Abstract
Background
The ABO blood group is reported to be associated with the incidence and patient survival for several types of malignancies. We conducted a retrospective study to evaluate the prognostic significance of the ABO blood group in patients with resected non-small cell lung cancer (NSCLC).
Methods
A total of 333 patients (218 men and 115 women) with resected NSCLC were included in this study. In addition to age, sex, smoking status, preoperative serum carcinoembryonic antigen (CEA) level, operative procedure, histology of tumors, pathological stage (p-stage), and adjuvant therapy, the association between the ABO blood group and survival was explored.
Results
The 5-year overall and disease-free survival rates were 83.0% and 71.6% for blood group O, 67.2% and 62.3% for blood group A, 68.8% and 68.8% for blood group B and 69.2% and 65.3% for blood group AB, respectively. A multivariate analysis for overall survival showed the ABO blood group (group A vs. group O: HR 2.47, group AB vs. group O: HR 3.62) to be an independent significant prognostic factor, in addition to age, sex, smoking status, p-stage, and serum CEA level. A multivariate analysis for disease-free survival also showed the ABO blood group to be an independent significant prognostic factor.
Conclusions
The ABO blood group is an independent prognostic factor in patients with resected NSCLC. Studies of other larger cohorts are therefore needed to confirm the relationship between the ABO blood group and the prognosis among patients with resected NSCLC.
Key words: non-small cell lung cancer, ABO blood group, surgery, prognostic factor
Abstract
【背景】
ABO血液型はいくつかのがん腫において、発生頻度や予後との関連が報告されている。この後ろ向き研究の目的は、非小細胞肺癌切除例における予後とABO血液型の関連を検討することである。
【方法】
本研究の対象は333例(男性218例、女性115例)の非小細胞肺癌切除例で、年齢、性別、喫煙状況、術前血清CEA値、術式、腫瘍の組織型、病理病期、術後補助療法、およびABO血液型と予後との関連を検討した。
【結果】
ABO血液型別の5年全生存率(Overall survival: OS)と5年無再発生存率(Disease free survival: DFS)は、それぞれO型83%、71.6%、A型67.2%、62.3%、B型68.8%、68.8%、AB型69.2%、65.3%であった。OSに対する多変量解析で、年齢、性別、喫煙状況、病理病期、術前血清CEA値と同様にABO血液型は独立した予後因子であった(A型 vs. O型のハザード比:2.47、AB型 vs. O型のハザード比:3.62)。DFSに対する多変量解析においても、ABO血液型は独立した予後因子であった。
【結論】
ABO血液型は非小細胞肺癌切除例における独立した予後因子である。ABO血液型と非小細胞肺癌切除例の予後との関連を検討するために、今後外部コホートや大規模な集団での検討が望まれる。
INTRODUCTION
Lung cancer is the leading cause of cancer death worldwide. In 2008, 1.6 million people received a new diagnosis of lung cancer, comprising 13% of all new cancer diagnoses, and 1.37 million people died of lung cancer, accounting for 18% of all cancer deaths in the world.1,2 Patients with lung cancer, especially non-small cell lung cancer (NSCLC) without metastatic disease, are considered to be candidates for surgical resection. Although complete resection is often achieved in such patients, some patients experience relapse after surgery. In order to improve the outcomes of surgically managed patients, new prognostic factors must be explored, in addition to established factors such as a high preoperative or postoperative serum carcinoembryonic antigen (CEA) level,3,4 positive results on pleural lavage cytology,5 and a high standardized uptake value on positron emission tomography.6
At the beginning of the 20th century, the Austrian scientist Karl Landsteiner identified the ABO blood group system. This discovery was the first detection of a genetic polymorphism in humans. Recently, an increasing number of studies have shown that the ABO blood group, in addition to its fundamental role in transfusion medicine, plays an important role in several human diseases, including venous thromboembolism (VTE),7 coronary heart disease,8 ischemic stroke,9 and neoplastic disorders. Some reports have evaluated the association between the ABO blood group and the incidence of various types of malignancies, including gastric cancer,10 pancreatic cancer,11 and renal cell carcinoma.12 In addition, there are two studies that evaluated the association between the ABO blood group and the prognosis of cancer patients, such as those with pancreatic cancer13 and renal cell carcinoma.14 Both studies found that the prognosis of blood group O patients is superior to that of non-blood group O patients.13,14 However, few studies have assessed the relationship between the ABO blood group and the prognosis among patients with lung cancer. The aim of the present study was to clarify the prognostic significance of the ABO blood group in patients with resected NSCLC.
METHODS
Between January 2004 and December 2007, 337 patients with NSCLC underwent pulmonary resections at Nagoya University Hospital. In order to evaluate both overall survival (OS) and disease-free survival (DFS), 4 patients who had pleural dissemination at the thoracotomy (R2 resection: macroscopic residual tumor) were excluded from this study. All of the eligible patients underwent an ABO blood group examination prior to surgery. The ABO blood group evaluations were carried out via agglutination technology using the ®BioVue system (Ortho Clinical Diagnostics Japan, Tokyo, Japan). All clinical and pathological data were collected using a clinical database and charts. In addition to the ABO blood group, examined factors included age, sex, smoking status, preoperative serum CEA level, operative procedures, histology, postoperative adjuvant therapy, and pathological stage (p-stage).
Fisher’s exact test and an analysis of variance were used to compare each variable between the blood groups, as appropriate. OS was calculated from the date of surgery to death. DFS was defined as the period from the date of surgery to the date of identification of recurrent disease or death from any cause. Two patients were excluded from DFS analysis because of missing data. The Kaplan-Meier method was used to calculate the survival rate with a 95% confidence interval (CI), and the log-rank test was used to compare the survival curves. A Cox proportional hazard model was used for the univariate and multivariate survival analyses. Reported P values were two-sided, and those less than 0.05 were considered statistically significant. The statistical analyses were performed using the computer software program STATA/SE Ver.12.1 (State Corp., College Station, TX, USA). The Institutional Review Board of Nagoya University Hospital approved this retrospective study.
RESULTS
The patient characteristics are shown in Table 1. This study included 218 men and 115 women, ranging in age from 31 to 85 years (median: 68 years). The median observation period in the survivors was 73 months (range: 1–107 months). The pathological characteristics were as follows: 210 tumors were adenocarcinomas (ADs), 93 tumors were squamous cell carcinomas (SQs), and 30 tumors were other NSCLCs (others). Meanwhile, 227 patients had p-stage I disease, 49 had p-stage II disease, and 57 had p-stage III disease. Sixty-eight patients (20.4%) received adjuvant therapy (chemotherapy and/or radiation therapy) after surgery.
Table 1. Patient characteristics.
| All | Blood group | ||||||||||
| Group O | Group A | Group B | Group AB | P value | |||||||
| n = 333 |
n = 108 (32.4%) |
% |
n = 140 (42.1%) |
% |
n = 59 (17.7%) |
% |
n = 26 (7.8%) |
% | |||
| Age, median (range) | 68 (31–85) | 68 (40–84) | 68 (31–85) | 67 (40–85) | 71 (40–83) | 0.511 | |||||
| Sex | Female | 115 | 38 | 35.2 | 55 | 39.3 | 13 | 22 | 9 | 34.6 | 0.128 |
| Male | 218 | 70 | 64.9 | 85 | 60.7 | 46 | 78 | 17 | 65.4 | ||
| Smoking status | Never | 93 | 31 | 28.7 | 43 | 30.7 | 13 | 22 | 6 | 23.1 | 0.585 |
| Ever/Current | 237 | 76 | 70.4 | 95 | 67.9 | 46 | 78 | 20 | 76.9 | ||
| Unknown | 3 | 1 | 0.9 | 2 | 1.4 | 0 | 0 | ||||
| CEA (ng/mL) | ≤5 | 219 | 73 | 67.6 | 90 | 64.3 | 39 | 66.1 | 17 | 65.4 | 0.986 |
| >5 | 103 | 34 | 31.5 | 41 | 29.3 | 19 | 32.2 | 9 | 34.6 | ||
| Unknown | 11 | 1 | 0.9 | 9 | 6.4 | 1 | 1.7 | 0 | |||
| Operative procedure | Wedge/Seg | 37 | 6 | 5.4 | 25 | 17.9 | 5 | 8.5 | 1 | 3.8 | 0.044 |
| Lob | 276 | 97 | 90 | 107 | 76.4 | 49 | 83 | 23 | 88.5 | ||
| Pn | 20 | 5 | 4.6 | 8 | 5.7 | 5 | 8.5 | 2 | 7.7 | ||
| Histology | AD | 210 | 60 | 55.6 | 94 | 67.1 | 37 | 62.7 | 19 | 73.1 | 0.353 |
| SQ | 93 | 34 | 31.5 | 34 | 24.3 | 19 | 32.2 | 6 | 23.1 | ||
| Others | 30 | 14 | 12.9 | 12 | 8.6 | 3 | 5.1 | 1 | 3.8 | ||
| p-stage | I | 227 | 72 | 66.7 | 98 | 70 | 37 | 62.7 | 20 | 77 | 0.037 |
| II | 49 | 10 | 9.3 | 20 | 14.3 | 16 | 27.1 | 3 | 11.5 | ||
| III | 57 | 26 | 24 | 22 | 15.7 | 6 | 10.2 | 3 | 11.5 | ||
| Adjuvant therapy | No | 265 | 81 | 75 | 113 | 80.7 | 52 | 88.1 | 19 | 73.1 | 0.17 |
| Yes | 68 | 27 | 25 | 27 | 19.3 | 7 | 11.9 | 7 | 26.9 | ||
AD, adenocarcinoma; CEA, carcinoembryonic antigen; Lob, lobectomy; Pn, pneumonectomy; p-stage, pathological stage; Seg, segmentectomy; SQ, squamous cell carcinoma; Wedge, wedge resection.
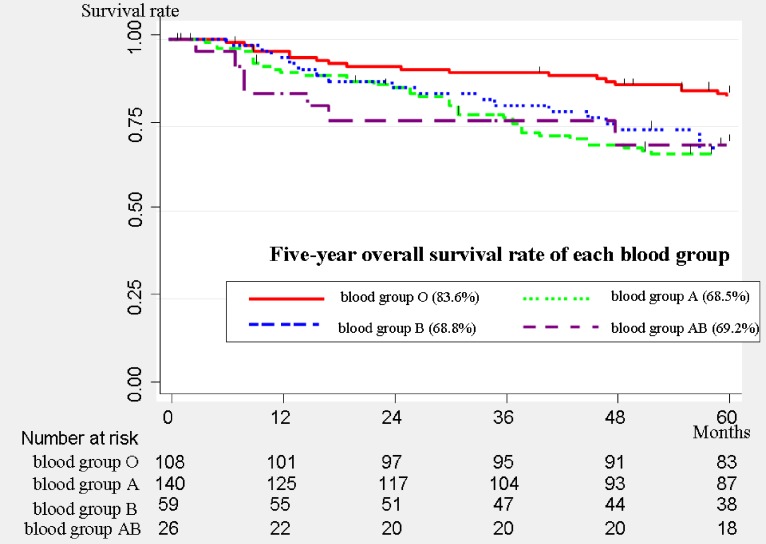
There were 108 (32.4%) patients with blood group O, 140 (42.1%) with blood group A, 59 (17.7%) with blood group B, and 26 (7.8%) with blood group AB. The distribution of each blood group was similar to that of the general population in Japan.15 There were significantly more advanced-stage patients in blood group O than in any other group (P = 0.037). The overall survival curves of each blood group are shown in Figure 1. The five-year overall survival (OS) rate was 83.6% (95% CI, 75.0%–89.5%) for blood group O, 68.5% (95% CI, 59.8%–75.6%) for blood group A, 68.8% (95% CI, 55.2%–79.1%) for blood group B, and 69.2% (95% CI, 47.8%–83.3%) for blood group AB. The patients in blood group O showed significantly better survival than the patients in non-O blood groups (P = 0.016). Stratified analysis by p-stage revealed that the association between the ABO blood group and overall survival was similar in each p-stage group.
Figure 1. Overall survival curves of all patients (n = 333). The 5-year overall survival rate was 83.6% (95% confidence interval [CI], 75%–89.5%) for blood group O, 68.5% (95% CI, 59.8%–75.6%) for blood group A, 68.8% (95% CI, 55.2%–79.1%) for blood group B, and 69.2% (95% CI, 47.8%–83.3%) for blood group AB. The patients in blood group O showed significantly better survival than the patients in the non-O blood groups (P = 0.016).
A univariate analysis for OS showed that age (per 1 year: hazard ratio [HR] 1.05), sex (male vs. female: HR 2.71), smoking status (ever/current vs. never: HR 1.16), preoperative serum CEA level (>5 vs. ≤5: HR 2.56), histology (SQ vs. AD: HR 2.3, others vs. AD: HR 2.16), p-stage (stage II vs. stage I: HR 2.12, stage III vs. stage I: HR 2.99), adjuvant therapy (Yes vs. No: HR 0.56), and blood group (group A vs. group O: HR 1.88) were significant prognostic factors (Table 2). Multivariate analysis for OS showed that age (per 1 year: HR 1.05), sex (male vs. female: HR 2.28), smoking status (ever/current vs. never: HR 1.26), p-stage (stage II vs. stage I: HR 2.21, stage III vs. stage I: HR 5.78), adjuvant therapy (Yes vs. No: HR 0.45), and blood group (group A vs. group O: HR 2.47; P = 0.001, group AB vs. group O: HR 3.62; P = 0.002) were independent significant prognostic factors (Table 2). Blood groups A and AB, which express the blood group A antigen on erythrocytes, were independently associated with poor OS among patients with resected NSCLC.
Table 2. Univariate and multivariate analysis for overall survival.
| Univariate analysis | Multivariate analysis | ||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Age (/year) | 1.05 | 1.02 | 1.07 | <0.001 | 1.05 | 1.02 | 1.08 | <0.001 | |
| Sex | Female | reference | reference | ||||||
| Male | 2.71 | 1.64 | 4.46 | <0.001 | 2.28 | 1.34 | 3.88 | 0.002 | |
| Smoking status | Never | reference | reference | ||||||
| Ever/Current | 1.16 | 1.01 | 1.32 | 0.03 | 1.26 | 1.02 | 1.55 | 0.029 | |
| CEA (ng/mL) | ≤5 | reference | reference | ||||||
| >5 | 2.56 | 1.71 | 3.82 | <0.001 | 1.09 | 0.99 | 1.19 | 0.052 | |
| Operative procedure | Wedge/Seg | reference | reference | ||||||
| Lob | 0.69 | 0.39 | 1.19 | 0.185 | 0.69 | 0.38 | 1.26 | 0.224 | |
| Pn | 1.1 | 0.47 | 2.6 | 0.827 | 0.68 | 0.25 | 1.91 | 0.47 | |
| Histology | AD | reference | reference | ||||||
| SQ | 2.3 | 1.52 | 3.49 | <0.001 | 1.55 | 0.95 | 2.53 | 0.08 | |
| Others | 2.16 | 1.15 | 4.07 | 0.017 | 1.98 | 0.99 | 3.91 | 0.05 | |
| p-stage | I | reference | reference | ||||||
| II | 2.12 | 1.27 | 3.56 | 0.004 | 2.21 | 1.26 | 3.89 | 0.006 | |
| III | 2.99 | 1.9 | 4.72 | <0.001 | 5.78 | 3.42 | 9.76 | <0.001 | |
| Adjuvant therapy | No | reference | reference | ||||||
| Yes | 0.56 | 0.32 | 0.98 | 0.031 | 0.45 | 0.24 | 0.82 | 0.01 | |
| Blood group | O | reference | reference | ||||||
| A | 1.88 | 1.15 | 3.08 | 0.012 | 2.47 | 1.45 | 4.21 | 0.001 | |
| B | 1.55 | 0.84 | 2.87 | 0.164 | 1.47 | 0.77 | 2.79 | 0.244 | |
| AB | 1.98 | 0.94 | 4.17 | 0.071 | 3.62 | 1.61 | 8.15 | 0.002 | |
AD, adenocarcinoma; CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; Lob, lobectomy; Pn, pneumonectomy; p-stage, pathological stage; Seg, segmentectomy; SQ, squamous cell carcinoma; Wedge, wedge resection.
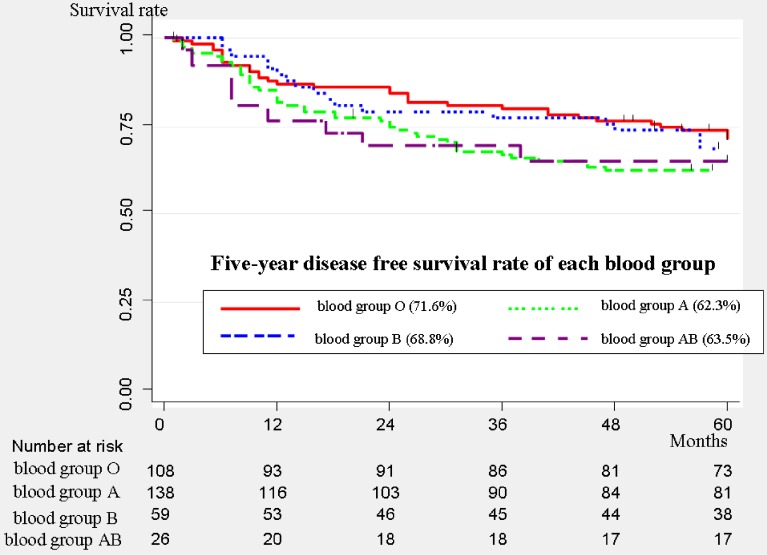
The DFS curves of each blood group are shown in Figure 2. The 5-year DFS rate was 71.6% (95% CI, 61.9%–79.2%) for blood group O, 62.3% (95% CI, 53.5%–69.8%) for blood group A, 68.8% (95% CI, 55.2%–79.1%) for blood group B, and 65.3% (95% CI, 44%–80.2%) for blood group AB.
Figure 2. Disease-free survival curves of all patients (n = 331). The 5-year disease-free survival rate was 71.6% (95% confidence interval [CI], 61.9%–79.2%) for blood group O, 62.3% (95% CI, 53.5%–69.8%) for blood group A, 68.8% (95% CI, 55.2%–79.1%) for blood group B, and 65.3% (95% CI, 44%–80.2%) for blood group AB.
Univariate analysis for DFS showed that age, sex, preoperative serum CEA level, histology, and p-stage were significant prognostic factors (Table 3). Multivariate analysis for DFS showed that age (per 1 year: HR 1.04), sex (male vs. female: HR 1.87), p-stage (stage II vs. stage I: HR 2.1, stage III vs. stage I: HR 6.08), and blood group (group A vs. group O: HR 1.78, P = 0.014; group AB vs. group O: HR 2.49, P = 0.016) were independent significant prognostic factors (Table 3). Similar to the results of multivariate OS analysis, blood groups A and AB were independently associated with a poor DFS among patients with resected NSCLC.
Table 3. Univariate and multivariate analysis for disease-free survival.
| Univariate analysis | Multivariate analysis | ||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Age (/year) | 1.03 | 1.01 | 1.06 | 0.002 | 1.04 | 1.01 | 1.06 | 0.002 | |
| Sex | Female | reference | reference | ||||||
| Male | 2.28 | 1.47 | 3.55 | <0.001 | 1.87 | 1.16 | 3 | 0.01 | |
| Smoking status | Never | reference | reference | ||||||
| Ever/Current | 1.12 | 0.99 | 1.28 | 0.073 | 1.19 | 0.97 | 1.44 | 0.09 | |
| CEA (ng/mL) | ≤5 | reference | reference | ||||||
| >5 | 1.1 | 1.01 | 1.19 | 0.028 | 1.09 | 0.99 | 1.19 | 0.055 | |
| Operative procedure | Wedge/Seg | reference | reference | ||||||
| Lob | 0.73 | 0.44 | 1.23 | 0.234 | 0.63 | 0.36 | 1.11 | 0.114 | |
| Pn | 1.45 | 0.68 | 3.09 | 0.339 | 0.63 | 0.25 | 1.56 | 0.317 | |
| Histology | AD | reference | reference | ||||||
| SQ | 1.91 | 1.29 | 2.8 | 0.001 | 1.24 | 0.78 | 1.95 | 0.364 | |
| Others | 1.89 | 1.04 | 3.45 | 0.037 | 1.57 | 0.83 | 2.98 | 0.164 | |
| p-stage | I | reference | reference | ||||||
| II | 1.91 | 1.18 | 3.19 | 0.009 | 2.1 | 1.22 | 3.61 | 0.007 | |
| III | 4.15 | 2.76 | 6.25 | <0.001 | 6.08 | 3.78 | 9.78 | <0.001 | |
| Adjuvant therapy | No | reference | reference | ||||||
| Yes | 0.97 | 0.62 | 1.52 | 0.897 | 0.73 | 0.45 | 1.21 | 0.222 | |
| Blood group | O | reference | reference | ||||||
| A | 1.44 | 0.94 | 2.21 | 0.097 | 1.78 | 1.12 | 2.84 | 0.014 | |
| B | 1.07 | 0.61 | 1.88 | 0.817 | 1.09 | 0.6 | 1.97 | 0.776 | |
| AB | 1.52 | 0.77 | 3 | 0.231 | 2.49 | 1.18 | 5.23 | 0.016 | |
AD, adenocarcinoma; CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; Lob, lobectomy; Pn, pneumonectomy; p-stage, pathological stage; Seg, segmentectomy; SQ, squamous cell carcinoma; Wedge, wedge resection.
DISCUSSION
Recently, several studies have suggested important roles for the ABO blood group in the development of hemostasis and neoplastic disease, as ABO antigens are highly expressed on the surface of a variety of human cells and tissues.16 As listed in Table 4, there are a number of reports regarding the relationship between the ABO blood group and the incidence of several types of cancers. Affirmation of this relationship has been reported by some studies of renal cell carcinoma,12 extrahepatic cholangiocarcinoma,17 nasopharyngeal carcinoma,18 ovarian cancer,19–21 breast cancer,22 gastric cancer,10,23,24 pancreatic cancer,11,25,26 and lung cancer.27 Meanwhile, no affirmation of the relationship has been reported for colorectal cancer28 and cervical/endometrial cancer.20 Trends in the relationship between blood group and incidence of various types of cancers have been noted; namely, blood groups O or non-A show a low incidence of cancer, while blood groups non-O or A demonstrate a higher incidence of cancer. Among these studies, the relationship between the ABO blood group and the incidence of gastric and/or pancreatic cancer is considered to be reliable and convincing, as the studies were large-scale meta-analyses.10,11
Table 4. Previously reported studies on the relationship between the ABO blood group and the incidence of malignant tumors.
| First author | Reference number |
Year | Country | Type of cancer | Type of study | Results | Blood group | |
| with low incidence | with high incidence | |||||||
| Joh | 12 | 2012 | USA | renal cell carcinoma | prospective cohort study | positive | O | non-O |
| Zhou | 17 | 2013 | China | extrahepatic cholangiocarcinoma | case-control study | positive | O | A |
| Sheng | 18 | 2013 | China | nasopharyngeal carcinoma | case-control study | positive | O | A, AB |
| Gates | 19 | 2011 | USA | ovarian cancer | prospective cohort study | positive | non-B | B |
| Yuzhalin | 20 | 2012 | Russia | ovarian cancer | case-control study | positive | O | premenopausal in A, postmenopausal in B and AB |
| Poole | 21 | 2012 | USA | ovarian cancer | meta analysis | positive | O | A |
| Miao | 22 | 2013 | China | breast cancer | meta analysis | positive | O | A |
| Edgren | 23 | 2010 | Sweden | gastric cancer | population-based cohort study | positive | O | A |
| Nakao | 24 | 2011 | Japan | gastric cancer | case-control study | positive | non-A | A |
| Wang | 10 | 2012 | China | gastric cancer | meta analysis | positive | non-A | A |
| Wolpin | 25 | 2009 | USA | pancreatic cancer | prospective cohort study | positive | O | non-O |
| Nakao | 26 | 2010 | Japan | pancreatic cancer | case-control study | positive | O | non-O |
| Risch | 11 | 2012 | USA | pancreatic cancer | meta analysis | positive | O | A |
| Urun | 27 | 2013 | Turkey | lung cancer | case-control study | positive | O | non-O |
| Khalili | 28 | 2011 | USA | colorectal cancer | prospective cohort study | negative | ||
| Yuzhalin | 19 | 2012 | Russia | cervical and endometrial cancer | case-control study | negative | ||
There are also a few studies regarding the relationship between the ABO blood group and the prognosis in patients with malignant tumors. Kaffenberger et al reported that the non-O blood type was found to be associated with a significantly decreased OS among 900 surgically managed patients with renal cell carcinoma according to a multivariate survival analysis (HR 1.68; 95% CI, 1.18–2.39).14 The authors also reported that the non-O blood type was associated with marginally decreased DFS in the same cohort (HR 1.53; 95% CI, 0.97–2.41). Rahbari et al analyzed a total of 627 patients who underwent resection for pancreatic ductal adenocarcinoma and revealed a favorable and independent impact of blood group O (vs. non-O) on OS according to a multivariate survival analysis (HR 0.78; 95% CI, 0.62–0.99).13 Furthermore, OuYang et al recently demonstrated the prognostic value of the ABO blood group in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy (IMRT) or conventional radiotherapy (CRT).29 In their multivariate survival analysis, patients with blood type A had a significantly lower OS and distant metastasis-free survival than those in the non-A group, among both the IMRT group (n = 924) and CRT group (n = 1193). These results are consistent with our observations, in which we found that the OS and DFS of the resected NSCLC patients in blood group A or AB are significantly poorer than that of patients in blood group O, despite the higher incidence of advanced cancer among blood group O individuals.
The mechanisms by which the ABO blood group influences the prognosis of cancer patients have not been fully investigated. The A and B antigens are expressed on the surface of red blood cells as well as numerous other tissues throughout the body,30 including lung cancer tissues.31 One hypothesis is that ABO antigens in tumor cells play an important role in intracellular adhesion and membrane signaling, both of which are critical to the progression and spread of malignant cells.32 Lee et al reported that the expression of blood group antigen A on lung cancer tissue is an important favorable prognostic factor in blood group A patients.31 We speculate that the A and B antibodies in the plasma of blood group O patients have protective effects against tumor cell progression.
Recently, two genome-wide association studies have suggested that single nucleotide polymorphisms at the ABO gene locus are associated with two serum markers, namely tumor necrosis factor-α (TNF-α) and soluble intracellular adhesion molecule-1 (sICAM-1).33,34 TNF-α is an inflammatory cytokine that affects tumor progression. In addition, the levels of sICAM-1 are known to be elevated in several types of malignancies and may play a role in escape from immune surveillance by tumor cells.35 Therefore, we also suspect that the ABO gene locus influences the prognosis of cancer patients via the effects of these serum proteins.
There are some limitations to our retrospective analysis. First, the number of study subjects was small; however, to our knowledge, this is the first report to show the prognostic significance of the ABO blood type in surgically managed NSCLC patients. Secondly, our data regarding the ABO blood groups were obtained using a serological technique based on the phenotype, not the genotype, of the blood group. Nakao et al reported that the number of non-O alleles was found to be associated with an increased risk of pancreatic cancer in a Japanese population.26 The number of non-O alleles may therefore have an additive effect on the prognosis of patients with NSCLC.
Conclusion
Our multivariate survival analysis showed the ABO blood group to be an independent prognostic factor in addition to age, sex, smoking status, p-stage, and serum CEA level. The blood group A antigen may have a negative effect on the prognosis of surgically managed patients with NSCLC. Studies using other larger cohorts are needed to confirm a robust relationship between ABO blood group and the prognosis of patients with resected NSCLC.
ONLINE ONLY MATERIAL
ACKNOWLEDGMENT
Conflicts of interest: None declared.
REFERENCES
- 1.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5Suppl):e1S–29S. 10.1378/chest.12-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3.Matsuguma H, Nakahara R, Igarashi S, Ishikawa Y, Suzuki H, Miyazawa N, et al. Pathologic stage I non-small cell lung cancer with high levels of preoperative serum carcinoembryonic antigen: clinicopathologic characteristics and prognosis. J Thorac Cardiovasc Surg. 2008;135(1):44–9. 10.1016/j.jtcvs.2007.09.032 [DOI] [PubMed] [Google Scholar]
- 4.Ozeki N, Fukui T, Taniguchi T, Usami N, Kawaguchi K, Ito S, et al. Significance of serum carcinoembryonic antigen level during the follow-up of patients with completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(4):687–92. 10.1093/ejcts/ezt424 [DOI] [PubMed] [Google Scholar]
- 5.Lim E, Clough R, Goldstraw P, Edmonds L, Aokage K, Yoshida J, et al. Impact of positive pleural lavage cytology on survival in patients having lung resection for non-small-cell lung cancer: an international individual patient data meta-analysis. J Thorac Cardiovasc Surg. 2010;139(6):1441–6. 10.1016/j.jtcvs.2009.05.048 [DOI] [PubMed] [Google Scholar]
- 6.Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol. 2011;6(1):43–7. 10.1097/JTO.0b013e3181f9abca [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Makris M. Non-O blood group: an important genetic risk factor for venous thromboembolism. Blood Transfus. 2013;11(2):164–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol. 2012;32(9):2314–20. 10.1161/ATVBAHA.112.248757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. Ischemic stroke is associated with the ABO locus: the EuroCLOT study. Ann Neurol. 2013;73(1):16–31. 10.1002/ana.23838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Liu L, Ji J, Zhang J, Yan M, Zhang J, et al. ABO blood group system and gastric cancer: a case control study and meta-analysis. Int J Mol Sci. 2012;13(10):13308–21. 10.3390/ijms131013308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risch HA, Lu L, Wang J, Zhang W, Ni Q, Gao YT, et al. ABO blood group and risk of pancreatic cancer: a study in Shanghai and meta-analysis. Am J Epidemiol. 2013;177(12):1326–37. 10.1093/aje/kws458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joh HK, Cho E, Choueiri TK. ABO blood group and risk of renal cell cancer. Cancer Epidemiol. 2012;36(6):528–32. 10.1016/j.canep.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahbari NN, Bork U, Hinz U, Leo A, Kirchberg J, Koch M, et al. ABO blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319–27. 10.1186/1471-2407-12-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaffenberger SD, Morgan TM, Stratton KL, Boachie AM, Barocas DA, Chang SS, et al. ABO blood group is a predictor of survival in patients undergoing surgery for renal cell carcinoma. BJU Int. 2012;110(11 Pt B):E641–6. 10.1111/j.1464-410X.2012.11366.x [DOI] [PubMed] [Google Scholar]
- 15.Fujita Y, Tanimura M, Tanaka K. The distribution of the ABO blood groups in Japan. Jinrui Idengaku Zasshi. 1978;23(2):63–109. 10.1007/BF02001790 [DOI] [PubMed] [Google Scholar]
- 16.Liumbruno GM, Franchini M. Hemostasis, cancer, and ABO blood group: the most recent evidence of association. J Thromb Thrombolysis. 2014;38(2):160–6. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Zhou Q, Lin Q, Chen R, Gong Y, Liu Y, et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer. 2013;133(8):1867–75. 10.1002/ijc.28196 [DOI] [PubMed] [Google Scholar]
- 18.Sheng L, Sun X, Zhang L, Su D. ABO blood group and nasopharyngeal carcinoma risk in a population of Southeast China. Int J Cancer. 2013;133(4):893–7. 10.1002/ijc.28087 [DOI] [PubMed] [Google Scholar]
- 19.Gates MA, Wolpin BM, Cramer DW, Hankinson SE, Tworoger SS. ABO blood type and incidence of epithelial ovarian cancer. Int J Cancer. 2011;128(2):482–6. 10.1002/ijc.25339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuzhalin AE, Kutikhin AG. ABO and Rh blood groups inrelation to ovarian, endometrial and cervical cancer risk among the population of South-East Siberia. Asian Pac J Cancer Prev. 2012;13(10):5091–6. 10.7314/APJCP.2012.13.10.5091 [DOI] [PubMed] [Google Scholar]
- 21.Poole EM, Gates MA, High BA, Chanock SJ, Cramer DW, Cunningham JM, et al. ABO blood group and risk of epithelial ovarian cancer within the Ovarian Cancer Association Consortium. Cancer Causes Control. 2012;23(11):1805–10. 10.1007/s10552-012-0059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao SY, Zhou W, Chen L, Wang S, Liu XA. Influence of ABO blood group and Rhesus factor on breast cancer risk: a meta-analysis of 9665 breast cancer patients and 244768 controls. Asia Pac J Clin Oncol. 2014;10(2):101–8. 10.1111/ajco.12083 [DOI] [PubMed] [Google Scholar]
- 23.Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172(11):1280–5. 10.1093/aje/kwq299 [DOI] [PubMed] [Google Scholar]
- 24.Nakao M, Matsuo K, Ito H, Shitara K, Hosono S, Watanabe M, et al. ABO genotype and the risk of gastric cancer, atrophic gastritis, and Helicobacter pylori infection. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1665–72. 10.1158/1055-9965.EPI-11-0213 [DOI] [PubMed] [Google Scholar]
- 25.Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101(6):424–31. 10.1093/jnci/djp020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakao M, Matsuo K, Hosono S, Ogata S, Ito H, Watanabe M, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102(5):1076–80. 10.1111/j.1349-7006.2011.01907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urun Y, Utkan G, Cangir AK, Oksuzoglu OB, Ozdemir N, Oztuna DG, et al. Association of ABO blood group and risk of lung cancer in a multicenter study in Turkey. Asian Pac J Cancer Prev. 2013;14(5):2801–3. 10.7314/APJCP.2013.14.5.2801 [DOI] [PubMed] [Google Scholar]
- 28.Khalili H, Wolpin BM, Huang ES, Giovannucci EL, Kraft P, Fuchs CS, et al. ABO blood group and Risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(5):1017–20. 10.1158/1055-9965.EPI-10-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang PY, Su Z, Mao YP, Liu Q, Xie FY. Prognostic value of ABO blood group in southern Chinese patients with established nasopharyngeal carcinoma. Br J Cancer. 2013;109(9):2462–6. 10.1038/bjc.2013.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szulman AE. The histological distribution of the blood group substances in man as disclosed by immunofluorescence: II. The H antigen and its relation to A and B antigens. J Exp Med. 1962;115(5):977–96. 10.1084/jem.115.5.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JS, Ro JY, Sahin AA, Hong WK, Brown BW, Mountain CF, et al. Expression of blood-group antigen A—a favorable prognostic factor in non-small-cell lung cancer. N Engl J Med. 1991;324(16):1084–90. 10.1056/NEJM199104183241603 [DOI] [PubMed] [Google Scholar]
- 32.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473(1):247–66. 10.1016/S0304-4165(99)00183-X [DOI] [PubMed] [Google Scholar]
- 33.Paré G, Chasman DI, Kellogg M, Zee RY, Rifai N, Badola S, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4(7):e1000118. 10.1371/journal.pgen.1000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4(5):e1000072. 10.1371/journal.pgen.1000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banks RE, Gearing AJ, Hemingway IK, Norfolk DR, Perren TJ, Selby PJ. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993;68(1):122–4. 10.1038/bjc.1993.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.