΔP while most of the phase-gradient functions measured in the presence of the off-frequency elicitors exhibited positive slopes yielding negative estimates of the group delay instead of the near-zero value expected for the effects of the MEMR.
The positive slope of the phase gradient function is consistent with the idea that the elicitor may have introduced an additional SFOAE at 6 kHz via a distortion process at the basal end of the cochlea (Guinan 1990). As the frequency of the probe used in the measurements of the phase-gradient function decreased, the peak of the traveling wave moved away from the place where the SFOAE resulting from the distortion may have been generated, thereby resulting in a smaller phase delay of the SFOAE. The explanation of the positive phase-gradient slopes in terms of the elicitor inducing additional SFOAE energy via a local distortion in the stereocilia is appealing because it could also account for the abnormally large residuals observed for the off-frequency elicitors. As mentioned above, this explanation is weakened by similarly large effects reported for notched noise elicitors with the notch around a 6-kHz probe (Walsh and Wojtczak 2014). Because of the frequency of occurrence and in many cases the consistency of the slope across elicitor C values (see red lines in the bottom panel for S7), these results do not appear to reflect mere measurement error and call for closer attention in a follow-up study.
Overall, the data from the two tests appear to suggest that the effects observed for the on-frequency elicitors were predominantly mediated by the MOCR, while a different mechanism was dominant for the more intense off-frequency elicitors. Despite performing both tests, it cannot be determined with certainty that the effects elicited by the off-frequency Schroeder-phase complexes were due to the activation of the stapedial muscle, mainly due to the often positive rather than near-zero slopes of the phase-gradient functions. Despite some uncertainties surrounding the interpretation of the data, they do not provide support for the hypothesis that phase effects observed in forward masking by the Schroeder-phase complexes by Wojtczak and Oxenham (2009b) were mediated by the mechanism(s) that produced changes in the ear-canal sound pressure at the probe frequency within this study.
EXPERIMENT 3: THE ROLE OF RESIDUAL COMPRESSION IN OFF-FREQUENCY FORWARD MASKING BY SCHROEDER-PHASE COMPLEXES
Rationale
The results from experiments 1 and 2 do not support the hypothesis that masker phase effects observed at 6 kHz with off-frequency maskers are due to differential activation of the MOCR. Therefore, alternative hypotheses must be sought. Wojtczak and Oxenham (2009b) argued that cochlear compression could not account for the effect, because they assumed that the off-frequency maskers in their study were processed linearly at the CF regions on the BM corresponding to the probe frequencies. This argument hinges on the assumption that nonlinearity in BM processing dominates psychophysical masking and other sources of cochlear nonlinearity associated with the inner hair cell (IHC) receptor potential and with nonlinearities in mechanical coupling of BM motion to IHC stereocilia (Stankovic and Guinan 1999; Guinan 2012; Zha et al. 2012) have negligible effects on masking. The assumption has support in results showing linear additivity of masking for low-level stimuli in listeners with normal hearing and in hearing impaired listeners with moderate hearing loss (Oxenham and Moore 1995; Plack et al. 2008) and in the fact that the slope ratio between growth of masking by on- an off-frequency maskers is similar to the slope of response growth on the BM (e.g., Oxenham and Plack 1997; Nelson et al. 2001). However, although the off-frequency maskers were centered one octave below the probe frequency, the highest masker components were only about half-an-octave below the signal frequency and thus may have fallen into the range of frequencies for which the response growth at the CF place on the BM corresponding to the probe frequency was compressive. Wojtczak and Oxenham argued that residual BM compression would have more likely resulted in significant effects of masker phase curvature for the lower probe frequencies, 1 and 2 kHz, for which no masker phase effects were observed, than for the 6-kHz probe for which the effects were significant. This argument was based on earlier reports that the relative bandwidth of the compressive region around the CF increases with decreasing CF (e.g., Lopez-Poveda et al. 2003), but no data were provided to rule out the role of residual BM compression. In this experiment, the role of BM compression of the off-frequency masker of a 6-kHz probe was examined by measuring forward masking produced by equally intense pure-tone and amplitude-modulated (AM) off-frequency maskers. It was assumed that if the effects observed for the off-frequency Schroeder-phase maskers at 6 kHz were due to residual compression of the masker waveform on the BM, then a negligible threshold difference should be observed between the AM and pure tone maskers since the highest component of the AM masker was nearly a full octave below the probe frequency. If, however, the effects were due to a mechanism that differentially responds to maskers with flat and fluctuating envelopes, then the AM masker should produce lower forward-masked thresholds than the pure tone with the same intensity, as was observed for zero-phase (fluctuating) and Schroeder-phase (flat) maskers.
Listeners
Five listeners (one male, four females) with normal hearing participated in the experiment. Three of the listeners (S1, S2, and S5) had also participated in experiment 1. All the listeners had hearing thresholds below 15 dB HL at audiometric frequencies between 250 and 8,000 Hz, as measured using an ANSI-certified audiometer (Madsen Conera). The listeners provided written informed consent before the participation and were given a brief (about 15 min) practice to become familiarized with the task.
Stimuli and Procedure
Forward masking of a 10-ms 6-kHz probe was measured for a 3-kHz pure-tone masker and a 100 % AM 3-kHz masker with the same overall level of 85 dB SPL. The modulation rate of the AM masker was 100 Hz, and thus, the highest component in its spectrum was 3,100 Hz (nearly an octave below 6 kHz). The starting phase of the modulation was randomly selected on each trial. Two masker durations were used, 200 and 30 ms, resulting in a total of four conditions (modulated, unmodulated × two durations). The probe and the maskers were gated using 5-ms raised-cosine onset and offset ramps. The probe was presented immediately after the masker (i.e., with a 0-ms delay between the 0-V amplitudes). Forward-masked thresholds were measured using an adaptive three-interval three-alternative forced-choice procedure combined with a two-down one-up tracking technique estimating the 70.7 % correct point on the psychometric function (Levitt 1971). Within each trial, two of the observation intervals contained only the masker and one, selected at random, contained the masker followed by the probe. The listener’s task was to select the interval with the probe. Visual feedback indicating the correct interval was provided after each trial. A run started with the probe presented at a clearly audible level. The probe level was decreased by 8 dB after two consecutive correct responses and increased by the same step after one incorrect response until the first two reversals were obtained. After that, the step was decreased to 4 dB for the subsequent two reversals and to 2 dB for the remaining eight reversals. A run terminated after the total of 12 reversals were obtained and a single-run threshold was estimated by averaging the level of the probe at the last eight reversal points. The final threshold was obtained by averaging three threshold estimates from single runs. The order of the four conditions was randomized for each listener and each repetition.
The stimuli were generated digitally on a PC with a sampling rate of 48 kHz and were played out via a 24-bit LynxStudio Lynx22 sound card. All the stimuli were presented monaurally (to the left ear) via a Sennheiser HD 580 headset. The listeners were tested in a double-walled sound-attenuating booth and responded via a computer keyboard or mouse.
Results and Discussion
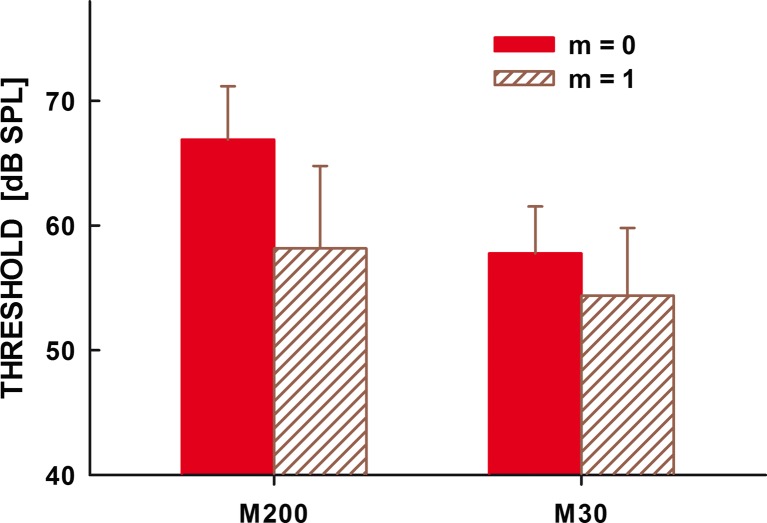
Figure 6 shows the forward-masked thresholds in the four conditions obtained by averaging the data from the five listeners tested. The left and right sets of bars represent thresholds for the 200- and 30-ms masker duration, respectively. The filled bars show thresholds for a pure-tone (unmodulated) masker, and the hatched bars show thresholds for a 100 % AM masker. The error bars show one standard error of the mean.
FIG. 6.
Forward-masked thresholds for a pure 3-kHz tone (filled bars) and an equal-intensity AM 3-kHz tone (hatched bars). The left set of bars shows data for the 200-ms maskers and the right set of bars for 30-ms maskers. The error bars represent one standard error of the mean.
Similar to the forward-masking data for the off-frequency Schroeder-phase maskers in the study by Wojtczak and Oxenham (2009b), thresholds for detecting the 6-kHz probe were lower when the off-frequency forward maskers were modulated than when their envelope was flat, even though the maskers had equal intensity. The difference in threshold between the two masker conditions was greater for the 200-ms (about 9 dB) than for the 30-ms maskers (about 4 dB). A repeated-measures ANOVA performed using the forward-masked thresholds with the main factors of condition (modulated vs unmodulated masker) and masker duration showed a significant effect of AM [F(1,4) = 26.31, p = 0.007] but no significant effect of masker duration [F(1,4) = 2.74, p = 0.17]. The interaction between the masker duration and condition just failed to reach significance [F(1,4) = 6.96, p = 0.058]. A paired t test performed on differences between forward-masked threshold observed for the pure-tone masker and that for the AM masker showed that the difference between the two thresholds was significantly smaller for the 30- than 200-ms maskers [t(4) = 4.09, p = 0.015].
Although the starting phase of the modulation was randomly varied between trials, when comparing thresholds for the pure-tone and AM maskers, it was implicitly assumed that for a modulation rate of 100 Hz, forward-masked threshold did not depend on whether the masker ended with a valley or a peak in the envelope before the onset of the probe. This assumption should hold based on the estimated equivalent rectangular duration of the temporal window used to fit combined effects of forward and backward masking of a 6-kHz tone by Oxenham and Moore (1994), which was in the range of 10–16 ms. However, to rule out the possibility that masked thresholds were lower for the AM maskers because in some proportion of trials the probe followed low intensity segments in the modulation cycle, forward-masked thresholds were measured for a 200-ms AM masker for the starting modulation phase in the masker fixed at 0, π/2, π, and (3/2) π in separate runs. Three out of the five listeners participating in experiment 3 completed the task. The differences between the highest and lowest thresholds across the four AM starting phases ranged between 2.2 and 3.6 dB across the three listeners. The results analyzed using a repeated-measures ANOVA with Greenhouse-Geisser correction for the violation of sphericity assumption showed no significant effect of the modulation phase on threshold [F(1.14,2.27) = 5.22, p = 0.14]. In addition, a one-tailed t test for groups with unequal variance showed that the differences between the thresholds observed for the AM maskers with a randomized envelope starting phase and pure-tone maskers were significantly greater than the differences between the maximum and minimum thresholds across the four fixed AM starting phases (p = 0.016). The lack of threshold dependence on the local intensity before the masker offset was consistent with that shown by Carlyon and Datta (1997) for a 1,100-Hz signal masked by Schroeder-phase positive and Schroeder-phase negative complexes ending at different points within the waveform cycle.
Lopez-Poveda et al. (2003) measured on- and off-frequency temporal-masking curves and concluded that once the probe frequency is about 4 kHz or higher, the growth of the cochlear response to an off-frequency masker an octave below the probe is linear, consistent with mechanical BM responses measured at the basal end of the cochlea (Ruggero et al. 1997; Russell and Nilsen 1997). Given their conclusion, the differences between thresholds observed with the modulated and unmodulated maskers in experiment 3 could not be explained in terms of BM compression. However, the psychophysical data obtained using methods for estimating compression that rely on a linear off-frequency reference (Oxenham and Plack 1997; Nelson et al. 2001; Lopez-Poveda et al. 2003) cannot rule out the possibility that the response to the masker an octave below the probe frequency is compressive at the output of the cochlea. Data from additivity of masking in a study by Plack and Arifianto (2010) indicated compression of responses to off-frequency maskers and showed that the amount of this compression increases with increasing masker level. Plack and Arifianto suggested that the compression reflects saturation of the IHC receptor potential (Cheatham and Dallos 2001). This type of compression would similarly affect responses to both the probe and the off-frequency masker, but it would have a differential effect on equally intense maskers with flat versus highly fluctuating envelopes in that the fluctuating maskers would produce a weaker response after the compression. Physiological studies have also provided evidence for other sources of nonlinearity in post-BM processing that may contribute to compressive growth of responses to off-frequency stimuli at the output of the cochlea. It has been shown that the tuning and nonlinear growth of motion measured on the reticular lamina, which contributes to the IHC response constituting the input to the higher-level processing, differs from that observed for the BM motion (Chen et al. 2011; Guinan 2012; Zha et al. 2012). Guinan (2012) considered several mechanical IHC drives that would result in nonlinearity of IHC responses that would increase with increasing level of stimulation. Because the off-frequency stimuli in experiment 3 and in the study by Wojtczak and Oxenham (2009b) were presented at a high level (85 dB SPL), it is possible that post-BM cochlear compression of the off-frequency maskers produced the lower masked thresholds for the maskers with fluctuating envelopes than the maskers with flat envelopes.
Although nonlinear IHC responses remain a possible candidate for the mechanism leading to lower forward-masked thresholds for the AM masker than the pure-tone masker in experiment 3, this account does not explain why off-frequency masker phase effects were observed for a 6-kHz probe but not for a 1- and 2-kHz probe in the study by Wojtczak and Oxenham (2009b). This account also does not predict masker duration effects observed in both studies.
GENERAL DISCUSSION
In this study, we set out to test the hypothesis that Schroeder-phase complexes with flat envelopes at the output of the cochlea are more effective at activating the MOCR than the complexes of the same overall level but with highly modulated envelopes, and that this difference in MOCR activation produced masker-phase-dependent thresholds in off-frequency forward masking of a 6-kHz probe in the study by Wojtczak and Oxenham (2009b). The data from experiment 1 showed large changes in the ear-canal pressure at 6 kHz in response to both on- and off-frequency Schroeder-phase complexes. Although in some cases the ΔP magnitude exceeded the magnitude of the SFOAE at 6 kHz measured using a suppression technique, the results from experiment 2 were generally consistent with the idea that on-frequency elicitors activated the MOCR thereby suppressing the probe-evoked SFOAE. In contrast, the results for the off-frequency elicitors were not consistent with the effects of the efferent feedback and the exact mechanism cannot be pinpointed by the data from this study. One reason why a different mechanism might have been activated by the off-frequency elicitors was their higher level (75- vs 65 dB SPL for the on-frequency elicitors). A straightforward explanation is that the off-frequency elicitor activated the MEMR and the changes in the ear-canal sound pressure at the probe frequency measured in experiment 1 reflected changes in the acoustic impedance at the tympanic membrane rather than changes in the SFOAE. However, this explanation is undermined by a lack of evidence that the MEMR significantly affects transmission at frequencies as high as 6 kHz (Feeney and Keefe 2001; Schairer et al. 2007). An alternative explanation is that the off-frequency elicitor produced an additional 6-kHz SFOAE by driving stereocilia into their nonlinear-processing region thereby generating energy at 6 kHz that traveled back through the middle ear to the ear canal. This possibility will be investigated in a follow-up study.
Another interesting aspect of the results from experiment 1 was that the changes in the ear-canal sound pressure at the probe frequency persisted for several seconds after the offset of the elicitor. Although the specific values of time constants for the decay of the effect were not estimated, visual inspection of the data from experiment 1 suggests that they were similar for the on- and off-frequency elicitors. This is surprising given that the results from experiment 2 suggest that the two types of elicitors may have activated different mechanisms. Backus and Guinan (2006) estimated that it takes about 200 ms for the cochlear response to recover from the effects of MOCR activation. This estimate was obtained by measuring changes in the SFOAE evoked by a 40-dB SPL 1-kHz probe that were elicited by a 60-dB SPL notched noise. In this study, the probe and elicitor levels were higher than those used by Backus and Guinan. Data from experiment 2 suggest that the effects dominating changes in the ear-canal sound pressure elicited by on-frequency Schroeder-phase complexes were of cochlear origin. If indeed the effects resulted from MOCR activation, the long recovery times for the 6-kHz probe may suggest frequency-dependent recovery times from efferent activation. However, the long recovery may also suggest that a mechanism different from the MOCR played a role. One earlier study, by Goodman and Keefe (2006), which used a double-evoked SFOAE assay (Keefe and Ling 1998) to measure changes in the so-called “nonlinear SFOAE component” due to an elicitor, reported long decay times of the effect of an ipsilateral notched noise elicitor on a high frequency probe (~3.5 kHz) in the absence of the elicitor’s effect on the simultaneously presented low-frequency tone (~250–300 Hz). Since no effect was observed at low frequencies, they assumed that the MEMR was absent and the effect observed at the higher frequency was due to a different mechanism. Even in the presence of the MEMR effect at the low frequency, the recovery time for the post-elicitor effect on the high-frequency probe was much longer, suggesting that different mechanisms produced changes in the ear-canal sound pressure for the low and high probe frequency. Goodman and Keefe (2006) provided no clear explanation for that result. Although they were confident that the effect on the low-frequency probe reflected activation of the MEMR, the effect with a longer decay time at the high frequency was hypothetically attributed to intrinsic cochlear processes that had been previously considered as mechanisms mediating DPOAE adaptation (Liberman et al. 1996; Kim et al. 2001). Although the ΔP magnitudes measured in this study were much larger than those suggested by the studies of DPOAE adaptation, the role of intrinsic cochlear processes cannot be ruled out by our experiments.
An alternative but also speculative explanation is that the off-frequency elicitors activated the tensor tympani. Very little is known about the effects of contraction of the tensor tympani in humans except that early studies of tympanic muscle effects have suggested that the MEMR in humans is only mediated by the stapedial muscle (Jepsen 1963; Møller 1964). What makes the tensor tympani worth considering is that direct and selective activation of the stapedial and the tensor-tympani muscles by electric pulses in guinea pig showed that the effects of contraction of the two muscles have different transfer characteristics across the audible frequency range (Nuttall 1974). While contraction of the stapedial muscle only produced changes in the transmission of frequencies below 2 kHz, contraction of the tensor tympani exhibited a nonmonotonic pattern of changes in the transmission magnitude, from attenuation at low frequencies to gain at frequencies between about 1.5 and about 5–6 kHz, and then attenuation in the highest frequency range. We were unable to find a study that reports the recovery time from activation of the tensor tympani in humans or in animals, but the recovery from activation of the acoustic reflex in humans was shown to be independent of the frequency and level of the activator, for frequencies up to 1.5 kHz (Hung and Dallos 1972). No difference was also found in recovery times between tonal and broadband noise activators in that study.
In summary, although the SFOAE measurements promised to be a convenient noninvasive probe into the cochlear mechanics and the role of the MOCR in perception, the data proved difficult to interpret. In particular, using psychoacoustically relevant stimulus parameters, such as medium-level probes and higher-level elicitors, runs the risk of introducing confounding factors when evaluating the SFOAEs. On the other hand, the use of the higher-level stimuli, like those typically used in psychophysical tasks, may reveal effects that are shown more clearly by the measurements of changes in sound pressure in the ear canal than by psychophysics, such as the unexpectedly long recovery times from the elicitor effects. Some of these effects may significantly affect psychophysical results in ways that have yet to be determined.
Assuming that for a given elicitor type (i.e., on- or off-frequency harmonic complex) the same mechanism mediated sound pressure changes in the ear canal for all three C values in Eq. 1, the lack of consistent effects of C suggests that neither MOCR nor MEMR was responsible for masker-phase-dependent thresholds in forward masking of a 6-kHz probe by off-frequency Schroeder-phase complexes. It is worth noting that listeners were passive observers in experiment 1, in that they were not required to pay attention to the stimuli during the recording from the ear canal. Because some past studies have shown small but significant effects of attention on the effects of efferent activation (Giard et al. 1994), it cannot be ruled out that a different result would have been observed had the listeners been involved in actively performing the forward masking task during the measurements of the SFOAEs.
Data from experiment 3 indicated that residual BM compression was unlikely to have been a factor in forward masking by the off-frequency Schroeder-phase complexes in the study by Wojtczak and Oxenham (2009b). This conclusion is also supported by the fact that off-frequency masker phase effects were not observed in that study at the two lower probe frequencies used, 1 and 2 kHz, for which residual compression of the off-frequency maskers would have been more likely. Compression in the IHC response remains a possible candidate for a mechanism underlying the off-frequency masker phase effects, although it too fails to account for why the effect is not observed at lower signal frequencies.
Acknowledgments
The authors thank John J. Guinan, Jr. and Christopher Shera for very helpful discussions on the data in this manuscript. We also thank John J. Guinan and an anonymous reviewer for insightful comments on the earlier version of the manuscript. This work was supported by grant R01 DC 010374 from the National Institutes of Health.
References
- Abbas PJ. Effects of stimulus frequency on adaptation in auditory-nerve fibers. J Acoust Soc Am. 1979;65:162–165. doi: 10.1121/1.382259. [DOI] [PubMed] [Google Scholar]
- Aguilar E, Eustaquio-Martin A, Lopez-Poveda EA. Contralateral efferent reflex effects on threshold and suprathreshold psychoacoustical tuning curves at low and high frequencies. J Assoc Res Otolaryngol. 2013;14:341–357. doi: 10.1007/s10162-013-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BC, Guinan JJ., Jr Time-course of the human medial olivocochlear reflex. J Acoust Soc Am. 2006;119:2889–2904. doi: 10.1121/1.2169918. [DOI] [PubMed] [Google Scholar]
- Brass D, Kemp DT. Suppression of stimulus frequency otoacoustic emissions. J Acoust Soc Am. 1993;93:920–939. doi: 10.1121/1.405453. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Datta AJ. Excitation produced by Schroeder-phase complexes: evidence for fast-acting compression in the auditory system. J Acoust Soc Am. 1997;101:3636–3647. doi: 10.1121/1.418324. [DOI] [PubMed] [Google Scholar]
- Charaziak KK, Souza P, Siegel JH. Stimulus-frequency otoacoustic emission suppression tuning in humans: comparison to behavioral tuning. J Assoc Res Otolaryngol. 2013;14:843–862. doi: 10.1007/s10162-013-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Dallos P. Inner hair cell response patterns: implications for low-frequency hearing. J Acoust Soc Am. 2001;110:2034–2044. doi: 10.1121/1.1397357. [DOI] [PubMed] [Google Scholar]
- Chen F, Zha D, Fridberger A, Zheng J, Choudhury N, Jacques SL, Wang RK, Shi X, Nuttall AL. A differentially amplified motion in the ear for near-threshold sound detection. Nat Neurosci. 2011;14:770–774. doi: 10.1038/nn.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GT, Cudahy EA. The time course of the acoustic reflex. Ear Hear. 1984;5:235–242. doi: 10.1097/00003446-198407000-00008. [DOI] [PubMed] [Google Scholar]
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res. 1990;43:251–261. doi: 10.1016/0378-5955(90)90232-E. [DOI] [PubMed] [Google Scholar]
- Collet L, Veuillet E, Bene J, Morgon A. Effects of contralateral white noise on click-evoked emissions in normal and sensorineural ears: Towards an exploration of the medial olivocochlear system. Audiology. 1992;31:1–7. doi: 10.3109/00206099209072897. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH. Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance, and power. Ear Hear. 2001;22:316–332. doi: 10.1097/00003446-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Giard MH, Collet L, Bouchet P, Pernier J. Auditory selective attention in the human cochlea. Brain Res. 1994;633:353–356. doi: 10.1016/0006-8993(94)91561-X. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Goodman SS, Keefe DH. Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions. J Assoc Res Otolaryngol. 2006;7:125–139. doi: 10.1007/s10162-006-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ., Jr . Changes in stimulus frequency otoacoustic emissions produced by two-tone suppression and efferent stimulation in cats. In: Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR, editors. Mechanics and biophysics of hearing. Madison: Springer; 1990. pp. 170–177. [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear Res. 2012;292:35–50. doi: 10.1016/j.heares.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IJ, Dallos P. Study of the acoustic reflex in human beings. I. Dynamic characteristics. J Acoust Soc Am. 1972;52:1168–1180. doi: 10.1121/1.1913229. [DOI] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA. Evaluating the effects of olivocochlear feedback on psychophysical measures of frequency selectivity. J Acoust Soc Am. 2012;132:2483–2496. doi: 10.1121/1.4742723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA, Heinz MG. Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking. J Acoust Soc Am. 2009;125:2172–2181. doi: 10.1121/1.3081383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen O. Middle ear muscle reflexes in man. In: Jerger J, editor. Modern developments in audiology. New York: Academic; 1963. pp. 193–223. [Google Scholar]
- Keefe DH, Ling R. Double-evoked otoacoustic emissions. II. Intermittent noise rejection, calibration and ear-canal measurements. J Acoust Soc Am. 1998;103:3499–3508. doi: 10.1121/1.423058. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Schairer KS, Ellison JC, Fitzpatrick DF, Jesteadt W. Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot. J Acoust Soc Am. 2009;125:1595–1604. doi: 10.1121/1.3068443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DO, Dorn PA, Neely ST, Gorga MP. Adaptation of distortion product otoacoustic emission in humans. J Assoc Res Otolaryngol. 2001;2:31–40. doi: 10.1007/s101620010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlrausch A, Sander A. Phase effects in masking related to dispersion in the inner ear. II. Masking period patterns of short targets. J Acoust Soc Am. 1995;97:1817–1829. doi: 10.1121/1.413097. [DOI] [PubMed] [Google Scholar]
- Krull V, Strickland EA. The effect of a precursor on growth of forward masking. J Acoust Soc Am. 2008;123:4352–4357. doi: 10.1121/1.2912440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol. 1997;78:3095–3106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- Lentz JJ, Leek MR. Psychophysical estimates of cochlear phase response: masking by harmonic complexes. J Assoc Res Otolaryngol. 2001;2:408–422. doi: 10.1007/s101620010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Rapid assessment of sound-evoked olivocochlear feedback: suppression of compound action potentials by contralateral sound. Hear Res. 1989;38:47–56. doi: 10.1016/0378-5955(89)90127-5. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Puria S, Guinan JJ., Jr The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1-f2 distortion product otoacoustic emission. J Acoust Soc Am. 1996;99:3572–3584. doi: 10.1121/1.414956. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci. 2014;34:4599–4607. doi: 10.1523/JNEUROSCI.4923-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol. 2009;10:459–470. doi: 10.1007/s10162-009-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol. 2009;101:1394–1406. doi: 10.1152/jn.90925.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J Neurophysiol. 2012;107:1598–1611. doi: 10.1152/jn.00549.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Plack CJ, Meddis R. Cochlear nonlinearity between 500 and 8,000 Hz in listeners with normal hearing. J Acoust Soc Am. 2003;113:951–960. doi: 10.1121/1.1534838. [DOI] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Andeol G, Gallego S, Collet L. Activation of medial olivocochlear efferent system in humans: influence of stimulus bandwidth. Hear Res. 2000;140:111–125. doi: 10.1016/S0378-5955(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci. 2013;33:5542–5552. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Champlin CA. Reductions in overshoot during aspirin use. J Acoust Soc Am. 1990;87:2634–2642. doi: 10.1121/1.399056. [DOI] [PubMed] [Google Scholar]
- Moleti A, Al-Maamury AM, Bertaccini D, Botti T, Sisto R. Generation place of the long- and short-latency components of transient-evoked otoacoustic emissions in a nonlinear cochlear model. J Acoust Soc Am. 2013;133:4098–4108. doi: 10.1121/1.4802940. [DOI] [PubMed] [Google Scholar]
- Møller AR. Effect of tympanic muscle activity on movement of the eardrum, acoustic impedance and cochlear microphonics. Acta Otolaryngol. 1964;58:525–534. doi: 10.3109/00016486409121413. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Schroder AC, Wojtczak M. A new procedure for measuring peripheral compression in normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2001;110:2045–2064. doi: 10.1121/1.1404439. [DOI] [PubMed] [Google Scholar]
- Norman M, Thornton AR. Frequency analysis of the contralateral suppression of evoked otoacoustic emissions by narrow-band noise. Br J Audiol. 1993;27:281–289. doi: 10.3109/03005369309076705. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Tympanic muscle effects on middle-ear transfer characteristic. J Acoust Soc Am. 1974;56:1239–1247. doi: 10.1121/1.1903414. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Dau T. Towards a measure of auditory-filter phase response. J Acoust Soc Am. 2001;110:3169–3178. doi: 10.1121/1.1414706. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Dau T. Reconciling frequency selectivity and phase effects in masking. J Acoust Soc Am. 2001;110:1525–1538. doi: 10.1121/1.1394740. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Dau T. Masker phase effects in normal-hearing and hearing-impaired listeners: evidence for peripheral compression at low signal frequencies. J Acoust Soc Am. 2004;116:2248–2257. doi: 10.1121/1.1786852. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Moore BC. Modeling the additivity of nonsimultaneous masking. Hear Res. 1994;80:105–118. doi: 10.1016/0378-5955(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Moore BCJ. Additivity of masking in normally hearing and hearing-impaired subjects. J Acoust Soc Am. 1995;98:1921–1934. doi: 10.1121/1.413376. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Plack CJ. A behavioral measure of basilar-membrane nonlinearity in listeners with normal and impaired hearing. J Acoust Soc Am. 1997;101:3666–3675. doi: 10.1121/1.418327. [DOI] [PubMed] [Google Scholar]
- Penner MJ, Glotzbach L, Huang T. Spontaneous otoacoustic emissions: measurement and data. Hear Res. 1993;68:229–237. doi: 10.1016/0378-5955(93)90126-L. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Arifianto D. On- and off-frequency compression estimated using a new version of the additivity of forward masking technique. J Acoust Soc Am. 2010;128:771–786. doi: 10.1121/1.3455844. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Oxenham AJ, Simonson AM, O’Hanlon CG, Drga V, Arifianto D. Estimates of compression at low and high frequencies using masking additivity in normal and impaired ears. J Acoust Soc Am. 2008;123:4321–4330. doi: 10.1121/1.2908297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roverud E, Strickland EA. The time course of cochlear gain reduction measured using a more efficient psychophysical technique. J Acoust Soc Am. 2010;128:1203–1214. doi: 10.1121/1.3473695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roverud E, Strickland EA. Accounting for nonmonotonic precursor duration effects with gain reduction in the temporal window model. J Acoust Soc Am. 2014;135:1321. doi: 10.1121/1.4864783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Temchin AN. Similarity of traveling-wave delays in the hearing organs of humans and other tetrapods. J Assoc Res Otolaryngol. 2007;8:153–166. doi: 10.1007/s10162-007-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC, Recio A, Narayan SS, Robles L. Basilar-membrane responses to tones at the base of the chinchilla cochlea. J Acoust Soc Am. 1997;101:2151–2163. doi: 10.1121/1.418265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Nilsen KE. The location of the cochlear amplifier: spatial representation of a single tone on the guinea pig basilar membrane. Proc Nat Acad Sci. 1997;94:2660–2664. doi: 10.1073/pnas.94.6.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer KS, Ellison JC, Fitzpatrick D, Keefe DH. Wideband ipsilateral measurements of middle-ear muscle reflex thresholds in children and adults. J Acoust Soc Am. 2007;121:3607–3616. doi: 10.1121/1.2722213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Collet L, Ulmer E, Chays A. On the role of the olivocochlear bundle in hearing: a case study. Hear Res. 1994;75:11–26. doi: 10.1016/0378-5955(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Chays A. On the role of the olivocochlear bundle in hearing: 16 case studies. Hear Res. 1997;103:101–122. doi: 10.1016/S0378-5955(96)00168-2. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am. 2003;113:2762–2772. doi: 10.1121/1.1557211. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Badri R (2002) Suppression of stimulus frequency emissions by tones. 25th ARO Midwinter Meeting, Abs# 320
- Siegel JH, Temchin AN, Ruggero M (2003) Empirical estimates of the spatial origin of stimulus-frequency otoacoustic emissions. 26th ARO Mindwinter Meeting, Abs# 1516
- Siegel JH, Cerka AJ, Temchin AN, Ruggero M (2004) Similar two-tone suppression patterns in SFOAEs and the cochlear microphonics indicate comparable spatial summation of underlying generators. 27th ARO Midwinter Meeting, Abs# 365
- Siegel JH, Cerka AJ, Recio-Spinoso A, Temchin AN, van Dijk P, Ruggero MA. Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. J Acoust Soc Am. 2005;118:2434–2443. doi: 10.1121/1.2005867. [DOI] [PubMed] [Google Scholar]
- Sisto R, Sanjust F, Moleti A. Input/output functions of different-latency components of transient-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am. 2013;133:2240–2253. doi: 10.1121/1.4794382. [DOI] [PubMed] [Google Scholar]
- Smith RL. Short-term adaptation in single auditory nerve fibers: some poststimulatory effects. J Neurophysiol. 1977;40:1098–1112. doi: 10.1152/jn.1977.40.5.1098. [DOI] [PubMed] [Google Scholar]
- Smith RL. Adaptation, saturation, and physiological masking in single auditory-nerve fibers. J Acoust Soc Am. 1979;65:166–178. doi: 10.1121/1.382260. [DOI] [PubMed] [Google Scholar]
- Smith RL, Brachman RL. Adaptation in auditory-nerve fibers: a revised model. Biol Cybern. 1982;44:107–120. doi: 10.1007/BF00317970. [DOI] [PubMed] [Google Scholar]
- Smith BK, Sieben UK, Kohlrausch A, Schroeder MR. Phase effects in masking related to dispersion in the inner ear. J Acoust Soc Am. 1986;80:1631–1637. doi: 10.1121/1.394327. [DOI] [PubMed] [Google Scholar]
- Stankovic KM, Guinan JJ., Jr Medial efferent effects on auditory-nerve responses to tail-frequency tones. I. Rate reduction. J Acoust Soc Am. 1999;106:857–869. doi: 10.1121/1.427102. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The temporal effect with notched-noise maskers: analysis in terms of input–output functions. J Acoust Soc Am. 2004;115:2234–2245. doi: 10.1121/1.1691036. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The relationship between precursor level and the temporal effect. J Acoust Soc Am. 2008;123:946–954. doi: 10.1121/1.2821977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland EA, Krishnan LA. The temporal effect in listeners with mild to moderate cochlear hearing impairment. J Acoust Soc Am. 2005;118:3211–3217. doi: 10.1121/1.2074787. [DOI] [PubMed] [Google Scholar]
- Summers V. Effects of hearing impairment and presentation level on masking period patterns for Schroeder-phase harmonic complexes. J Acoust Soc Am. 2000;108:2307–2317. doi: 10.1121/1.1318897. [DOI] [PubMed] [Google Scholar]
- Summers V, Leek MR. Masking of tones and speech by Schroeder-phase harmonic complexes in normally hearing and hearing-impaired listeners. Hear Res. 1998;118:139–150. doi: 10.1016/S0378-5955(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol. 1991;65:724–735. doi: 10.1152/jn.1991.65.3.724. [DOI] [PubMed] [Google Scholar]
- von Klitzing R, Kohlrausch A. Effect of masker level on overshoot in running- and frozen-noise maskers. J Acoust Soc Am. 1994;95:2192–2201. doi: 10.1121/1.408679. [DOI] [PubMed] [Google Scholar]
- Walsh KP, Wojtczak M. Time-course of recovery from the effects of a notched-noise on the ear-canal pressure at different frequencies. J Acoust Soc Am. 2014;135(Pt.2):2386. doi: 10.1121/1.4877887. [DOI] [Google Scholar]
- Walsh KP, Pasanen EG, McFadden D. Overshoot measured physiologically and psychophysically in the same human ears. Hear Res. 2010;268:22–37. doi: 10.1016/j.heares.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EH, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hear Res. 1989;37:105–122. doi: 10.1016/0378-5955(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Wojtczak M, Oxenham AJ. Pitfalls in behavioral estimates of basilar-membrane compression in humans. J Acoust Soc Am. 2009;125:270–281. doi: 10.1121/1.3023063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak M, Oxenham AJ. On- and off-frequency forward masking by Schroeder-phase complexes. J Assoc Res Otolaryngol. 2009;10:595–607. doi: 10.1007/s10162-009-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha D, Chen F, Ramamoorthy S, Fridberger A, Choudhury N, Jacques SL, Wang RK, Nuttall AL. In vivo outer hair cell length changes expose the active process in the cochlea. PLoS One. 2012;7:e32757. doi: 10.1371/journal.pone.0032757. [DOI] [PMC free article] [PubMed] [Google Scholar]