Abstract
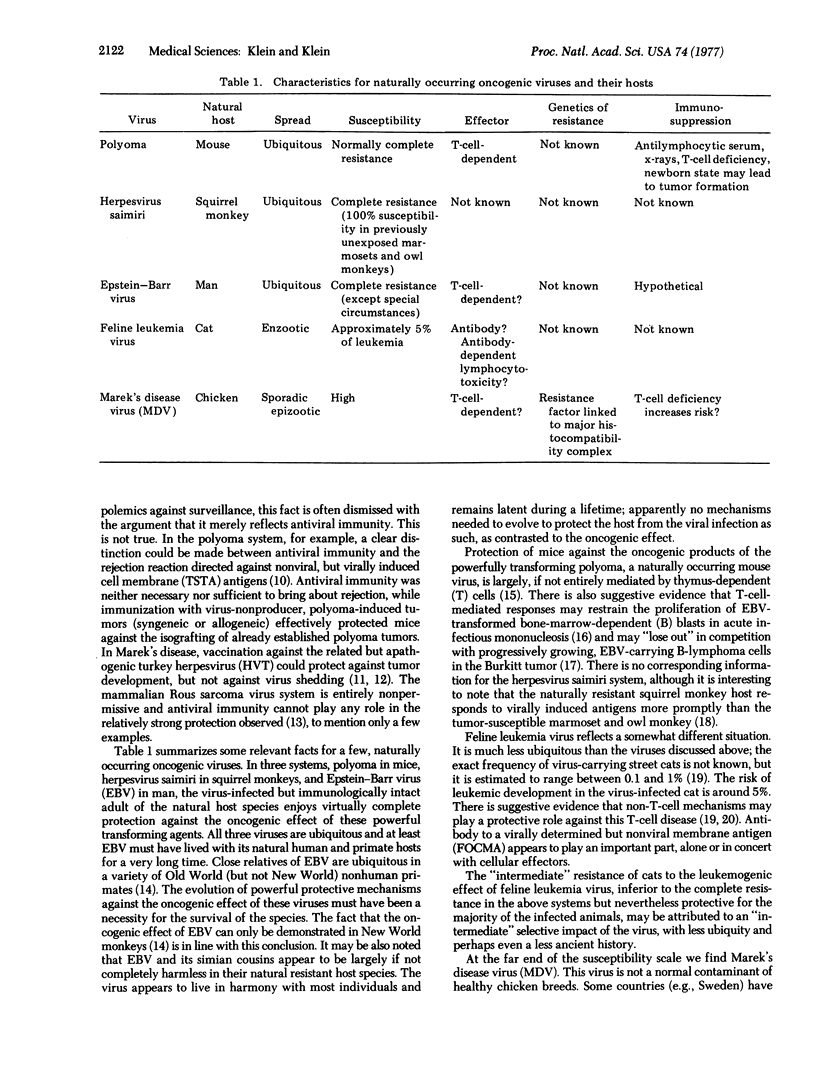
Spontaneous tumours are defined as tumors that develop in the absence of all experimental interference. In contrast to the widely documented, strong rejection reactions against most virus-induced tumors, spontaneous tumors evoke little or no detectable rejection reaction in intact or preimmunized syngeneic hosts. The difference can be viewed in relation to the contrasting natural history of the two conditions. Spontaneous tumors evolve in several steps, as a fule. "Tumor progression" is a microevolutionary process at the level of the somatic tissue where successive clonal variants replace each other. Each new variant gains the upper hand due to its greater independence of some restricting host mechanism. Independence of immune restrictions must be part of this process. Host selection for immune resistance apparently plays no major role here, presumably because most of the naturally occurring tumors arise after the host has passed the peak of its reproductive period. Protection against the oncogenic effects of ubiquitous tumor viruses is, on the other hand, the result of host selection for immune mechanisms favoring prompt rejection of virus-transformed cells. This is neither synonymous with nor related to protection against the viral infection per se, which is frequently successful and usually quite harmless. A certain relationship can be perceived between the degree of viral ubiquity and the strength of immune protection against the corresponding tumor cells. Natural selection for host recognition of commonly occurring, virally induced changes in neoplastic cell membranes can be surmised to occur, at least in part, by the fixation of appropriate immune responsiveness (Ir) genes. The role of Ir genes for tumor recognition can be approached by the genetic analysis of the F1 hybrid resistance effect. Unresponsiveness to spontaneous tumors may be overcome by target-cell modification, e.g., by chemical coupling, somatic cell hybridization, or viral "xenogenization".
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. W. Immunological aspects of chemical carcinogenesis. Adv Cancer Res. 1973;18:1–75. doi: 10.1016/s0065-230x(08)60750-2. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W. Tumour-specific immunity against spontaneous rat tumours. Int J Cancer. 1966 May 15;1(3):257–264. doi: 10.1002/ijc.2910010305. [DOI] [PubMed] [Google Scholar]
- Basilico C. Temperature-sensitive mutations in animal cells. Adv Cancer Res. 1977;24:223–266. doi: 10.1016/s0065-230x(08)61016-7. [DOI] [PubMed] [Google Scholar]
- Bauer H. Virion and tumor cell antigens of C-type rna tumor viruses. Adv Cancer Res. 1974;20:275–341. doi: 10.1016/s0065-230x(08)60112-8. [DOI] [PubMed] [Google Scholar]
- Biggs P. M. Vaccination against oncogenic herpesviruses - a review. IARC Sci Publ. 1975;(11 Pt 2):317–327. [PubMed] [Google Scholar]
- Deinhardt F. W., Falk L. A., Wolfe L. G. Simian herpesviruses and neoplasia. Adv Cancer Res. 1974;19(0):167–205. doi: 10.1016/s0065-230x(08)60054-8. [DOI] [PubMed] [Google Scholar]
- Dofuku R., Biedler J. L., Spengler B. A., Old L. J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1515–1517. doi: 10.1073/pnas.72.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. From the molecular biology of oncogenic DNA viruses to cancer. Science. 1976 Apr 30;192(4238):437–440. doi: 10.1126/science.1257779. [DOI] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Essex M., Sliski A., Cotter S. M., Jakowski R. R., Hardy W. D., Jr Immunosurveillance of naturally occurring feline leukemia. Science. 1975 Nov 21;190(4216):790–792. doi: 10.1126/science.173019. [DOI] [PubMed] [Google Scholar]
- FOULDS L. The natural history of cancer. J Chronic Dis. 1958 Jul;8(1):2–37. doi: 10.1016/0021-9681(58)90039-0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Hewitt H. B., Blake E. R., Walder A. S. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976 Mar;33(3):241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitotsumachi S., Rabinowitz Z., Sachs L. Ciromosomal control of reversion in transformed cells. Nature. 1971 Jun 25;231(5304):511–514. doi: 10.1038/231511a0. [DOI] [PubMed] [Google Scholar]
- Jarvis J. E., Ball G., Rickison A. B., Epstein M. A. Cytogenetic studies on human lymphoblastoid cell lines from Burkitt's lymphomas and other sources. Int J Cancer. 1974 Dec 15;14(6):716–721. doi: 10.1002/ijc.2910140604. [DOI] [PubMed] [Google Scholar]
- Jondal M., Svedmyr E., Klein E., Singh S. Killer T cells in a Burkitt's lymphoma biopsy. Nature. 1975 May 29;255(5507):405–407. doi: 10.1038/255405a0. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Klein G., Wigzel H. Genetic variation of in vitro cytolytic activity and in vivo rejection potential of non-immunized semi-syngeneic mice against a mouse lymphoma line. Int J Cancer. 1975 Jun 15;15(6):933–940. doi: 10.1002/ijc.2910150608. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Kärre K., Jondal M., Tracey D., Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976 Apr 1;143(4):772–780. doi: 10.1084/jem.143.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Harris H. Expression of polyoma-induced transplantation antigen in hybrid cell lines. Nat New Biol. 1972 Jun 7;237(75):163–164. doi: 10.1038/newbio237163a0. [DOI] [PubMed] [Google Scholar]
- Klein G. Immunological surveillance against neoplasia. Harvey Lect. 1973;(69):71–102. [PubMed] [Google Scholar]
- Klein G., Pearson G., Rabson A., Ablashi D. V., Falk L., Wolfe L., Dienhardt F., Rabin H. Antibody reactions to herpesvirus saimiri (HVS)-induced early and late antigens (EA and LA) in HVS-infected squirrel, marmoset and owl monkeys. Int J Cancer. 1973 Jul 15;12(1):270–289. doi: 10.1002/ijc.2910120128. [DOI] [PubMed] [Google Scholar]
- LAW L. W. Present status of nonviral factors in the etiology of reticular neoplasms of the mouse. Ann N Y Acad Sci. 1957 Oct 21;68(2):616–635. doi: 10.1111/j.1749-6632.1957.tb56109.x. [DOI] [PubMed] [Google Scholar]
- Manolov G., Manolova Y. Marker band in one chromosome 14 from Burkitt lymphomas. Nature. 1972 May 5;237(5349):33–34. doi: 10.1038/237033a0. [DOI] [PubMed] [Google Scholar]
- Mark J. Chromosomal abnormalities and their specificity in human neoplasms: an assessment of recent observations by banding techniques. Adv Cancer Res. 1977;24:165–222. doi: 10.1016/s0065-230x(08)61015-5. [DOI] [PubMed] [Google Scholar]
- Mitelman F., Mark J., Levan G., Levan A. Tumor etiology and chromosome pattern. Science. 1972 Jun 23;176(4041):1340–1341. doi: 10.1126/science.176.4041.1340. [DOI] [PubMed] [Google Scholar]
- Oth D., Burg C. Persistence of syngeneic preference given by a methylcholanthrene-induced sarcoma after four consecutive passages in F1 hybrids. Nature. 1967 Apr 22;214(5086):418–419. doi: 10.1038/214418a0. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. Tumor progression and homeostasis. Adv Cancer Res. 1976;23:203–236. doi: 10.1016/s0065-230x(08)60547-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Genetic factors in the natural history of murine leukemia virus infection: G. H. A. Clowes Memorial Lecture. Cancer Res. 1973 Dec;33(12):3061–3068. [PubMed] [Google Scholar]
- SNELL G. D., STEVENS L. C. Histocompatibility genes of mice. III. H-1 and H-4, two histocompatibility loci in the first linkage group. Immunology. 1961 Oct;4:366–379. [PMC free article] [PubMed] [Google Scholar]
- Sanford B. H. Evidence for immunological resistance to a parental line tumor by F. hybrid hosts. Transplantation. 1967 May;5(3):557–560. [PubMed] [Google Scholar]
- Sanford B. H., Soo S. F. Resistance to transplants of recent spontaneous parental line tumors by F1 hybrid hosts. J Natl Cancer Inst. 1971 Jan;46(1):95–101. [PubMed] [Google Scholar]
- Sjögren H. O. Transplantation methods as a tool for detection of tumor-specific antigens. Prog Exp Tumor Res. 1965;6:289–322. [PubMed] [Google Scholar]
- Stutman O. Immunodepression and malignancy. Adv Cancer Res. 1975;22:261–422. doi: 10.1016/s0065-230x(08)60179-7. [DOI] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Knufermann H., Wunderlich F. Membrane aspects of neoplasia. FEBS Lett. 1973 Jul 15;33(3):275–280. doi: 10.1016/0014-5793(73)80210-8. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]



