Abstract
Introduction
Many options exist for the surgical treatment of lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH), including transurethral resection of the prostate (TURP), laser surgery, and open adenomectomy. Recently, endoscopic techniques have been used in the treatment of BPH.
Material and methods
We reviewed clinical studies in PubMed describing minimally invasive endoscopic procedures for the treatment of BPH.
Results
Laparoscopic adenomectomy (LA) and robotic–assisted simple prostatectomy (RASP) were introduced in the early 2000s. These operative techniques have been standardized and reproducible, with some individual modifications. Studies analyzing the outcomes of LA and RASP have reported significant improvements in urinary flow and decreases in patient International Prostate Symptom Score (IPSS). These minimally invasive approaches have resulted in a lower rate of complications, shorter hospital stays, smaller scars, faster recoveries, and an earlier return to work.
Conclusions
Minimally invasive techniques such as LA and RASP for the treatment BPH are safe, efficacious, and allow faster recovery. These procedures have a short learning curve and offer new options for the surgeon treating BPH.
Keywords: benign prostatic hyperplasia (BPH), adenomectomy, simple prostatectomy, laparoscopic adenomectomy (LA), robotic–assisted simple prostatectomy (RASP)
INTRODUCTION
Lower urinary tract symptoms (LUTS) are common, especially in older men, and are most often caused by benign prostatic hyperplasia (BPH) which results in benign prostatic enlargement (BPE) and bladder outlet obstruction (BOO).
Patients with severe LUTS unresponsive to pharmacological management, and those with BPH complications are candidates for surgical therapy. Transurethral resection of the prostate (TURP) has been considered the surgical “gold standard” in terms of efficacy and re–treatment rate. However, recent scientific and technological advances have challenged the traditional surgical approach to BPH. The morbidity and late complications of TURP were the major impetus driving the development of new techniques such as bipolar TURP and laser enucleation or vaporization. However, these techniques were not applicable for the treatment of larger prostates. Open prostatectomy, recommended by The European Association of Urology for patients with prostate volumes >80 mL [1], is associated with a prolonged hospital stay and serious complications such as severe blood loss and infection. Additionally, patients may require subsequent surgical revision. Despite the wide acceptance of open prostatectomy; blunt dissection from the capsule, particularly in the apical area, may be technically challenging for the less experienced surgeons and is frequently associated with complications.
Laparoscopic, and more recently robotic techniques, have provided a minimally invasive alternative to open prostatectomy, with equal efficacy, faster recovery, and shorter hospital stay. There is less pain and improved cosmetic results, although operative time (OT) and estimated blood loss are dependent on surgical expertise [2]. These endoscopic procedures allow improved visualization and reduced morbidity and are well established in the management of prostate carcinoma, providing a rationale for their use in the treatment of BPH [3–5].
METHODS
Articles for this review were identified through PubMed searches from January 2004 through December 2013. Various algorithms were used including: “benign prostatic hyperplasia”, “adenomectomy”, “simple prostatectomy”, “laparoscopic adenomectomy”, and “robotic–assisted simple prostatectomy.” Articles were also identified through the references of these articles. Reference lists of retrieved articles were reviewed to ensure inclusion of all pertinent studies. Only papers published in English were reviewed. The final reference list was approved by authors RS and TB based on originality and relevance to the scope of this review article.
RESULTS
Pre– and postoperative evaluation
Prior to endoscopic procedures, patients underwent a detailed history assessment, a thorough physical examination, and an appropriate laboratory assessment. Transrectal ultrasonography was performed to evaluate the prostate and adenoma volume. Cystoscopy and urodynamic evaluation were not routinely performed. The severity of LUTS was evaluated by most authors using the International Prostate Symptom Score (IPSS) questionnaire and the maximal flow rate (Qmax). For precise postoperative functional evaluation, some authors used the Sexual Health Inventory For Men (SHIM), typically 3, 6, and 12 months postoperatively [6, 7]. Prostate biopsy was performed as a standard procedure in some studies, but in other studies it was only performed in selected cases [8, 9]. Peri– and postoperative assessment included OT, blood loss, transfusion requirements, catheterization time, and duration of hospital stay. Complications were evaluated based on their severity, usually according to Clavien et al. [10]. The longest follow–up period was several years in patients after LA, and up to 10 months in patients after robotic–assisted adenomectomy [11, 12].
Laparoscopic adenomectomy (LA): Initial studies
In 2002, Mariano et al. described the first LA in a 71–year–old man with an ultrasonography–estimated 173–mL prostate and a 26.6 ng/mL prostate–specific antigen (PSA) level [13]. The procedure was performed with five intraperitoneal trocars, with anterior prostate capsulotomy and placement of hemostatic sutures at the 5 and 7 o'clock positions after adenoma removal. Mean OT was 138 min, and blood loss was estimated at 330 mL. IPSS and Qmax showed definite improvement.
In 2004 Van Velthoven et al. described their technique of LA, which included hemostatic control of the lateral venous vesicoprostatic pedicles, transverse anterior incision of the prostatic capsule, adenoma enucleation using the harmonic scalpel, and reconstruction of the posterior bladder neck and prostatic capsule [14]. The mean OT was 145 min and blood loss was 192 mL. The authors reported very good functional outcomes; mean Qmax was increased by 4.3 mL/s postoperatively.
In 2005, Sotelo et al. presented 17 cases of laparoscopic simple retropubic prostatectomy using 5 ports [15]. Stepwise, the technique included transverse cystotomy just proximal to the prostatovesical junction, subcapsular plane development, prostatic adenomectomy, prostatic fossa trigonization, and prostatic capsule suture repair. The mean OT was 156 min and blood loss was 516 mL (100–2500 ml). A 7 mL/s increase in Qmax was noted postoperatively.
Since these initial reports, laparoscopic simple prostatectomy has been more widely adopted, offering surgeons an extra– or intraperitoneal approach, following a transcapsular or transvesical route. In selected patients, finger assistance has been used for rapid enucleation of large adenomas [2, 16, 17, 8].
LA procedure
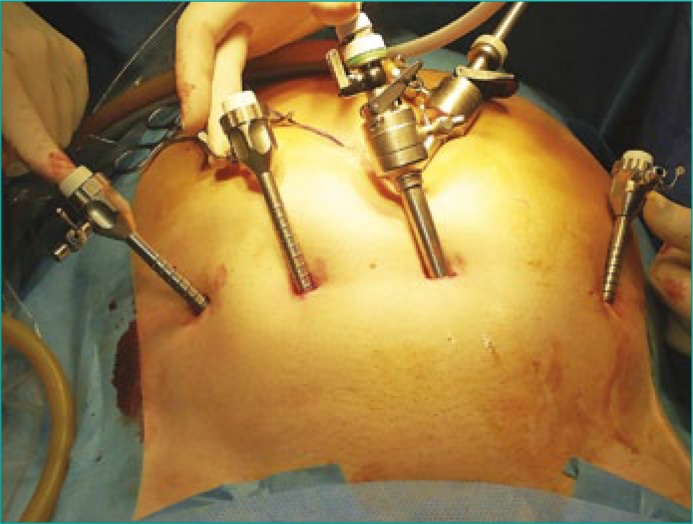
Several laparoscopic techniques are used to create a preperitoneal space. One technique involves an incision under the umbilicus with carbon dioxide insufflation into the extraperitoneal space via a Veress needle to 12 mmHg [19]. A second technique involves making a 2–cm vertical midline incision above the pubic arch followed by blunt dissection of the preperitoneal and Retzius space with an index finger and a 700–mL self–dilating balloon [2]. In a third technique, the preperitoneal space is created after insertion of a balloon dissector. The retroperitoneal space is then bluntly dissected with an 800–1200–mL infusion of sterile saline solution into the balloon [4]. A Hasson trocar is introduced under the umbilicus. The operation can also begin with the primary insertion of a 10–mm infraumbilical port and laparoscope. Then, dissection of the preperitoneal space is completed with the aid of the laparoscope and insufflation. Usually, 4 trocars, 5 mm or 12 mm, are inserted in a fan shape, to introduce a needle for suturing, as in extraperitoneal radical prostatectomy [4]. A single 10–mm port is inserted infraumbilically as the camera port (Figure 1). The pneumoextraperitoneum is usually created at 12 mm Hg. The pelvic fascia and the anterior wall of the prostate are exposed. The dorsal vein complex is assessed and then carefully coagulated using bipolar forceps cranially, keeping an appropriate distance from the puboprostatic ligaments. In a fourth technique, two hemostatic sutures are applied to these vessels [2]. Using the bladder catheter or a special metal guide inserted into the urethra as a reference point, the interface between the bladder neck and prostate base is identified. If necessary, two cross–stitch hemostatic sutures are placed on the lateral surface of the prostate at the level of the bilateral vesicoprostatic vessels.
Figure 1.
Location of the ports during extraperitoneal laparoscopic adenomectomy.
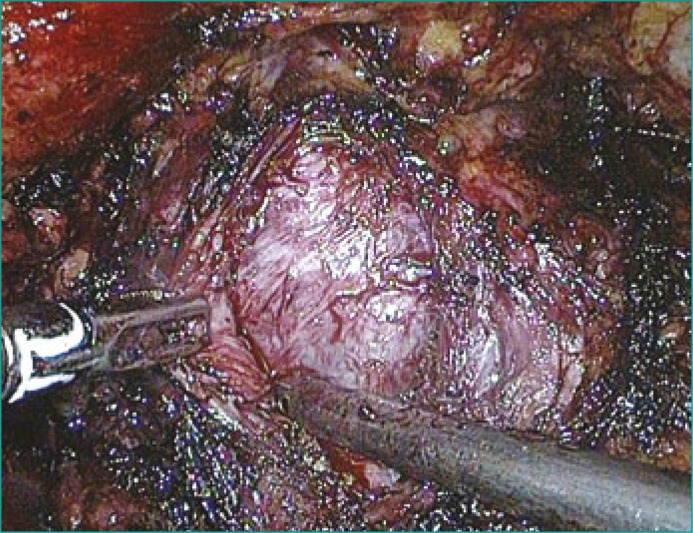
The prostatic capsule is opened 3–4 cm transversally and 1 cm distal to the bladder neck. The puboprostatic ligaments are avoided to prevent bleeding from the dorsal vascular plexus. Hemostatic sutures are placed at the 5 and 7 o'clock positions [19]. The capsular incision is carried to a depth that first reveals the off–white tissue of the adenoma [17]. Monopolar scissors and the suction–irrigation cannula are used to develop the plane between the prostatic adenoma and the capsule (Figure 2). The anterior plane is then developed, followed by lateral and posterior dissection. In some cases, where improved exposure is required, the incision is extended in the shape of an inverted “T” on the prostatic capsule [21]. To enucleate the lateral lobes, a circumferential incision is made in the urethral mucosa at the bladder neck. The lateral lobes are grasped using a laparoscopic claw grasper. A harmonic scalpel is used to develop the surgical avascular capsular plane in the distal projection towards the apex, the lateral projection to the posterior plane, and the cranial projection to the bladder neck, in a fashion similar to that used in open surgery. In a modified version of the procedure, two lateral stay sutures are used between the cut prostatic capsule and the Cooper ligament, providing a clear visualization of the fossa and the cleavage plan [11].
Figure 2.
Laparoscopic enucleation of adenoma tissue from the surgical capsule.
Any bladder stones are removed during the intervention through a capsular incision. The adenoma is excised, and then one or two corresponding specimens are placed outside the capsule in the lateral prostatic fossa, e.g., in close proximity to the obturator fossa, to be removed later. Hemostasis is obtained with stitches for transcapsular arteries and bipolar or monopolar electrocoagulation for minor vessels. The prostatic fossa is inspected for any remaining adenoma nodules.
To facilitate the re–epithelialization of the prostatic fossa and to achieve more effective control of hemostasis, the prostatic fossa is trigonized using 2–4 stitches between the sacral lip of the bladder neck and posterior surgical capsule [19]. The bladder catheter is then replaced, usually by an irrigation or Foley catheter. An interrupted or running suture is used for prostatic capsule reconstruction, followed by the introduction of a bag via the lateral 10–mm port for collection of the fragmented adenoma [17]. When the adenoma is too large to be removed as a whole, morcellation is performed before extraction. A drain (Redon type) is inserted via the port, the infraumbilical incision is enlarged to enable intact retrieval, and the bag with the specimen is removed. The catheter is removed in 1–5 days. The prostatic capsule can also be opened through a longitudinal opening in the anterior aspect of the bladder and extended to the anterior aspect of the prostatic capsule using bipolar diathermy, scissors, or a harmonic scalpel [11]. Stay sutures are placed between the edges of the open bladder on each side of the Cooper ligament [16].
Most published series describe the extraperitoneal technique for LA, but a transperitoneal approach has also been described. With the patient in a steep Trendelenburg position, the pneumoperitoneum is created, and five intraperitoneal trocars are placed in a “W” fashion [22]. After the peritoneum is incised and the Retzius space is dissected, the bladder and prostate are taken down. A midline incision covering the anterior aspect of the prostatic capsule and bladder neck exposes the adenoma [11]. The hyperplastic tissue is bluntly dissected and the adenoma is removed. Retrigonization of the mucosa and hemostatic sutures of the bladder neck are performed with intracorporeal sutures [11].
Some authors described another modification with an extraperitoneal transvesical approach [17, 23]. In this case, a transverse cystotomy incision is made proximal to the junction of the bladder and prostate. In this manner, the anterior bladder neck is incised and entry is gained into the bladder lumen[19]. Next, a circular incision is made on the vesicle mucosa overlying the prostate lobes, and made deeper to reach the prostatic adenoma. Then the adenoma is surgically exposed using the subcapsular approach. The procedure ends with closure of the transverse cystotomy to ensure water tightness [19].
In some patients, when the layer for surgical exposure of a large adenoma is unclear, or if the surgeon is inexperienced, the adenoma can be dissected using the finger–assisted technique [2, 7, 16, 18, 22]. After opening the prostatic capsule and developing the plane of cleavage between the prostatic adenoma and capsule, gas flow is stopped and the index finger is introduced through a 2–3 cm suprapubic incision into this developed plane. The adenoma is then digitally enucleated. This can be performed easily and is assisted by digital rectal examination (DRE) [18, 19, 23]. Insufficient finger reach may be a problem, particularly in obese patients. The specimen is removed via the incision, and insufflation is restarted after closure of the suprapubic incision [2, 22].
Laparoscopic techniques have a learning curve, and require more time when first performed. Increased prostate weight, and hence prostate volume, have been correlated with increased OT [15]. Excellent visualization and bloodless resection of the adenoma limits complications and decreases OT, especially with larger lesions [11]. The use of vascular control, bladder neck and capsular incisions, and ultrasonic scissors as a sharp and blunt dissection instrument allow easier enucleation of larger adenomas [11]. There have been no reports of conversion to open surgery due to unexpected difficulties corresponding to prostate size. In a recently published series, after excluding the first 10 cases of LA, univariate analysis of the next 78 patients with a large prostate (>90 mL) showed a correlation of prostate volume and OT with complications. However, multivariate analysis did not confirm this result [7]. Even with a large prostate (>90 mL), LA provides excellent operative and perioperative results and patient satisfaction (Table 1).
Table 1.
Series (with >15 cases) on laparoscopic simple prostatectomy
| Reference | N | Operation approach | Prostate size (ml) | Operative time (min) | Catheter (days) | Hospital stay (days) | I–PSS | Qmax | Blood loss, ml | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | ||||||||
| Van Velthoven, 2004 [14] | 18 | E | 95 | 145 | NR | 7 | NR | NR | 4.3 | 17.9 | 192 |
| Sotelo, 2005 [15] | 17 | E | 93 | 156 | 6.3 | 2 | 24.5 | 9.9 | 7 | 22.8 | 516 |
| Mariano, 2006 [11] | 60 | T | 144.5 | 138.5 | 4.6 | 4.6 | 28.3 | 5.15 | 4.78 | 19.9 | 330 |
| Zhou, 2009 [18] | 45 | E | 85.4 | 105 | 4.6 | NR | 25.5 | 2.4 | 6.1 | 18.7 | 360 |
| Castillo, 2011 [24] | 59 | E | 108.5 | 123 | 4.2 | 4.2 | 18 | 2 | NR | NR | 415 |
| Porpiglia, 2011 [7] | 78 | E | 96 | 103 | 3.5 | 5.4 | 18.1 | 8.1 | 9.8 | 22.7 | 333 |
N – number of patients; T – transperitoneal approach; E – extraperitoneal approach; Catheter – catheterization time after the procedure; I–PSS – International Prostate Symptom Score; Qmax – the maximal flow rate; NR – not received
A major benefit of LA includes improved control of bleeding, possibly because of improved visualization and vascular compression from insufflated gas. Morbidity and pain are reduced compared to those in the open, procedure as incisions are smaller and there is no need for retraction [19]. LA has better esthetic results, reduced need for analgesics, fewer wound infections, shorter hospital stay, and an earlier return to normal activities compared with the open procedure (Table 1).
Intraoperative complications, mostly bleeding, are rare (<2.5%) with no effect on clinical outcome and a decreased need for transfusion [4, 22]. Intraoperative blood loss does not usually correlate with the amount of enucleated tissue [4] (Table 1). Decreased blood loss is achieved via gas compression of the venous system, aiding hemostasis, and allowing more precise dissection and coagulation of the adenomatous cleavage plane [11, 13]. Furthermore, better visualization improves hemostasis [14]. The early complication rate of 14% is acceptable, particularly since most are less than Clavien grade II [5].
Long–term complications rarely occur (2.5% at the 12–month follow–up, <5% at the 30–month follow–up), and mainly include new obstructive urinary symptoms including urinary tract infections such as pyelonephritis, prostatitis, or epididymitis that are usually treated with conservative methods, and short presphincteric urethral stenosis usually treated with endoscopic urethrotomy [7].
Functional outcomes of LA confirm the efficacy of this procedure for treating large prostates. Most researchers observed a significant increase in the Qmax in comparison to preoperative values (mean increased Qmax 14.4 mL/s) and a marked decrease in IPSS (mean decrease 17.2) postoperatively. IPSS, IPSS in the quality of life domain, and the Expanded Prostate Cancer Index Composite (EPIC) questionnaire score remained stable at 3, 6, and >12 months postoperatively. Patient satisfaction was high throughout the follow–up period [7]. Erectile function, evaluated with IIEF–5 or other tools, did not change significantly as a result of LA [20]. Persistent retrograde ejaculation was a consequence of the surgery, but had no significant impact on sexual function [11]. PSA levels were significantly different preoperatively and postoperatively, and later PSA levels remained stable during follow–up [7]. The demand for analgesics after LA was significantly lower.
Most investigators use the extraperitoneal technique based on the Millin technique employed in open procedures [7, 11, 14, 15, 17–19]. With this approach, the risk of bladder tamponade by clots is avoided along with the additional anesthesiology risks associated with the steep Trendelenburg position that is necessary in the transperitoneal approach. The transperitoneal technique was used by some investigators [11, 13, 17], but is associated with a risk of ileus, peritonitis, and bowel injuries along with urine leakage and possible urine peritonitis from the bladder suture. No studies have compared extra– and transperitoneal laparoscopic adenomectomy in the way laparoscopic or robotic radical prostatectomy has been compared [24–26]. However, in LA, these two approaches give comparable results (Table 1).
LA has a relatively short learning curve in comparison with radical laparoscopic prostatectomy, and has been estimated at 5–10 surgeries [19]. If conversion is required during a procedure using the preperitoneal access, no new access is needed because the surgery can be continued within the same space. As the number of surgeries performed and laparoscopic skills increase, there is a clear reduction in OT [11, 15, 21].
Comparison studies between LA and open prostatectomy
Studies comparing LA and open surgery have shown that both procedures offer the same benefits in terms of functional results, but that LA performed by experienced surgeons [4] provided greater perioperative benefits such as reduced bleeding and transfusions, shorter irrigation and catheterization times, shorter hospital stays, lower analgesic requirements, shorter recovery times, and improved cosmetic results [4, 20, 21, 27] (Table 2).
Table 2.
Comparative series between open and laparoscopy adenomectomy from published series
| Reference | N | Operative time (min) | p Value for operative time | Hospital stay (days) | p Value of Hospital Stay | Catheter (days) | P Value of Catheter | Blood loss, (ml) | p Value of Blood loss |
|---|---|---|---|---|---|---|---|---|---|
| Porpiglia, 2006 [4] | 0–20 L – 20 | 0–95.5 L – 107.2 | 0.6 | 0–7 L – 7.8 | 0.8 | 0–6.6 L – 6.3 | 0.9 | 0–687 L – 411 | 0.004 |
| Baumert, 2006 [21] | 0–30 L – 30 | 0–54 L – 115 | <0.01 | 0–8 L – 5.1 | 0.003 | 0–6.8 L – 4 | 0.004 | 0–643 L – 367 | 0.045 |
| McCullough, 2009 [27] | 0–184 L – 96 | 0–54.7 L – 95.1 | <0.0001 | 0–7.7 L – 6.3 | <0.0001 | 0–6.4 L – 5.2 | 0.001 | 0–400 L – 350 | 0.387 |
| Garcia–Segui, 2012 [20] | 0–18 L – 17 | 0–101 L – 135 | 0.022 | 0–6.6 L – 3.7 | 0.006 | 0–7.5 L – 5.5 | 0.030 | 0–493 L – 250 | 0.004 |
N – number of patients; O – open adenomectomy, L – laparoscopy adenomectomy, catheter – catheterization time after the procedure
Robotic–assisted simple prostatectomy (RASP)
The introduction of the da Vinci® Robotic Surgical System [Intuitive Surgical, Inc., Sunnyvale, CA, USA] for urological procedures, including radical prostatectomy, has been a major step towards a minimally invasive approach. In 2008, Sotelo et al. used a newly developed technique for RASP in 7 patients with a mean prostate volume of 77.66 mL [28]. Mean OT was 205 min and mean intraoperative blood loss was 298 mL. The functional outcomes were very good with reduction in the IPSS by 14.5 points and an increase in the Qmax by 37.75 mL/min The authors concluded that robotic simple prostatectomy is a feasible and reproducible procedure for symptomatic BPH [28]. In the same year, Yuh reported RASP using a technique similar to conventional Millin surgery [9]. Subsequently, RASP has become popular as another option for endoscopic prostatectomy. Many clinical studies and case series using RASP have been published, but all have a small sample size and non–comparative designs.
Port placement and surgical access in RASP are similar to those used in robotic–assisted radical prostatectomy [6]. After exposure of the retropubic space, the endopelvic fascia is opened bilaterally to expose the puboprostatic ligaments. The dorsal venous complex is ligated and access to the adenoma is achieved through the transvesical [28–31] or prevesical approach [9, 12]. In the prevesical approach, the anterior bladder neck is horizontally incised just proximal to the vesicoprostatic junction [6]. The plane between the adenoma and the prostatic capsule is identified and dissected, and the prostatic urethra is carefully transected to prevent external sphincter damage. Finally, the adenoma is removed and retrigonization is achieved by suturing the posterior edge of the bladder neck mucosa to the posterior edge of the urethra [12]. In another technique, a horizontal cystotomy of the prostatic capsule is made [28]. After removing the adenoma, some authors suggest a modification of retrigonization by folding the posterior prostatic capsule, suturing the anterior prostatic capsule to the anterior bladder wall, and performing a modified Van Velthoven continuous vesicourethral anastomosis [6].
Patients treated with RASP show significant increases in Qmax and reductions in IPSS after surgery. OT is usually slightly longer or comparable to that in LA, although in some reports, OT was >3 h. Blood loss has also been comparable to that reported in LA, and in most cases no blood transfusions were necessary [30, 32]. Hospital stays were short, and most patients were discharged 1–2 days after surgery (range 1–3.2 days) [6, 9, 30, 32]. Reported follow–up periods have been short and it is difficult to precisely define the long–term outcome and complications.
The laparoendoscopic single site (LESS) procedure is also used to remove prostatic adenomas [33, 34]. The initial procedures used classical laparoscopic instruments with modifications, including the use of a port device for pre– or transvesical access to the prostate [35–38]. To avoid the basic limitations of LESS, collision of the robot's arms and small operative space, single–site instruments designed for the da Vinci® Surgical System were used to perform single port transvesical enucleation of the prostate (STEP) [36, 37]. In recent years, several clinical studies and case reports have been published on LESS [35, 37–42]. Preliminary functional outcomes are encouraging, but the procedure is associated with a high risk of complications, and its role has yet to be determined [37].
CONCLUSIONS
Despite its recent development, LA has become a well–established option for the surgical treatment of BPH patients. LA is standardized and reproducible and offers good functional results and a minimal complication rate along with other benefits of minimally invasive surgery. RASP offers the benefits of robotic surgery, and a shorter hospital stay, faster recovery, and quicker return to work than LA. Additionally, robotic surgery offers a number of benefits including stereoscopic vision and 6 degrees of freedom. Longer terms studies of the functional outcomes, complications, and cost analysis of RASP will further define this procedure's place in the urological surgeon's armamentarium.
References
- 1.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel M, et al. Guidelines on the Management of Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO) European Association of Urology. 2012:45–48. [Google Scholar]
- 2.Chlosta PL, Varkarakis IM, Drewa T, Dobruch J, Jaskulski J, Antoniewicz AA, et al. Extraperitoneal laparoscopic Millin prostatectomy using finger enucleation. J Urol. 2011;186:873–876. doi: 10.1016/j.juro.2011.04.080. [DOI] [PubMed] [Google Scholar]
- 3.Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic Radical Prostatectomy for Localized Prostate Cancer: A Systematic Review of Comparative Studies. J Urol. 2011;175:2017. doi: 10.1016/S0022-5347(06)00265-5. [DOI] [PubMed] [Google Scholar]
- 4.Porpiglia F, Terrone C, Renard J, Grande S, Musso F, Cossu M, et al. Transcapsular adenomectomy (Millin): a comparative study, extraperitoneal laparoscopy versus open surgery. Eur Urol. 2006;49:120–126. doi: 10.1016/j.eururo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Slojewski M, Golab A, Gałęski M, Sikorski A. Laparoscopic adenomectomy in the treatment of benign prostatic hyperplasia. Urol Pol. 2008;61:48–54. [Google Scholar]
- 6.Coelho RF, Chauhan S, Sivaraman A, Palmer KJ, Orvieto MA, Rocco B, et al. Modified technique of robotic–assisted simple prostatectomy: advantages of a vesico–urethral anastomosis. BJU Int. 2012;109:426–433. doi: 10.1111/j.1464-410X.2011.010401.x. [DOI] [PubMed] [Google Scholar]
- 7.Porpiglia F, Fiori C, Cavallone B, Morra I, Bertolo R, Scarpa RM. Extraperitoneoscopic transcapsular adenomectomy: complications and functional results after at least 1 year of followup. J Urol. 2011;185:1668–1673. doi: 10.1016/j.juro.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Matei DV, Brescia A, Mazzoleni F, Spinelli M, Musi G, Melegari S, et al. Robot–assisted simple prostatectomy (RASP): does it make sense? BJU Int. 2012;110:E972–979. doi: 10.1111/j.1464-410X.2012.11192.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuh B, Laungani R, Perlmutter A, Eun D, Peabody JO, Mohler JL, et al. Robot–assisted Millin's retropubic prostatectomy: case series. Can J Urol. 2008;15:4101–4105. [PubMed] [Google Scholar]
- 10.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 11.Mariano MB, Tefilli MV, Graziottin TM, Morales CM, Goldraich IH. Laparoscopic prostatectomy for benign prostatic hyperplasia––a six–year experience. Eur Urol. 2006;49:127–131. doi: 10.1016/j.eururo.2005.09.018. disc 31–32. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland DE, Perez DS, Weeks DC. Robot–assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol. 2011;25:641–644. doi: 10.1089/end.2010.0528. [DOI] [PubMed] [Google Scholar]
- 13.Mariano MB, Graziottin TM, Tefilli MV. Laparoscopic prostatectomy with vascular control for benign prostatic hyperplasia. J Urol. 2002;167:2528–2529. [PubMed] [Google Scholar]
- 14.van Velthoven R, Peltier A, Laguna MP, Piechaud T. Laparoscopic extraperitoneal adenomectomy (Millin): pilot study on feasibility. Eur Urol. 2004;45:103–109. doi: 10.1016/j.eururo.2003.07.002. disc 9. [DOI] [PubMed] [Google Scholar]
- 15.Sotelo R, Spaliviero M, Garcia–Segui A, Hasan W, Novoa J, Desai MM, et al. Laparoscopic retropubic simple prostatectomy. J Uro. 2005;173:757–760. doi: 10.1097/01.ju.0000152651.27143.b0. [DOI] [PubMed] [Google Scholar]
- 16.Slojewski M, Petrasz P, Golab A, Leminski A, Sikorski A. “Finger–assisted” laparoscopic adenomectomy – a feasible method of removal of large prostatic adenomas. Eur Urol Meet. 2007;2:22. [Google Scholar]
- 17.Asimakopoulos AD, Mugnier C, Hoepffner JL, Lopez L, Rey D, Gaston R, et al. Laparoscopic treatment of benign prostatic hyperplasia (BPH): overview of the current techniques. BJU Int. 2011;107:1168–1182. doi: 10.1111/j.1464-410X.2011.10157.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou LY, Xiao J, Chen H, Zhu YP, Sun YW, Xuan Q. Extraperitoneal laparoscopic adenomectomy for benign prostatic hyperplasia. World J Urol. 2009;27:385–387. doi: 10.1007/s00345-008-0359-8. [DOI] [PubMed] [Google Scholar]
- 19.Rey D, Ducarme G, Hoepffner JL, Staerman F. Laparoscopic adenectomy: a novel technique for managing benign prostatic hyperplasia. BJU Int. 2005;95:676–678. doi: 10.1111/j.1464-410X.2005.05361.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia–Segui A, Gascon–Mir M. [Comparative study between laparoscopic extraperitoneal and open adenomectomy] Actas Urol Esp. 2012;36:110–116. doi: 10.1016/j.acuro.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Baumert H, Ballaro A, Dugardin F, Kaisary AV. Laparoscopic versus open simple prostatectomy: a comparative study. J Urol. 2006;175:1691–1694. doi: 10.1016/S0022-5347(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 22.Hoepffner J, Gaston R, Piechaud T, Rey D. Finger Assisted Laparoscopic Retropubic Prostatectomy (Millin) Eur Urol Suppl. 2006;6:962–967. [Google Scholar]
- 23.Rehman J, Khan SA, Sukkarieh T, Chughtai B, Waltzer WC. Extraperitoneal laparoscopic prostatectomy (adenomectomy) for obstructing benign prostatic hyperplasia: transvesical and transcapsular (Millin) techniques. J Endourol. 2005;19:491–496. doi: 10.1089/end.2005.19.491. [DOI] [PubMed] [Google Scholar]
- 24.Castillo OA, Bolufer E, Lopez–Fontana G, Sanchez–Salas R, Foneron A, Vidal–Mora I, et al. [Laparoscopic simple prostatectomy (adenomectomy): experience in 59 consecutive patients] Actas Urol Esp. 2011;35:434–437. doi: 10.1016/j.acuro.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Cathelineau X, Cahill D, Widmer H, Rozet F, Baumert H, Vallancien G. Transperitoneal or extraperitoneal approach for laparoscopic radical prostatectomy: a false debate over a real challenge. J Urol. 2004;171:714–716. doi: 10.1097/01.ju.0000103885.71434.02. [DOI] [PubMed] [Google Scholar]
- 26.Atug F, Castle EP, Woods M, Srivastav SK, Thomas R, Davis R. Transperitoneal versus extraperitoneal robotic–assisted radical prostatectomy: is one better than the other? Urology. 2006;68:1077–1081. doi: 10.1016/j.urology.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.McCullough TC, Heldwein FL, Soon SJ, Galiano M, Barret E, Cathelineau X, et al. Extraperitoneal laparoscopic prostatectomy (adenomectomy) for obstructing benign prostatic hyperplasia: transvesical and transcapsular (Millin) techniques. J Endourl. 2009;23:129–133. [Google Scholar]
- 28.Sotelo R, Clavijo R, Carmona O, Garcia A, Banda E, Miranda M, et al. Robotic simple prostatectomy. J Urol. 2008;179:513–515. doi: 10.1016/j.juro.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 29.John H, Bucher C, Engel N, Fischer B, Fehr JL. Preperitoneal robotic prostate adenomectomy. Urology. 2009;73:811–815. doi: 10.1016/j.urology.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Vora A, Mittal S, Hwang J, Bandi G. Robot–assisted simple prostatectomy: multi–institutional outcomes for glands larger than 100 grams. J Endourol. 2012;26:499–502. doi: 10.1089/end.2011.0562. [DOI] [PubMed] [Google Scholar]
- 31.Uffort E, Jensen J. Robotic–assisted laparoscopic simple prostatectomy: an alternative minimal invasive approach for prostate adenoma. J Robotic Surg. 2010;4:7–10. doi: 10.1007/s11701-010-0180-4. [DOI] [PubMed] [Google Scholar]
- 32.Banapour P, Patel N, Kane CJ, Cohen SA, Parsons JK. Robotic–assisted simple prostatectomy: a systematic review and report of a single institution case series. Prostate Cancer Prostatic Dis. 2014;17:1–5. doi: 10.1038/pcan.2013.52. [DOI] [PubMed] [Google Scholar]
- 33.Autorino R, Sosnowski R, De Sio M, Omero S, Khalifeh A, Kaouk J. Laparo–endoscopic single–site surgery: recent advances in urology. Cent European J Urol. 2012;65:204–211. doi: 10.5173/ceju.2012.04.art5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez–Salas R, Clavijo R, Barret E, Sotelo R. Laparoendoscopic single site in pelvic surgery. Indian J Urol. 2012;28:54–59. doi: 10.4103/0970-1591.94958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai MM, Berger AK, Brandina R, Aron M, Irwin BH, Canes D, et al. Laparoendoscopic single–site surgery: initial hundred patients. Urology. 2009;74:805–812. doi: 10.1016/j.urology.2009.02.083. [DOI] [PubMed] [Google Scholar]
- 36.Autorino R, Kaouk JH, Stolzenburg JU, Gill IS, Mottrie A, Tewari A, et al. Current status and future directions of robotic single–site surgery: a systematic review. Eur Urol. 2013;63:266–280. doi: 10.1016/j.eururo.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Fareed K, Zaytoun OM, Autorino R, White WM, Crouzet S, Yakoubi R, et al. Robotic single port suprapubic transvesical enucleation of the prostate (R–STEP): initial experience. BJU Int. 2012;110:732–737. doi: 10.1111/j.1464-410X.2012.10954.x. [DOI] [PubMed] [Google Scholar]
- 38.Desai MM, Aron M, Canes D, Fareed K, Carmona O, Haber GP, et al. Single–port transvesical simple prostatectomy: initial clinical report. Urology. 2008;72:960–965. doi: 10.1016/j.urology.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Desai MM, Fareed K, Berger AK, Astigueta JC, Irwin BH, Aron M, et al. Single–port transvesical enucleation of the prostate: a clinical report of 34 cases. BJU Int. 2010;105:1296–1300. doi: 10.1111/j.1464-410X.2009.09106.x. [DOI] [PubMed] [Google Scholar]
- 40.Rao PDM, Sotelo R, Rao P, Liu C. Hybrid LESS Prostatectomy for BPH: A Combined Technique. Urology. 2011;78:S64. [Google Scholar]
- 41.Sotelo RJ, Astigueta JC, Desai MM, Canes D, Carmona O, De Andrade RJ, et al. Laparoendoscopic single–site surgery simple prostatectomy: initial report. Urology. 2009;74:626–630. doi: 10.1016/j.urology.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Oktay B, Vuruskan H, Koc G, Danisoglu ME, Kordan Y. Single–port extraperitoneal transvesical adenomectomy: initial operative experience. Urol Int. 2010;85:131–134. doi: 10.1159/000314896. [DOI] [PubMed] [Google Scholar]