Abstract
Introduction
We sought to evaluate the cost effectiveness of perioperative use of alvimopan in cystectomy and urinary diversion. A recent randomized controlled trial demonstrated the efficacy of alvimopan in reducing postoperative ileus and length of stay in cystectomy; however, a major limitation was the exclusion of epidural analgesia.
Materials and methods
Eighty–six cystectomy and urinary diversion procedures performed by seven surgeons were analyzed between January 2008 and April 2012. The first 50 patients did not receive alvimopan perioperatively, while the subsequent 36 received a single dose of 12 mg preoperatively and then 12 mg every 12 hours for 15 doses or until discharge.
Results
The groups were equal with respect to age, gender, indication, surgeon, and type of diversion. Patients who received alvimopan experienced a shorter length of stay (LOS) versus those in who did not receive alvimopan (10.5 vs. 8.6 days, p = 0.005, 95% CI 0.6–3.3). Readmission for ileus was low in both alvimopan and control groups (0% and 4.4%, respectively). Costs were significantly lower in the alvimopan group than the control groups (2012 USD 32,443 vs. 40,604 p <0.001). This difference stood up to multivariate analysis with a $7,062 difference in hospital stay.
Conclusions
Use of alvimopan in the routine perioperative care of our cystectomy and urinary diversion patients has decreased LOS by 1.9 days. Additionally, institution of routine perioperative alvimopan has reduced costs by $7,062 per admission (20% reduction). This demonstrates a real world application of alvimopan at a moderate volume center.
Keywords: cystectomy, alvimopan, bladder cancer, urinary diversion, length–of–stay, cost–effectiveness
INTRODUCTION
Cystectomy and urinary diversion is a procedure frequently (10–23% incidence) complicated by postoperative ileus [1–7]. Ileus can result in pain limiting mobility, vomiting with increased risk of dehiscence and aspiration, threatened nutritional status, and is associated with a greater risk of additional complications and death. Costs are increased in the setting of ileus because of increased testing and LOS [8]. Recovery of bowel function governs length of stay in many bowel surgeries but also in cystectomy and urinary diversion. Numerous interventions to prevent postoperative ileus and hasten recovery of bowel function have been practiced and studied including early feeding, ambulation [9], avoidance of nasogastric tube [10], omission of mechanical bowel prep [11], chewing gum [12, 13], and optimized fluid management [14]. Multimodal perioperative pathways have been developed to achieve early recovery from surgery, focusing on perioperative nutrition, minimizing mechanical bowel preparation, and analgesic alternatives to systemic opioids [15, 16]. These interventions have had mixed success in speeding bowel recovery and decreasing LOS.
Costs are increased in the setting of ileus [8]. Hospitals are running at capacity and early discharges are requested by hospital management. Strategies that shorten LOS will facilitate bed turnover and minimize profit loss. LOS is important from both a cost saving and hospital bed turnover standpoint. Alvimopan is a peripherally acting mu opioid receptor antagonist [17]. In May 2008, the Food and Drug Administration approved alvimopan for accelerating upper and lower gastrointestinal tract recovery after partial large–bowel or small–bowel resection with primary anastomosis. Several studies have examined the efficacy in reducing length of stay and readmission rates of patients receiving alvimopan who have undergone bowel resection [18–21] as well as the cost–effectiveness [22, 23]. One smaller retrospective study showed a diminished length of stay in patients treated with alvimopan as part of a multimodal postoperative pathway in patients undergoing cystectomy [24]. However, no studies have evaluated the cost–effectiveness of alvimopan alone in a patient cohort undergoing cystectomy and urinary diversion in a real–world setting of moderate volume center and epidural analgesia.
MATERIAL AND METHODS
Subjects
Between January 2008 and January 2012, 98 consecutive patients underwent cystectomy and urinary diversion for benign and oncologic indications at a single academic institution by 7 surgeons. This was a retrospective cohort analysis. Twelve of these patients suffered Clavien 3b or greater complications (death (2), laparotomy for dehiscence/evisceration (2), cardiogenic shock (2), stroke requiring carotid endarterectomy, ischemic leg requiring embolectomy, laparotomy for small bowel obstruction, laparotomy for delayed rectal injury, status epilepticus, pulmonary hemorrhage) thus making LOS independent from bowel function and were eliminated from analysis as their LOS was unlikely to be governed by bowel function. Patients with muscle–invasive bladder cancer were referred to oncology to discuss neoadjuvant chemotherapy. The use of neoadjuvant chemotherapy was not standardized among all patients by strict criteria. All active smokers were counseled on smoking cessation preoperatively. Preoperatively, bowel preparation was performed with 4 liters of polyethylene glycol and two days of clear liquid diet. An epidural catheter was placed unless the patient refused or had contraindications.
Operative details
Radical or supratrigonal cystectomy was performed with ileal conduit, Indiana pouch, transverse colon conduit, or Mainz neobladder. Cystectomy technique was performed through a low midline incision or via a robotic–assisted laparoscopic approach but all diversion was performed extracorporally. The adoption of a robotic–assisted technique occurred during this study period. A Blake drain and 8 French ureteral catheters were left in all patients. Patients with continent diversions were left with two urinary drainage catheters. Patients were treated without nasogastric tube postoperatively. Patients received care in the surgical intensive care unit or the acute care floor postoperatively. Patients were kept nil per os (NPO) until passage of flatus in most cases, but in younger, healthier patients this was determined by surgeon discretion.
Intervention
In 2010, all surgeons agreed to institute alvimopan. Alvimopan was administered with a sip of water at a 12 mg dose preoperatively and then 12 mg every 12 hours for up to 15 doses or until discharge. All patients were eligible for the drug; with no patients with stage 5 chronic kidney disease or severe hepatic impairment (Child–Pugh class C). The Medical Center is a participant in the ENTEREG Access Support and Education Program for Risk Evaluation and Monitoring of Safety for alvimopan.
Outcomes
Discharge criteria were: tolerating solid food without nausea or vomiting, pain controlled on oral pain medications, ambulatory, laboratory values and vital signs within normal limits, and urinary diversion teaching performed. Most patients had a bowel movement prior to discharge, but only a flatus was a firm discharge criteria.
The cost analysis was from the institutional perspective with hospital cost data obtained from the Medical Center Finance Department. Total costs (not charges) were obtained and included direct variable, overhead, and fixed variable costs. The health system would not provide a breakdown by components. Cost analysis was performed using the F–test for amount billed, amount billed minus drug cost, and insurance paid. Cost was adjusted for pre– and perioperative characteristics in regression analysis. All costs were adjusted to 2012 US dollars using the medical care component of the Consumer Price Index [25].
Statistical analisys
Analysis was performed on an intent–to–treat basis. The Kruskal–Wallis test was used to evaluate differences between the groups with regards to outcomes. The Wald test was used in a Poisson regression to determine alvimopan administration effect on LOS in univariate analysis. Multivariate analysis was performed using Poisson regression by taking into account variables with p <0.05 due to high number of variables relative to the number of subjects. Poisson regression based on quartiles of propensity scores on variables with p <0.1. For all testing, P value of <0.05 was deemed to be significant.
RESULTS
Pre– and perioperative characteristics
After removal of the Clavien IIIb or higher complications, there were 36 patients in the alvimopan group and 50 patients in the pre–alvimopan cohort. Upon detailed review of the medication reconciliation, we found that, although ordered, patients did not always receive the drug. The median number of doses was 10 (range 0–15). Importantly, all but 6 received the preoperative dose. No patient stopped alvimopan due to side effects.
The entire cohort was 77% male and had a mean age of 63.5 years and a mean BMI of 28.4 kg/m2. Seventy percent of patients were active smokers at the time of surgery. This demographic data was the same for both groups. Indications were oncologic in 91% of patients. Neoadjuvant chemotherapy was administered in 28% of patients. Ileal conduit was the dominant diversion type performed in 52% of the pre–alvimopan cohort and 72% of the alvimopan cohort (Table 1).
Table 1.
Preoperative variables
| Pre–alvimopan | Alvimopan | P | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Gender | Male | 39 | 78% | 27 | 75% | 0.745 |
| Female | 11 | 22% | 9 | 25% | ||
| Smoker | No | 37 | 77% | 23 | 64% | 0.096 |
| Yes | 11 | 23% | 13 | 36% | ||
| Neoadjuvant | No | 36 | 73% | 26 | 76% | 0.757 |
| Yes | 13 | 27% | 8 | 24% | ||
| Mean (SD) | Range | Mean (SD) | Range | P | ||
| Age (years) | 64.5 (12.5) | 35–91 | 62.2 (11.5) | 41–82 | 0.335 | |
| BMI (kg/m2) | 28.0 (5.1) | 19.9–41.8 | 28.8 (5.7) | 14.8–40.2 | 0.458 | |
Trends that increased over the time period between the pre–alvimopan and alvimopan era included a shift from increased epidural catheters (82% vs. 60%, p = 0.032) to patient controlled analgesia (7% vs. 31%, p = 0.004). There was increased disposition of patients from the OR to the intensive care unit in the alvimopan group (44% vs. 66%, p = 0.048). Additionally, use of robotic technique increased markedly in the alvimopan group (8% vs. 25%, p = 0.030). Perioperative characteristics of estimated blood loss and OR time were no different between the cohorts.
Efficacy
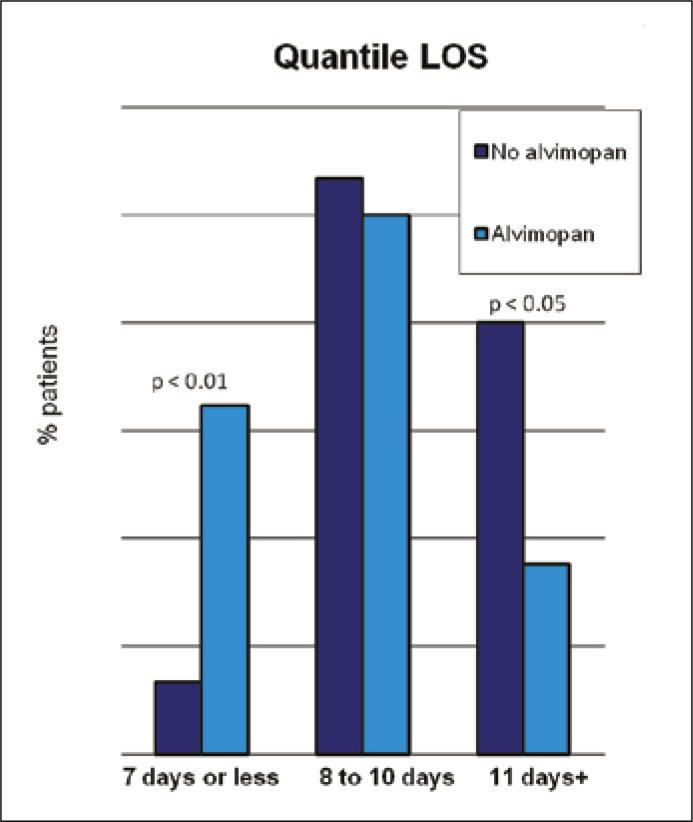
With regards to outcomes, readmission for ileus (4.4% pre–alvimopan vs. 0% alvimopan, p = 0.26) was similar in the groups. Utilization of total parenteral nutrition was higher in the pre–alvimopan group (38.8% pre–alvimopan 20.6% vs. alvimopan, p = 0.010). Time to clear liquid diet was 5.3 and 3.7 days in the pre–alvimopan and alvimopan groups, respectively (p = 0.058). Time to regular diet was significantly longer in the pre–alvimopan cohort at 7.1 days compared to 4.8 days in the treatment group (p = 0.020). Length of stay was reduced by 1.9 days (18% reduction in LOS) in the alvimopan group (10.5 vs. 8.6 days, p = 0.005, 95% CI 0.6–3.3) (see Table 2). While most patients stayed 8 to 10 days, the alvimopan group had one third of patients leaving at 7 days or earlier, while the pre–alvimopan group LOS was 11 days or greater in 40% of patients (Figure 1). No dose response effect was noted when comparing the number of doses administered versus LOS beyond a single dose. On multivariate analysis taking into account robotic technique, OR time, EBL, ICU disposition, epidural use, and patient controlled analgesia, there was a 16% reduction in LOS (p = 0.048, 95% CI 0.1–29%). Importantly, the robotic technique did not affect LOS and thus could not account for the difference of LOS between the pre–and post–alvimopan cohorts. Using propensity scoring Poisson regression to take into account additional variables, the magnitude of LOS reduction was again 16% (95% CI 0.0–30%, p = 0.050).
Table 2.
Treatments
| Pre–alvimopan | Alvimopan | P | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Robotic | No | 46 | 92% | 27 | 75% | 0.030 |
| Yes | 4 | 8% | 9 | 25% | ||
| Diversion Type | Ileal conduit | 26 | 52% | 26 | 72% | 0.069 |
| Indiana pouch | 8 | 16% | 6 | 17% | ||
| Neobladder | 16 | 32% | 4 | 11% | ||
| Epidural | No | 8 | 18% | 14 | 40% | 0.032 |
| Yes | 36 | 82% | 21 | 60% | ||
| PCA | No | 41 | 93% | 24 | 69% | 0.004 |
| Yes | 3 | 7% | 11 | 31% | ||
| ICU First Night | No | 27 | 56% | 12 | 34% | 0.048 |
| Yes | 21 | 44% | 23 | 66% | ||
| Surgeon | 1 | 30 | 60% | 17 | 47% | 0.340 |
| Others | 20 | 40% | 19 | 53% | ||
| Mean (SD) | Range | Mean (SD) | Range | P | ||
| OR time (minutes) | 637.8 (148.3) | 276–977 | 581.7 (153.6) | 259–858 | 0.098 | |
| EBL (milliliters) | 1353.6 (866.3) | 200–4000 | 1221.4 (1206.1) | 100–5500 | 0.146 | |
Figure 1.
Quantile LOS.
Cost–effectiveness
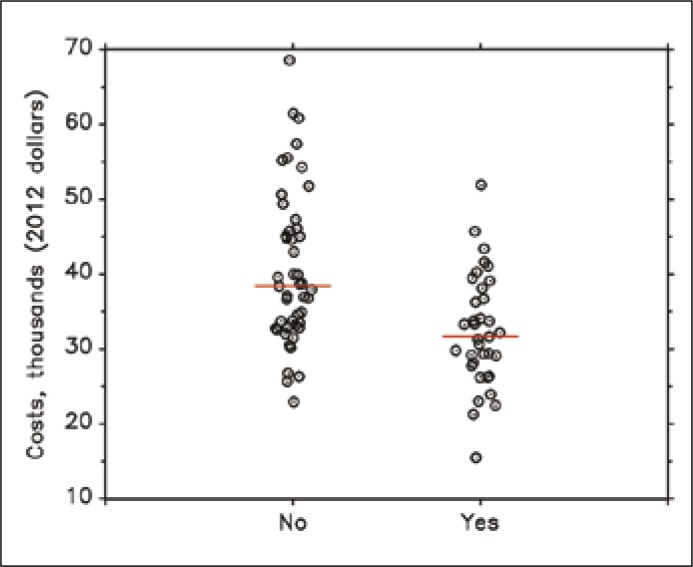
Costs were lower in the alvimopan group in the 35 patients with available cost data versus the 47 in the pre–alvimopan cohort. The mean cost was $40,604 in the pre–alvimopan group (2012 USD) compared to the alvimopan group mean of $32,443 (p <0.001) for a savings of $8,161 or 20% reduction of cost. When adjusting for the significantly different preoperative and perioperative variables of epidural, PCA, indication, robotic technique, OR time, EBL, and ICU disposition, the mean costs remained lower in the alvimopan group by $7,062 (p = 0.003) (Figure 2).
Figure 2.
Cost per hospitalization with and without alvimopan (thousands 2012 USD).
DISCUSSION
Our study has three key findings. First, it confirms the reduction in LOS conferred by routine administration of alvimopan. Second, this reduction in LOS does translate to reduction in costs. Finally, this demonstrates a real–world implementation of alvimopan administration which realizes the same advantages as a randomized controlled trial.
Postoperative ileus is a vexing problem in surgery. Its pathogenesis is multifactorial and its treatment is frustrating to patients and clinicians alike. It is associated with increased LOS, increased costs, and increased GI complications. Patients experience pain, nausea, vomiting, and malnutrition. Innumerable interventions have been studied to reduce postoperative ileus with mixed results. Chewing gum administered early in the perioperative period after bowel surgery has been evaluated in meta–analyses with the conclusion that it is a low cost safe modality that decreases time to flatus and bowel movement but not LOS [26]. Multimodal therapy has been attempted to combat postoperative ileus with moderate success. Alvimopan is the only pharmacologic agent shown to speed GI recovery after bowel surgery.
Cystectomy and urinary diversion is a surgery fraught with complications with an inpatient complication rate of 49–64% and a 30 day readmission rate around 25% [27]. GI complications, specifically postoperative ileus account for the plurality of this morbidity [28]. Interventions to decrease postoperative ileus can be in the pre–, peri–, and post–operative period. Forgoing mechanical bowel preparation has been shown to reduce prolonged ileus and LOS after cystectomy by Shafii et al. [11]. Intraoperatively, transesophageal doppler optimized fluid management yielded a marked reduction in postoperative ileus as well as a two day reduction in time to flatus and bowel movement but no change in LOS [14]. Pruthi and colleagues observed that institution of routine chewing gum postoperatively led to reduction in time to flatus and bowel movement by about half a day each [13]. Similarly, Choi and colleagues demonstrated in a randomized trial of 50 patients that chewing gum reduces time to flatus by 18% and time to bowel movement by 18% [12]. This was true regardless of open or robotic technique. Brodner and colleagues demonstrated that compared to traditional management, epidural analgesia, forced mobilization, and early enteral feeding leads to earlier time to first bowel movement after radical cystectomy [15]. Wallen and colleagues describe their perioperative pathway for reducing LOS and hastening bowel recovery with metoclopramide, ketorolac, chewing gum, and early oral feeding as the cornerstones of their care plan [16]. Similar multimodal pathways spanning the pre–, peri–, and postoperative period have been described with success in decrease time to flatus or bowel movement and mixed results in diminishing LOS. Such pathways have challenges such as acceptance of implementation and cost.
Alvimopan was first reported in 2001 in 14 healthy volunteers to attenuate the delayed enteric transit time but not the analgesic effects of morphine [17]. Wolff reported in 2004 in a randomized–controlled study that alvimopan 6 mg and 12 mg doses reduced time to return bowel function and the 12 mg dose reduced time to discharge order by almost 1 full day in 510 patients undergoing laparotomy for bowel resection or hysterectomy [21]. Additional phase III studies reinforced this. Alvimopan was FDA–approved in 2008 to accelerate bowel recovery after bowel surgery [18]. It has been shown to decrease LOS in small and large bowel surgery by 1 or 2 days. Of note, the promise of alvimopan for chronic opioid use associated bowel dysfunction was cut short by a study revealing 3 cardiac events in a chronically dosed alvimopan group which could neither be deemed causal nor were reproducible [29]. Nevertheless, long term use of alvimopan has been avoided because of this. No cardiac toxicities have been noted with the short–term use of alvimopan described in this study.
Vora and colleagues demonstrated in 50 patients that alvimopan administration decreases times to discharge order, flatus, bowel movement, clear liquid diet, and regular diet with magnitude of effect (2 days) similar to the current study. However, nasogastric decompression, an intervention fraught with increased complications and delayed GI recovery, was utilized in the control group but not the alvimopan group, thus confounding the findings. Additionally, this study does not assess the cost of the intervention [24].
Hilton et al. performed a sensitivity analysis assuming a reduction in rate of postoperative ileus by 50% which revealed the conditions under which alvimopan would be cost–effective [30]. However, this was a purely theoretical study and did not include novel patient data. They estimate an incremental cost of a hospital day is $1110 before taking into account additional tests, nutrition, or procedures. The authors recognized the cost–effectiveness of alvimopan would be contingent upon the rate of postoperative ileus and the degree to which alvimopan reduces the rate of postoperative ileus. Unfortunately, postoperative ileus tends to be a nebulous term which essentially means extended cessation of bowel function beyond the period of the expected transient cessation of bowel function. In studies assessing prevention of postoperative ileus definitions have varied widely suggesting the need for a standardized definition [26]. It is for these very reasons that we chose to exclude this as an endpoint in favor of more objective outcomes such as LOS, time to clear liquid diet, time to regular diet, use of parenteral nutrition, and readmission for ileus. Furthermore, these endpoints are of more utility in a retrospective study where time to flatus and time to first bowel movement are not always well documented.
The strength of our study is related to the results of a multicenter RCT confirming reduction of LOS by a mean 2.6 days [31]. However, RCT are often not as generalizable in the community. For example, the RCT excluded patients with epidural analgesia. Our experience contributes to the literature by demonstrating the advantage of alvimopan in decreasing LOS by 1.9 days even in the setting of epidural analgesia and when all 15 doses of alvimopan were not received.
Limitations of the current study include the retrospective nature. While a new intervention was implemented empirically based on translation from a different discipline, this was not performed for study purposes. The sequential rather than randomized nature thus limits interpretation of this data due to nonrandom biases that are due to chronology of the cases. This is addressed with multivariate analysis to reduce the impact of known variables such as robotic technique and epidural analgesia. Interestingly, contrary to most contemporary literature, there was not a diminished LOS in the robotic technique in this series. This likely reflects the early end of the learning curve in our robotic cystectomy experience and mandates future study. Nevertheless, unknown biases may be present and unaccounted for. This is a relatively small and single center series.
CONCLUSIONS
Alvimopan has been shown to decrease LOS after bowel surgery. In our experience, alvimopan is associated with decrease LOS by almost 2 days after cystectomy and urinary diversion. Furthermore, this is reflected in cost of stay with a 20% reduction in cost. This is important as health care policy changes place increasing scrutiny on costs. Alvimopan is an effective adjunct to early ambulation, epidural analgesia, optimized fluid management and early feeding to combat postoperative ileus after cystectomy and urinary diversion.
References
- 1.Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–174. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Chang SS, Wells N, Parekh DJ, Smith JA., Jr Complications of radical cystectomy for nonmuscle invasive disease: comparison with muscle invasive disease. J Urol. 2003;169:101–104. doi: 10.1016/S0022-5347(05)64045-1. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeck BK, Miller DC, Taub D, Dunn RL, Khuri SF, Henderson WG, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174:1231–1237. doi: 10.1097/01.ju.0000173923.35338.99. [DOI] [PubMed] [Google Scholar]
- 4.Svatek RS, Fisher MB, Williams MB, Matin SF, Kamat AM, Grossman HB, et al. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology. 2010;76:1419–1424. doi: 10.1016/j.urology.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Lee KL, Freiha F, Presti JC, Jr, Gill HS. Gender differences in radical cystectomy: complications and blood loss. Urology. 2004;63:1095–1099. doi: 10.1016/j.urology.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, et al. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010;57:274–281. doi: 10.1016/j.eururo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Soulié M, Straub M, Gamé X, Seguin P, De Petriconi R, Plante P, Hautmann RE. A multicenter study of the morbidity of radical cystectomy in select elderly patients with bladder cancer. J Urol. 2002;167:1325–1328. [PubMed] [Google Scholar]
- 8.Konety BR, Allareddy V. Influence of post–cystectomy complications on cost and subsequent outcome. J Urol. 2002;177:280–287. doi: 10.1016/j.juro.2006.08.074. [DOI] [PubMed] [Google Scholar]
- 9.Maffezzini M, Gerbi G, Campodonico F, Parodi D, Capponi G, Spina A, Guerrieri AM. Peri–operative management of ablative and reconstructive surgery for invasive bladder cancer in the elderly. Surg Oncol. 2004;13:197–200. doi: 10.1016/j.suronc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Inman BA, Harel F, Tiguert R, Lacombe L, Fradet Y. Routine nasogastric tubes are not required following cystectomy with urinary diversion: a comparative analysis of 430 patients. J Urol. 2003;170:1888–1891. doi: 10.1097/01.ju.0000092500.68655.48. [DOI] [PubMed] [Google Scholar]
- 11.Shafii M, Murphy DM, Donovan MG, Hickey DP. Is mechanical bowel preparation necessary in patients undergoing cystectomy and urinary diversion? BJU Int. 2002;89:879–881. doi: 10.1046/j.1464-410x.2002.02780.x. [DOI] [PubMed] [Google Scholar]
- 12.Choi H, Kang SH, Yoon DK, Kang SG, Ko HY, Moon du G, et al. Chewing gum has a stimulatory effect on bowel motility in patients after open or robotic radical cystectomy for bladder cancer: a prospective randomized comparative study. Urology. 2011;77:884–890. doi: 10.1016/j.urology.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 13.Kouba EJ, Wallen EM, Pruthi RS. Gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion. Urology. 2007;70:1053–1056. doi: 10.1016/j.urology.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 14.Pillai P, McEleavy I, Gaughan M, Snowden C, Nesbitt I, Durkan G, et al. A double–blind randomized controlled clinical trial to assess the effect of Doppler optimized intraoperative fluid management on outcome following radical cystectomy. J Urol. 2011;86:2201–2206. doi: 10.1016/j.juro.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 15.Brodner G, Van Aken H, Hertle L, Fobker M, Von Eckardstein A, Goeters C, et al. Multimodal perioperative management–combining thoracic epidural analgesia, forced mobilization, and oral nutrition–reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth Analg. 2001;92:1594–1600. doi: 10.1097/00000539-200106000-00049. [DOI] [PubMed] [Google Scholar]
- 16.Pruthi RS, Nielsen M, Smith A, Nix J, Schultz H, Wallen EM. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. J Am Coll Surg. 2010;210:93–99. doi: 10.1016/j.jamcollsurg.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Liu SS, Hodgson PS, Carpenter RL, Fricke JR., Jr ADL 8–2698, a trans–3,4–dimethyl–4–(3–hydroxyphenyl) piperidine, prevents gastrointestinal effects of intravenous morphine without affecting analgesia. Clin Pharmacol Ther. 2001;69:66–71. doi: 10.1067/mcp.2001.112680. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig K, Enker WE, Delaney CP, Wolff BG, Du W, Fort JG, et al. Gastrointestinal tract recovery in patients undergoing bowel resection: results of a randomized trial of alvimopan and placebo with a standardized accelerated postoperative care pathway. Arch Surgery. 2008;143:1098–1105. doi: 10.1001/archsurg.143.11.1098. [DOI] [PubMed] [Google Scholar]
- 19.Wolff BG, Viscusi ER, Delaney CP, Du W, Techner L. Patterns of gastrointestinal recovery after bowel resection and total abdominal hysterectomy: pooled results from the placebo arms of alvimopan phase II North American clinical trials. J Am Coll Surg. 2007;205:43–51. doi: 10.1016/j.jamcollsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Delaney CP, Wolff BG, Viscusi ER, Senagore AJ, Fort JG, Du W, et al. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase II studies. Ann Surg. 2007;245:355–363. doi: 10.1097/01.sla.0000232538.72458.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff BG, Michelassi F, Gerkin TM, Techner L, Gabriel K, Du W, Wallin BA, et al. Alvimopan, a ovel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double–blind, placebo–controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240:728–734. doi: 10.1097/01.sla.0000141158.27977.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell TJ, Poston SA, Kraft MD, Senagore AJ, Delaney CP, Techner L. Economic analysis of alvimopan in North Anerican Phase III efficacy trials. Am J Health Syst Pharm. 2009;66:1362–1368. doi: 10.2146/ajhp080329. [DOI] [PubMed] [Google Scholar]
- 23.Poston S, Broder MS, Gibbons MM, Maclaren R, Chang E, Vandepol CJ, et al. Impact of alvimopan (entereg) on hospital costs after bowel resection: results from a large inpatient database. P&T. 2011;36:209–220. [PMC free article] [PubMed] [Google Scholar]
- 24.Vora AA, Harbin A, Rayson R, Christiansen K, Ghasemian R, Hwang J, et al. Alvimopan provides rapid gastrointestinal recovery without nasogastric tube decompression after radical cystectomy and urinary diversion. Can J Urol. 2012;19:6293–6298. [PubMed] [Google Scholar]
- 25.US Bureau of Labor Statistics. Consumer Price Index. Available at: http://www.bls.gov/cpi/. Accessed: March 5, 2013.
- 26.Ramirez JA, McIntosh AG, Strehlow R, Lawrence VA, Parekh DJ, Svatek RS. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: A systematic review. Eur Urol. 2013;64:588–597. doi: 10.1016/j.eururo.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs BL, Zhang Y, Tan HJ, Ye Z, Skolarus TA, Hollenbeck BK. Hospitalization trends after prostate and bladder surgery: implications of potential payment reforms. J Urol. 2013;189:59–65. doi: 10.1016/j.juro.2012.08.182. [DOI] [PubMed] [Google Scholar]
- 28.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, et al. Comparative analysis of outcomes and costs following open radical cystectomy versus robot–assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol. 2012;61:1239–1244. doi: 10.1016/j.eururo.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Webster L, Jansen JP, Peppin J, Lasko B, Irving G, et al. Alvimopan, a peripherally acting mu–opioid receptor (PAM–OR) antagonist for the treatment of opioid–induced bowel dysfunction: results from a randomized, double–blind, placebo–controlled, dose–finding study in subjects taking opioids for chronic non–cancer pain. Pain. 2008;137:428–440. doi: 10.1016/j.pain.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Hilton WM, Lotan Y, Parekh DJ, Basler JW, Svatek RS. Alvimopan for prevention of postoperative paralytic ileus in radical cystectomy patients: a cost–effectiveness analysis. BJU Int. 2012;111:1054–1060. doi: 10.1111/j.1464-410X.2012.11499.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee CT, Chang SS, Kamat AM, et al. Alvimopan Accelerates Gastrointestinal Recovery After Radical Cystectomy: A Multicenter Randomized Placebo–Controlled Trial. Eur Urol. 2014;14:S0302–28380. doi: 10.1016/j.eururo.2014.02.036. [DOI] [PubMed] [Google Scholar]