Abstract
The 2009/10 pandemic (pH1N1) highlighted the need for vaccines conferring heterosubtypic immunity against antigenically shifted influenza strains. Although cross-reactive T cells are strong candidates for mediating heterosubtypic immunity, little is known about the population-level prevalence, frequency, and cytokine-secretion profile of heterosubtypic T cells to pH1N1. To assess this, pH1N1 sero-negative adults were recruited. Single-cell IFN-γ and IL-2 cytokine-secretion profiles to internal proteins of pH1N1 or live virus were enumerated and characterised. Heterosubtypic T cells recognising pH1N1 core proteins were widely prevalent, being detected in 90% (30 of 33) of pH1N1-näive individuals. Although the last exposure to influenza was greater than 6 months ago, the frequency and proportion of the IFN-γ-only-secreting T-cell subset was significantly higher than the IL-2-only-secreting subset. CD8+ IFN-γ-only-secreting heterosubtypic T cells were predominantly CCR7−CD45RA− effector-memory phenotype, expressing the tissue-homing receptor CXCR3 and degranulation marker CD107. Receipt of the 2008–09 influenza vaccine did not alter the frequency of these heterosubtypic T cells, highlighting the inability of current vaccines to maintain this heterosubtypic T-cell pool. The surprisingly high prevalence of pre-existing circulating pH1N1-specific CD8+ IFN-γ-only-secreting effector memory T cells with cytotoxic and lung-homing potential in pH1N1-seronegative adults may partly explain the low case fatality rate despite high rates of infection of the pandemic in young adults.
Keywords: Heterosubtypic immunity, Influenza vaccines, Pandemic influenza, T-cell memory
Introduction
Despite the successful development of strain-specific influenza vaccines, influenza continues to be a major public health problem affecting an estimated 15% of the global population [1]. Infection results in protective neutralizing antibodies specific to the surface glycoproteins (haemagglutinin and neuraminidase) of the infecting strain. Pandemics occur when genetic reassortments between influenza viruses leads to the emergence of a new antigenically shifted strain infecting a susceptible näive population lacking protective humoral immunity to the new reassortant strain.
During pandemics, such as with the pandemic H1N1 (pH1N1) strain [2], cross-reactive immune responses to conserved regions of the new pandemic strain generated through prior exposure to a different virus subtype may provide some heterosubtypic immunity [3,4]. The role of cross-reactive T-cell-mediated heterosubtypic immunity is well documented in animal models [5-7] and is associated with decreased viral shedding following experimental challenge in humans [8-10]. The recent recognition of decreased severity of pandemic influenza in young adults with prior seasonal H1N1 infection in the absence of cross-reactive antibodies provides further compelling but circumstantial evidence for the protective role of heterosubtypic T cells [11].
However, little is known about naturally occurring heterosubtypic cellular immunity in humans. Although cross-reactive memory T cells to pH1N1 have recently been demonstrated in a small number of individuals [12-15], there are no data on the prevalence, population-level frequency and functional profile of cytokine-secreting memory T cells to influenza A virus. Moreover, the durability of circulating heterosubtypic T cells after infection and vaccination is currently unknown.
Antigen-specific T cells can be categorised by distinct functional cytokine profiles into three key memory T-cell subsets [16]. In view of the central importance of these key functional T-cell subsets in protective immunity, viral clearance and vaccine development [16,17], defining their respective contributions to heterosubtypic immunity to influenza is a priority. However, the low frequency of influenza-specific cross-reactive memory T cells precludes their reliable quantification by conventional flow-cytometry-based single-cell cytokine profiling. Measurement of influenza-specific memory T cells has therefore largely relied upon the IFN-γ ELISPOT assay which, though sensitive for detecting low-frequency responses, provides no information on the polyfunctional nature of the T-cell response. We therefore exploited the high sensitivity of the immunospot platform, combining it with fluorophore-tagged anti-cytokine antibodies to simultaneously measure IFN-γ and IL-2 secretion at the single cell level [18] in order to evaluate the functional cytokine signature of heterosubtypic memory T cells to pH1N1.
Influenza is an acute infection with rapid antigen clearance [16,19]. In our study population where circulation of influenza A virus ceased greater than 6 months prior to recruitment [20], we hypothesised that pH1N1-specific T cells would be predominantly IL-2 cytokine secreting, corresponding to a shift from functionally defined effector to central memory cells following resolution of infection and antigen clearance [16].
The emergence of the pH1N1 strain provided a unique opportunity to study heterosubtypic immunity to influenza. We therefore recruited pH1N1 sero-negative healthy individuals prior to the onset of the second wave of the pandemic in the UK to specifically investigate heterosubtypic T cells to pH1N1 virus and its three most immunodominant and conserved proteins: polymerase basic protein 1 (PB1), nucleoprotein (NP) and matrix protein 1 (M1) [15,21]. Our investigation of heterosubtypic T-cell memory to pH1N1 is in the largest group of individuals studied to date providing the first population-level comprehensive data on the prevalence, frequency and cytokine-secretion profile of pH1N1 cross-reactive memory T cells.
Results
Cohort characteristics
The 33 participants had a median age of 30 years (range 18–73 years). Haemagglutination inhibition assay to detect pH1N1-specific antibodies undertaken independently at two different national laboratories was negative in all individuals. None were vaccinated for pH1N1 at the time of recruitment and only one individual gave a history of flu-like illness in the preceding three months prior to recruitment.
High prevalence of IFN-γ-only-secreting cross-reactive memory T cells to pH1N1 internal proteins
Based on the model of influenza as an acute infection with rapid antigen clearance [16], we hypothesised that our cohort of pH1N1-naive individuals unexposed to influenza antigen for greater than 6 months (since February 2009) when assessed for cross-reactive T cells to three core proteins (PB1, M1 and NP) of the pH1N1 virus would have memory T cells with a cytokine signature that would be predominantly IL-2 secreting.
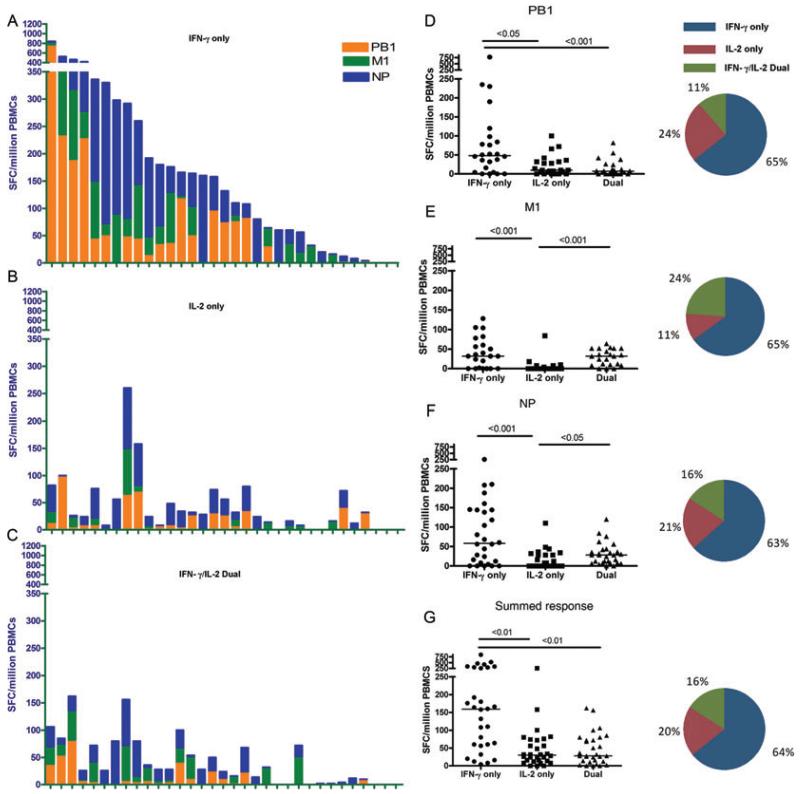
T-cell responses measured with fluorescence-immunospot to one or more of the three core proteins of pH1N1 were found in 30 out of 33 participants (91%; 95% CI 75.7 – 98.1) (Fig. 1A–C). All the individuals with detectable T-cell responses had IFN-γ-only-secreting heterosubtypic T cells to core proteins of pH1N1 (Fig. 1A), while 27/33 (81%) individuals had detectable IL-2 only (Fig. 1B) and IFN-γ/IL-2 dual (Fig. 1C) secreting T cells, respectively.
Figure 1. Pre-existing cross-reactive memory T-cell responses to pH1N1 core proteins in pH1N1 sero-negative individuals.
Total magnitude of ex vivo PBMC responses from the (A) IFN-γ-only, (B) IL-2-only, and (C) IFN-γ/IL-2 dual cytokine-secreting subsets to overlapping peptide pools of PB1, M1 and NP of pH1N1 (A/California/04/09) virus in each individual was measured by fluorescence immunospot. Individuals are shown in the same order in (A through C) (n = 33). PB1: polymerase basic protein 1, M1: matrix protein 1, NP: nucleoprotein, SFCs: spot forming cells, PBMCs: peripheral blood mononuclear cells. Haemagglutination inhibition assay was performed to confirm sero-negativity to H1 of A/England/195/09 and A/California/04/09. Magnitude of ex vivo PBMC responses from the IFN-γ only, IL-2 only and IFN-γ/IL-2 dual-secreting subsets to overlapping peptide pools of (D) PB1, (E)M1, (F) NP, and (G) the summed response to PB1, M1 and NP of pH1N1 (A/California/04/09) virus. Each symbol represents a single individual and horizontal lines represent the median response. Differences between subset responses were estimated by Kruskall-Wallis test. Pie charts represent mean proportions of cytokine-secreting responses. Non-responders to antigens excluded, PB1: n = 24, M1: n = 21, NP: n = 28, All antigens: n = 30.
The frequency of the total T-cell response summed to all three proteins, NP, PB1 and M1, was significantly higher (p<0.01) in the IFN-γ-only-secreting (median 159 SFC (spot forming cell)/million peripheral blood mononuclear cells (PBMCs); IQR: 58–295) compared to the IL-2-only (median 30 SFC/million; IQR: 13–73) and IFN-γ/IL-2 dual (median 28 SFC/million; IQR: 11–76) cytokine-secreting T-cell subsets (Fig. 1G) and significantly higher for each individual protein (Fig. 1D–F).
The relative proportion of the IFN-γ-only-secreting subset (64% of total IFN-γ/IL-2 cytokine-secreting cells) was significantly higher compared to the IL-2-only and IFN-γ/IL-2 dual-secreting subset (Fig. 1, Supporting Information Fig. 1A–D) for each of the three core proteins. However, despite this predominance of IFN-γ-only-secreting T-cell subset in our cohort, we observed individual-level heterogeneity in the proportions of the cytokine-secreting T-cell subsets. In approximately 25% of individuals, the predominant cytokine-secreting T-cell subsets were IL-2 only or IFN-γ/IL-2 dual cytokine secreting (Supporting Information Fig. 1E–H).
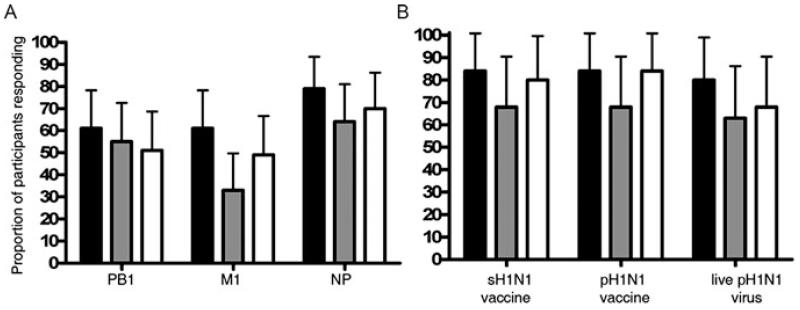
The prevalence of cross-reactive T cells was highest to the NP protein (79%) followed by PB1 (61%) and M1 (61%) proteins (Fig. 2A) consistent with the reported number of predicted epitopes conserved between seasonal and pH1N1 strains in each of these three internal proteins [15]. There was a similarly high proportion (80%) of individuals with cross-reactive T cells responses to live pH1N1 virus, pH1N1 vaccine strain and the sH1N1 vaccine strain (Fig. 2B).
Figure 2. Prevalence of pH1N1 cross-reactive memory T-cell responses.
The proportion of pH1N1 sero-negative individuals with ex vivo PBMC responses to overlapping peptide pools of (A) PB1, M1 and NP of pH1N1 (A/California/04/09) and (B) sH1N1 vaccine strain, pH1N1 vaccine strain and live pH1N1 virus from IFN-γ-only (black bars), IL-2-only (grey bars) and IFN-γ/IL-2 dual (white bars) cytokine-secreting subsets (n = 33) was evaluated by fluorescence immunospot. Each bar represents the average proportion and error bars represent upper 95% confidence interval.
Cross-reactive memory T cells recognising live pH1N1 virus predominantly secrete only IFN-γ
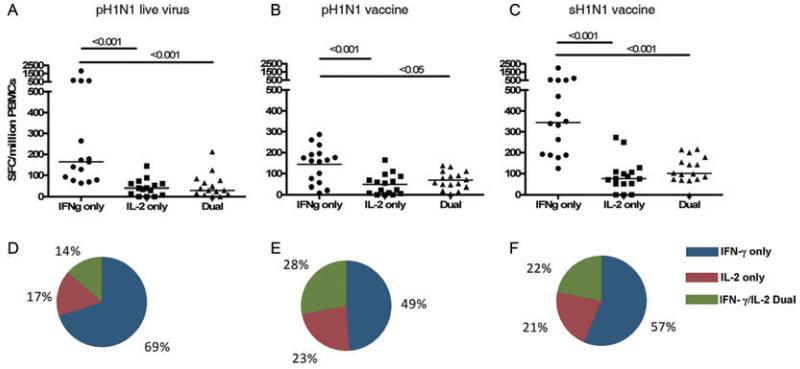
We assessed cross-reactive T-cell memory in 19 of our 33 pH1N1 sero-negative individuals, in whom cryopreserved PBMCs remained after responses to core proteins were measured, to live pH1N1 virus (A/England/195/09) and the inactivated reassortant virus strain used to manufacture the pH1N1 vaccine to confirm whether influenza-specific memory T cells that recognise synthetic peptides also recognise naturally processed peptides following infection of antigen-presenting cells (APCs) with live virus and recombinant viral proteins, respectively.
Despite absence of prior exposure to the pH1N1 virus or pH1N1 vaccine, the majority of individuals had memory T cells that recognised naturally processed peptides presented by APCs infected with live pH1N1 virus (16/19, 84%) or the pandemic vaccine strain (15/19, 79%). Similar to responses specific to core proteins of pH1N1, the frequency and proportion of antigen-specific T-cell responses to naturally processed antigens of pH1N1 virus was dominated by the IFN-γ-only cytokine-secreting subset (Fig. 3A, B, D, E). The median frequency of the IFN-γ-only-secreting T-cell response to live pH1N1 virus was 164 SFC/million (IQR: 78–620) and significantly greater than frequency of the IL-2-only (median 40 SFC/million (IQR: 8–60)) and IFN-γ/IL-2 dual-secreting subsets (median 30 SFC/million (IQR: 12–76)).
Figure 3. Cross-reactive memory T-cell responses to naturally processed pH1N1 epitopes.
The magnitude of ex vivo PBMC responses from the IFN-γ only, IL-2 only, and IFN-γ/IL-2 dual cytokine-secreting subsets to (A) pH1N1 live virus (A/England/195/2009), (B) pH1N1 vaccine strain (A/California/07/09, NYMCX-179A) and (C) sH1N1 vaccine strain (A/Brisbane/10/2007, IVR-148) in pH1N1 sero-negative individuals was determined by fluorescence immunospot. Each symbol represents a single individual and horizontal lines represent medians. Pie charts represent mean proportions of cytokine-secreting responses to (D) pH1N1 vaccine strain, (E) pH1N1 live virus and (F) sH1N1 vaccine strain. Differences between subset responses were estimated by Kruskall-Wallis test. Non-responders to stimulation excluded, pH1N1 vaccine n = 16, pH1N1 live virus n = 15, sH1N1 vaccine = 16.
We investigated whether this predominance of IFN-γ-only-secreting T cells was restricted to cross-reactive responses by stimulating PBMCs with inactivated sH1N1 vaccine strain, which was the virus strain circulating prior to the emergence of pH1N1 strain. Although T-cell responses to sH1N1 vaccine strain were also predominantly of a IFN-γ-only-secreting subset (Fig. 3C and F), the frequency of T-cell responses to sH1N1 vaccine strain was significantly higher (p<0.05) than T-cell frequencies to live pH1N1 virus and inactivated pH1N1 vaccine strain, as would be expected given the greater exposure of individuals to the pre-pandemic circulating sH1N1 strain. However, there was no difference in the relative proportions of cytokine-secreting T-cell subsets of the homosubtypic response to sH1N1 virus strain compared to heterosubtypic responses to pH1N1 live virus and inactivated virus strain (Fig. 3D–F).
IFN-γ-only-secreting pH1N1-specific T cells are predominantly of an effector memory phenotype
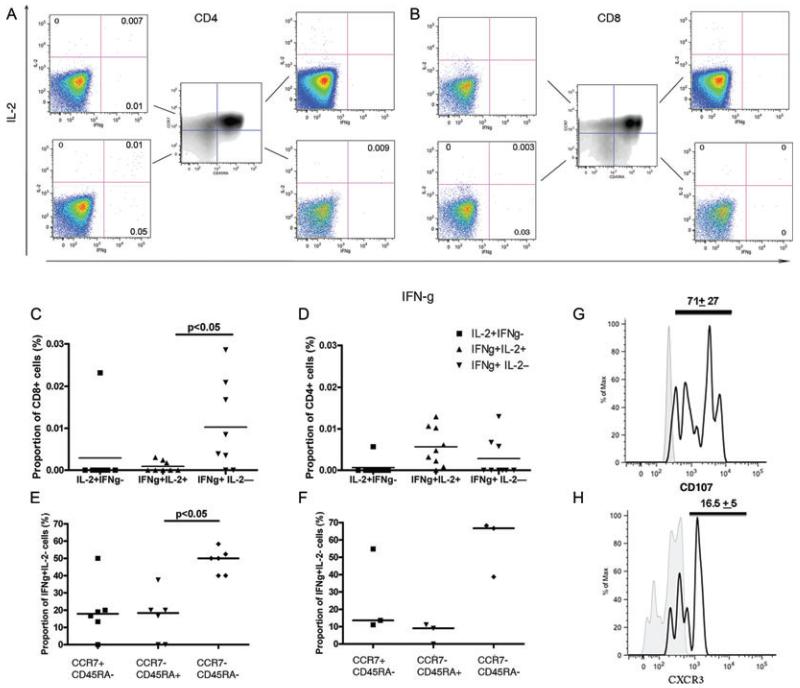
Given the predominance of IFN-γ-only-secreting T cells following stimulation of PBMCs with peptides, live virus and inactivated virus, we comprehensively characterised the phenotype of the IFN-γ-only-secreting influenza-specific T cells with flow cytometry. In 12 individuals chosen for a positive response to live pH1N1 virus on fluorescence immunospot (median 285 SFCs/million, range 64–424) and available PBMC sample, antigen-specific cytokine responses above background were detected by flow cytometry and intracellular cytokine staining in only 8 out of the 12 individuals.
The influenza virus-specific CD3+CD8+ T-cell response was predominantly IFN-γ-only-secreting (average proportion 0.01% of CD8+ T cells). CD3+CD8+ T cells secreting IFN-γ but not IL-2 were in significantly higher frequency (p<0.05) and more prevalent (6/8 individuals had antigen-specific response) compared with CD8+ T cells that were dual IFN-γ+IL-2+ secreting (Fig. 4C). In contrast, although the frequency of influenza virus-specific CD3+CD4+ T cells was similar between IFN-γ+IL-2− and dual IFN-γ+IL-2+ CD4+ cells, more individuals (7/12) had dual IFN-γ+IL-2+ CD4+ T cells compared with only 3/12 individuals with an IFN-γ+IL-2− CD4+ T-cell response (Fig. 4D).
Figure 4. pH1N1-specific cross-reactive memory IFN-γ+IL-2− CD4+ and CD8+ T cells are predominantly effector memory.
PBMCs stimulated with live pH1N1 virus or allantoic fluid (unstimulated) for 16 h were stained for surface markers of memory and intracellular cytokines. PBMCs were gated on live, single cells, lymphocytes, CD3+, CD4+ or CD8+. The frequency of IL-2 and IFN-γ secreting cells was analysed separately for CD4+ and CD8+ cells in each pH1N1 sero-negative donor. (A-B) A representative example from one pH1N1 sero-negative donor stimulated with pH1N1 live virus (A/England/195/2009) is shown. Gates for CD45RA and CCR7 were identified from FMO control samples. PBMCs were gated on (A) CD3+CD4+CD8− or (B) CD3+CD4−CD8+ lymphocytes, cells stained for CD45RA and CCR7 in each quadrant are shown in central plot. Frequency of IFN-γ and IL-2 secretion for the different memory subsets based on CD45RA and CCR7 expression is shown. Number in the plot represents the frequency of cytokine-secreting cells in the stimulated sample minus the negative control sample. (C-D) Frequency of IFN-γ and IL-2 secretion for each cytokine subset for (C) CD8+ and (D) CD4+ cells in 12 individuals is shown. Individual responses are represented by symbols and horizontal lines represent median responses. Differences between groups were analysed by Kruskall-Wallis test accounting for multiple comparisons. (E-F) Proportion of (E) CD8+ and (F) CD4+ IFN-γ+IL-2− T cells that were effector memory (CCR7−CD45RA−), effector (CCR7−CD45RA+) and central memory (CCR7+CD45RA−) phenotype was determined by flow cytometry. Responses for individuals with a positive IFN-γ+IL-2− CD8+ (six individuals) and CD4+ (three individuals) T-cell response (C and D) are represented by symbols and horizontal lines represent median responses. Differences between groups were analysed by Kruskall-Wallis test accounting for multiple comparisons. (G) Expression of CD107 on CD8+ IFN-γ-only-secreting T cells. Histogram shows the staining of CD107 on stimulated cells (black line histogram) compared to unstimulated (grey-filled histogram) for one sample representative of 12 individuals. The number represents the average proportion and standard deviation of CD107+ cells from 11 individuals. (H) Representative example of expression of lung trafficking receptor CXCR3 on CD8+ IFN-γ-only-secreting cells stimulated with live pH1N1 virus. Histogram shows the staining of CXCR3 on stimulated cells (black line histogram) compared to fluorescence minus one control (grey-filled histogram). Number at the top of the histogram represents the average proportion and standard deviation of CD8+ IFN-γ-only-secreting cells that are CXCR3+. Example shown is representative of four individuals.
Further phenotypic investigation for memory surface markers to determine the memory phenotype of our IFN-γ-only-secreting CD4+ and CD8+ population [22] revealed that IFN-γ-only-secreting CD4+ and CD8+ T cells were predominantly of an effector memory CD45RA−CCR7− population (Fig. 4E and F).
In light of our finding of high prevalence of circulating rapidly activated pH1N1-specific CD8+ T cells that are suggested to offer some protection against severe pandemic influenza [15], we investigated their degranulating potential using CD107a and CD107b [23] and migratory capacity to the site of the lung inflammation with CXCR3 [24]. A large proportion of influenza-specific IFN-γ-only-secreting CD8+ T cells (average 80%) expressed CD107 on their surface (Fig. 4G). We found live pH1N1 virus stimulated IFN-γ-only-secreting CD8+ T cells (Fig. 4H) to express CXCR3 (average 16% of IFN-γ-only-secreting CD8+ cells), which taken together with the ability to undergo granulocytosis suggests potential to traffic to the respiratory airways upon influenza infection to initiate viral clearance.
Seasonal influenza vaccination does not maintain cross-reactive T-cell memory
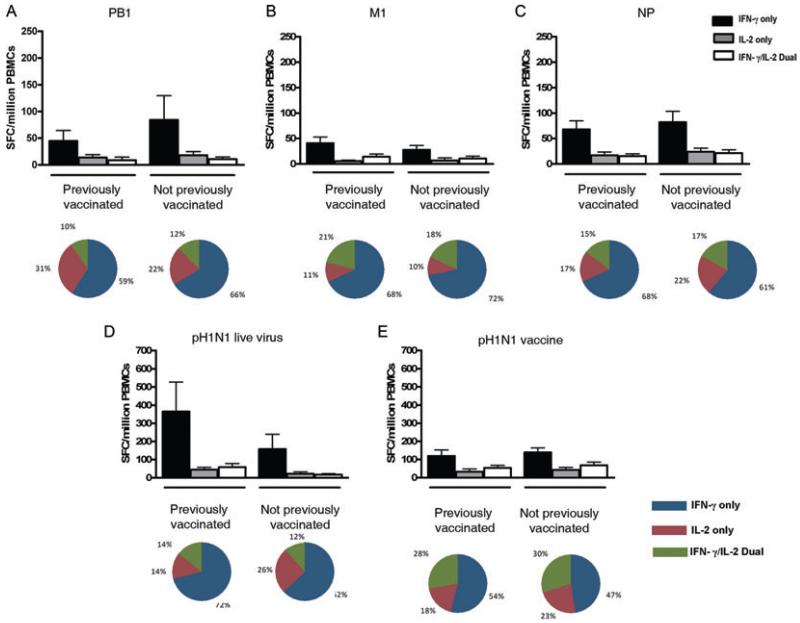
Seasonal influenza vaccines have been shown to induce cross-reactive T-cells to pH1N1 up to 1 month post-vaccination [13], but do not maintain cross-reactive antibody responses to the pandemic virus [25]. Whether they maintain cross-reactive T-cell memory to pH1N1 is unknown. Of our 33 pH1N1 sero-negative individuals, 14 (58%) reported seasonal influenza vaccination in 2008. We found no difference in the magnitude (Fig. 5A–C) or the functional profile of cytokine-secreting T-cell subsets to PB1, NP and M1 antigens, pH1N1 live virus and pH1N1 vaccine strain (Fig. 5D and E) between previously vaccinated and unvaccinated individuals.
Figure 5. Previous influenza vaccination does not affect magnitude or cytokine-secreting profile of cross-reactive T-cell memory responses to pH1N1.
(A–C) Magnitude of ex vivo PBMC responses from the IFN-γ only, IL-2 only and IFN-γ/IL-2 dual-secreting subsets to overlapping peptide pools of (A) PB1, (B) M1, and (C) NP of pH1N1 (A/California/04/09) virus, (D) pH1N1 live virus or (E) pH1N1 vaccine strain in pH1N1 sero-negative individuals among those who reported having the 2008 seasonal influenza vaccine (n = 14) and those who did not have the vaccine (n = 17) as determined by fluorescence immunospot. Bars show mean responses with standard error of the mean. Pie charts represent mean proportions of cytokine-secreting responses.
Discussion
Our investigation of heterosubtypic memory to pH1N1 in a cohort of pH1N1-naive healthy young adults identified circulating pre-existing memory T cells to at least one of the three immunodominant pH1N1 core proteins (PB1, M1 and NP) in 90% of individuals. Moreover, this first cohort-level assessment of the functional profile of influenza-specific heterosubtypic T-cell memory revealed an unexpected predominance in the frequency, proportion and prevalence of IFN-γ-only-secreting T cells.
T-cell-derived IFN-γ is an important contributor to antiviral immunity in influenza [26-28] with IFN-γ demonstrated to mediate influenza viral clearance and protection against pathology in animal models [29,30]. Influenza-specific cytotoxic T cells have been shown to reduce viral shedding but not prevent infection [8,31] and peptide-specific memory T cells that are rapidly activated to produce IFN-γ have been identified in humans [28]. We found a remarkably high prevalence of cross-reactive IFN-γ-only-secreting T cells specific for pH1N1 core proteins and live virus in our cohort of young healthy pH1N1-naive adults using fluorescence-immunospot assay and flow cytometry. The high proportion of conserved epitopes in the immunodominant core proteins between seasonal and pandemic influenza virus may in part explain the high prevalence of cross-reactive T cells we observed to peptides from pH1N1 M1, NP and PB1 as well as live pH1N1 [15]. Phenotypic analysis of these pH1N1-specific CD4+ and CD8+ IFN-γ-only-secreting T cells revealed that they predominantly comprised CD45RA−CCR7− effector-memory cells, consistent with recent reports of pH1N1-specific T cells [13,15], but in contrast to previous reports of a predominance of central memory cells [21,32]. Experiments in mice have shown that circulating effector memory CD8+ T cells generated following a respiratory viral infection play a vital role in recall responses in the lung following secondary respiratory infection [33] and these effector memory T cells also mediate protection against other intracellular pathogens [34,35]. We therefore analysed the cytotoxic potential of these influenza-specific IFN-γ-only-secreting cells. The majority of pH1N1-specific cross-reactive CD8+ IFN-γ-only-secreting T cells expressed the degranulation marker CD107 on their surface, similar to a recent paper reporting a similar predominance of influenza epitope-specific CD107a+CD8+ T cells [36]. Interestingly, a proportion of these CD8+ IFN-γ-only-secreting T cells also displayed upregulated expression of CXCR3, a tissue-homing receptor associated with T-cell trafficking to the lung. To our knowledge, there are only two other reports measuring CXCR3 expression on influenza-specific CD8+ T cells in humans [13,37], both of which reported M1-epitope-specific responses in contrast to our study reporting global CD8+ T cells to live virus.
Our findings indicate the presence of pre-existing heterosubtypic effector memory T cells in the peripheral blood of healthy adults with the potential to traffic to the lung during the inflammatory response following an influenza infection, undergo rapid degranulation and secrete IFN-γ on encounter with pH1N1-infected host cells. These attributes are consistent with a role in local viral containment or clearance and limiting the severity of symptoms following influenza infection. Epidemiological observations during the pandemic revealed high rates of infection, associated with low prevalence of cross-reactive antibodies, but low case fatality rates in adults (<45 years) suggestive of protective heterosubtypic immunity against severe disease [38]. Our data, for the first time, reports the high prevalence of heterosubtypic CD8+ IFN-γ-only-secreting T cells in pH1N1 sero-negative adults, which taken together with other recent studies [13,15] provides key evidence to support the notion that heterosubtypic CD8+ cellular immunity may have in part accounted for the decreased case fatality rate in adults during the 2009–2010 pandemic. Although, alternative reasons such as a less pathogenic pandemic strain and lower comorbidities cannot be ruled out, recent evidence of the association between pre-existing T cells and limited illness severity following experimental influenza challenge study in humans [9] adds further weight to the notion that heterosubtypic T cells may limit disease severity during pandemics but this remains to be demonstrated.
Cross-reactive immune memory develops as a result of previous exposure to influenza antigen, either through infection or vaccination. Although, seasonal vaccination does not maintain cross-reactive antibodies to pH1N1 [25], the effect on pH1N1-specific cross-reactive T-cell memory is little investigated. Influenza vaccination induces cross-reactive CD8+ T cells for dominant epitopes detectable 1 month later, but the durability of these responses is not known [13,39]. Our findings using a case-control study design, with its inherent limitations, imply that currently available inactivated influenza vaccines may be unable to maintain (beyond 6 months post-vaccination) a cross-reactive circulating memory T-cell pool to pH1N1. These findings in conjunction with existing literature [40,41] suggest that while infection and vaccination can both induce heterosubtypic T cells, only influenza infection is able to maintain durable cross-reactive T-cell responses to influenza. However, only a longitudinal cohort study following individuals after vaccination to compare pre- and post-vaccination heterosubtypic T-cell responses over time could confirm or refute this hypothesis.
IFN-γ and IL-2 cytokine-secretion profiles of T cells can help differentiate effector and memory T-cell populations [42]. More recently, a paradigm for different models of viral infection based on antigen load and exposure reflected by distinct functional cytokine signatures of T cells has been proposed [16]. In this paradigm, a predominantly IFN-γ secreting T-cell signature is associated with conditions of acute antigen exposure or uncontrolled chronic persistent infections (e.g. HIV) while a predominant IL-2 secreting T-cell signature is associated with acute cleared infections (e.g. influenza) [17,43,44].
In view of the current model of influenza [16,19] and considering that circulation of influenza A virus in our study population ceased by the end of February 2009 (>6 months prior to recruitment) [20], we expected to observe a predominant IL-2 or predominant dual IFN-γ/IL-2 cytokine-secreting T-cell response corresponding to a shift from effector to memory cells following resolution of infection and antigen clearance [16]. Our finding of predominance of IFN-γ-only-secreting cells, a signature of effector T-cell populations associated with acute infection and high-antigen load, [17,45] was surprising.
We therefore investigated whether this unexpected finding might have been an artefact of our experimental system. A predominance of peptide-specific IFN-γ-only-secreting T cells from a PBMC population might reflect relative predominance of CD8+ T cells inherently more efficient than CD4+ T cells at producing IFN-γ. However, regardless of the mode of antigen stimulation, whether PBMCs were stimulated with live virus to mimic naturally processed antigens or inactivated virus to simulate processing of recombinant proteins for preferential recognition by CD4+ T cells, the heterosubtypic response was dominated by IFN-γ-only-secreting T cells. Further, comparison of the magnitude of CD4+ and CD8+ T cells secreting IFN-γ-only on flow cytometry showed no significant difference. The predominance of IFN-γ-only-secreting T cells to homosubtypic sH1N1 and heterosubtypic pH1N1 suggests a phenomenon generally applicable to influenza A viruses. A recent report also found a similar predominance of IFN-γ-only-secreting influenza virus-specific CD8+ T cells [46]. The possibility that fluorescence-immunospot assay preferentially enumerates IFN-γ rather than IL-2 thereby biasing our results is also unlikely given that this assay revealed a predominance of IFN-γ/IL-2 dual-secreting T cells in response to Mycobacterium tuberculosis antigens in patients with latent TB infection [18].
The surprising predominance of IFN-γ-only-secreting T cells seems real. One possible explanation for this unexpected finding may be that influenza, like other acute respiratory viral infections, causes repeated infections and cumulative antigen exposure over a lifetime in humans. Murine models of repeated acute infections demonstrate that each repeated antigen exposure results in a preferential diminution of antigen-specific memory T cells secreting IL-2 without a concomitant decrease in IFN-γ-secreting cells along with a movement of memory cells to non-lymphoid compartments including the blood and peripheral organs [47,48]. This implies that each antigen exposure increases the proportion of peripherally circulating antigen-specific IFN-γ-only-secreting to IL-2-only-secreting memory T cells, consistent with our findings in humans. Despite recurrent acute infections being the commonest form of infection, our model of cumulative antigenic exposure from multiple repeated acute infections is hitherto undescribed in humans, in contrast to other well-defined models of acute, chronic and latent infections [16,49,50]. Our conclusions suggest that recurrent acute infections skew development of antigen-experienced memory T-cells toward an activated circulating IFN-γ-only-secreting effector memory functional subset primed to protect against inevitable subsequent infections, analogous to what has been observed following respiratory viral infections in mice [51].
If our model of influenza infection as one of increasing cumulative antigen exposure is appropriate, it follows that fewer antigen exposures or remote encounters would be associated with an increased proportion of IL-2-secreting cells. Interestingly, we identified a subgroup of individuals (approximately 25% of the cohort) in whom the proportion of antigen-specific IL-2-only and IFN-γ/IL-2-dual cytokine response was higher than the IFN-γ-only response. Ascertaining whether an immunological profile of influenza-specific memory dominated by IL-2-only or IFN-γ/IL-2-dual cytokine-secreting T cells reflects few remote influenza exposures while an IFN-γ-only dominant profile marks multiple repeated antigen exposures will require a long-term immuno-epidemiological follow-up of individuals over multiple influenza seasons. Alternatively, given our current lack of knowledge of the time required for development of T-cell memory as manifested by development of IL-2-secreting T cells in the setting of natural viral infection in humans, our findings may instead reflect a slow and variable kinetic for the shift from IFN-γ-secreting effector to IL-2-secreting memory T-cell predominance following influenza infection in humans.
In summary, our study, the first to report the prevalence of cytokine-secreting heterosubtypic cellular immune responses to influenza, revealed a high prevalence of pH1N1-reactive T cells and a surprising predominance of IFN-γ-only-secreting T cells in pH1N1 sero-negative adults. This novel immunological observation offers a hint toward an additional model of recurrent acute viral infections in humans that progressively biases development of T-cell memory toward an effector memory IFN-γ-only-secreting population, as observed in murine models. If heterosubtypic T-cell populations do indeed mediate protection against symptoms and disease severity, the high prevalence of IFN-γ-only-secreting effector memory CD8+ T cells with lung homing and cytotoxic potential may possibly explain key epidemiological observations of the current pandemic.
Materials and methods
Study population
Healthy adult (>18 years) staff and students at Imperial College London were recruited after providing written informed consent between September and November 2009. Individuals likely to be vaccinated with the pandemic influenza vaccine were excluded. Frontline healthcare workers, pregnant women, individuals with asthma, diabetes or chronic respiratory disease and individuals on immunosuppressive medication were specifically excluded. No participants were vaccinated with pandemic H1N1 influenza vaccine at the time of recruitment. Demographic information, information on risk factors for acquiring influenza infection and history of previous influenza vaccination were recorded at the time of recruitment. This study was approved by the North West London Research Ethics Committee on 9th September 2009 (study reference number 09/H0724/27).
Sample collection and processing
Blood was collected for isolation of PBMCs and serum. PBMCs were isolated by Ficoll-Paque PLUS (Amersham Biosciences) density centrifugation, washed twice in RPMI-1640 (Sigma-Aldrich) and suspended in RPMI-1640 supplemented with 10% foetal calf serum (Invitrogen). PBMCs were cryopreserved in heat-inactivated foetal calf serum supplemented with 10% DMSO (Sigma-Aldrich) at −180°C in liquid nitrogen. All assays were undertaken using cryopreserved PBMCs.
Virus, vaccines and peptides
Fifteen-mer overlapping peptides (overlapping by 10 aa) representing the entire length of NP, M1 and PB1 of A/California/04/09 virus strain were synthesised (Pepceuticals, Oxford, UK). The A/California/04/09 is the reference strain for the pandemic H1N1 virus and was isolated at the start of the 2009–2010 pandemic. Peptide pools with 25 peptides per pool were prepared with each peptide at a final concentration of 5 μg/well. Live pH1N1 virus was obtained by growing A/England/195/09 strain in eggs. The inactivated reassortant strain NYMCX-179A and IVR-148 used for making the pH1N1 vaccine and sH1N1 vaccine, respectively in 2009–2010 were purchased from NIBSC, UK.
Fluorescence immunospot
The fluorescence-immunospot assay was used to enumerate T cells expressing IFN-γ and IL-2 cytokines and a detailed protocol has been previously described [18]. PBMCs (250,000 cells/well in 120 μL) were stimulated with only media (negative control), 5 μg/mL PHA (positive control), overlapping peptide pools, live pH1N1 virus (MOI = 1), mock-infected allantoic fluid (negative control when live pH1N1 virus was used), pH1N1 vaccine strain or sH1N1 vaccine strain (10 μg/well) for 18 h. SFCs were counted using an ELISPOT IFL-reader (AID iSpot, Autoimmun Diagnostika GmbH). Size and intensity settings of the counted cells were predefined and unchanged throughout.
Individuals were deemed as responders if the IFN-γ and IL-2 antigen-specific response was greater than 20 and 28 SFC/million PBMCs, respectively and greater than two times the average of the individual’s negative control well. These cut-offs equate to 2 standard deviations of the mean value of the background wells in 62 independent observations generated from this study and therefore encompass 95% of standard variation for a negative result. For antigens where either the IFN-γ or IL-2 response was deemed to have been positive, the frequency of antigen-specific response was calculated by subtracting the frequency of response in the antigen well from the average frequency of the negative-control wells. The magnitude of response to each individual protein (i.e. NP, PB1 and M1) was calculated as the sum total of the antigen-specific response of all pools containing only peptides corresponding to each individual protein.
Flow cytometry
PBMCs were stimulated with mock-infected allantoic fluid (negative control), phorbol myristate acetate (PMA)/Ionomycin (positive control) or live pH1N1 (A/England/09/195) virus (MOI = 1) for 16 h to maintain consistency with the Fluorescence-immunospot assay Monesin A (Sigma-Aldrich) which was added 1 h after addition of stimulus and cells were incubated for 16 h.
Staining with CD107a (clone H4A3, BD Biosciences) and CD107b (clone H4B4, BD Biosciences) was undertaken at the time of stimulation. Cells were stained with aqua amine-reactive viability dye (Invitrogen) as a live/dead marker and surface staining was undertaken using a suitable combination of fluorochrome-labelled anti-human CD3 (clone SK7, BD Biosciences), CD4 (clone S3.5, Invitrogen), CD8 (clone RPA-T8, BD Biosciences), CCR7 (clone 3D12, BD Biosciences), CD45RA (clone MEM-56, Invitrogen) and CXCR3 (clone 1C6, BD Biosciences) markers. Intracellular cytokine staining (ICS) was performed with BD cytofix/cytoperm plus kit according to the manufacturer’s instructions and cells were stained with IFN-γ (cloneB27, BD Biosciences) and IL-2 (clone MQ1–17H12, BD Biosciences) and TNF-α (clone 6401.111, BD Biosciences) antibodies. Fluorescence minus one controls stimulated with live pH1N1 virus were used for identifying positive populations of CCR7, CD107, CXCR3 and CD45RA. In all samples, at least 0.5 million events were collected and analysed. Antigen-specific cytokine responses were calculated only if the responses were greater than 0.001% of the parent population and greater than twice the negative-control sample. Flow cytometric analyses were performed using an LSRII (BD Biosciences) and data were analysed with Flow Jo (Tree Star) software.
Haemagglutination inhibition assay
Antibody responses were detected by use of Haemagglutination inhibition assay according to standard methods at the Centre for Infections, Health Protection Agency (London, UK) and National Institute for Health and Welfare (Helsinki, Finland). The virus strains used were the A/England/195/2009 and the A/California/07/2009.
Statistical analysis
Mann-Whitney 2-tailed U-test or Kruskall-Wallis test was used to identify statistically significant differences between groups, accounting for multiple comparisons. For the analysis of flow cytometry results, due to the sample size, results from the different subgroups were compared using two-sided matched pair non-parametric one-way ANOVA. Graphpad prism version 4 was used for statistical analysis.
Supplementary Material
Acknowledgements
The authors take this opportunity to thank all the willing participants of this study. We express our grateful appreciation to Marie Bautista, Sharleen Bowes, Bianca Fortunaso and Sharna Lloyd-James for processing all the samples. We extend our grateful thanks to Katrina Pollock and Robert Sampson for their assistance with flow cytometry and Rosalyn Casey for help with the fluorescence-immunospot assay. We thank Charles Bangham, Suzie Hingley-Wilson and Fiona Culley for their input and insightful discussions during the preparation of this manuscript. We wish to acknowledge the support of the Wellcome Trust Strategic Award 083567/Z/07/Z (Centre for Respiratory Infection). This work was funded by the National Institute of Health Research, the Medical Research Council and Imperial College Healthcare NHS Trust. AL is a Wellcome Trust Senior Research Fellow in Clinical Science.
Abbreviations
- M1
matrix protein 1
- NP
nucleoprotein
- PBMC
peripheral blood mononuclear cell
- PB1
polymerase basic protein 1
- pH1N1
pandemic H1N1
- SFC
spot forming cell
Footnotes
Supporting Information available online
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.World Health Organization WHO position paper on influenza vaccines 2005. Wkly Epidemiol Record. 33:279–287. http://www.who.int/immunization/wer8033influenza_August2005_position_paper.pdf [Google Scholar]
- 2.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 3.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 2006;193:49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 4.Slepushkin AN. The effect of a previous attack of A1 influenza on susceptibility to A2 virus during the 1957 outbreak. Bull. World Health Organ. 1959;20:297–301. [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen JP, Doherty PC, Branum KC, Riberdy JM. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J. Virol. 2000;74:11690–11696. doi: 10.1128/jvi.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo SH, Webster RG. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 2001;75:2516–2525. doi: 10.1128/JVI.75.6.2516-2525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill E, Krauss SL, Riberdy JM, Webster RG, Woodland DL. Heterologous protection against lethal A/Hong Kong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J. Gen. Virol. 2000;81:2689–2696. doi: 10.1099/0022-1317-81-11-2689. [DOI] [PubMed] [Google Scholar]
- 8.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, et al. Preexisting influenza-specific CD4(+) T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 10.Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, Hamill M, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 2012;55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couch RB, Atmar RL, Franco LM, Quarles JM, Nino D, Wells JM, Arden N, et al. Prior infections with seasonal influenza A/H1N1 virus reduced the illness severity and epidemic intensity of pandemic H1N1 influenza in healthy adults. Clin. Infect. Dis. 2012;54:311–317. doi: 10.1093/cid/cir809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge X, Tan V, Bollyky PL, Standifer NE, James EA, Kwok WW. Assessment of seasonal influenza A specific CD4 T cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J. Virol. 2010;84:3312–3319. doi: 10.1128/JVI.02226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, Chan PL, et al. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J. Virol. 2010;84:6527–6535. doi: 10.1128/JVI.00519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards KA, Topham D, Chaves FA, Sant AJ. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J. Immunol. 2010;185:4998–5002. doi: 10.4049/jimmunol.1001395. [DOI] [PubMed] [Google Scholar]
- 15.Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, Vita R, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. USA. 2009;106:20365–20370. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 17.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 18.Casey R, Blumenkrantz D, Millington K, Montamat-Sicotte D, Kon OM, Wickremasinghe M, Bremang S, et al. Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS One. 2010;5:e15619. doi: 10.1371/journal.pone.0015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halwani R, Doroudchi M, Yassine-Diab B, Janbazian L, Shi Y, Said EA, Haddad EK, et al. Generation and maintenance of human memory cells during viral infection. Springer Semin. Immunopathol. 2006;28:197–208. doi: 10.1007/s00281-006-0027-2. [DOI] [PubMed] [Google Scholar]
- 20.Health Protection Agency Surveillance of influenza and other respiratory viruses in the United Kingdom: October 2008 to April 2009. 2009 http://www.hpa.org.uk/hpr/archives/Infections/2009/respiratory09.htm#flu0809.
- 21.Lee LY-H, Ha DLA, Simmons C, de Jong MD, Chau NVV, Schumacher R, Peng YC, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Investigation. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 23.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 24.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, Woodland DL. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J. Immunol. 2009;183:4378–4384. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 26.Morris AG, Lin YL, Askonas BA. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982;295:150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- 27.Seo SH, Peiris M, Webster RG. Protective cross-reactive cellular immunity to lethal a/goose/Guangdong/1/96-like H5N1 influenza virus is correlated with the proportion of pulmonary CD8+ T cells expressing gamma interferon. J. Virol. 2002;76:4886–4890. doi: 10.1128/JVI.76.10.4886-4890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bot A, Bot S, Bona CA. Protective role of gamma interferon during the recall response to influenza virus. J. Virol. 1998;72:6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 32.Lucas M, Day CL, Wyer JR, Cunliffe SL, Loughry A, McMichael AJ, Klenerman P. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. J. Virol. 2004;78:7284–7287. doi: 10.1128/JVI.78.13.7284-7287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J. Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 34.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puissant-Lubrano B, Bossi P, Gay F, Crance JM, Bonduelle O, Garin D, Bricaire F, et al. Control of vaccinia virus skin lesions by long-term-maintained IFN-gamma+TNF-alpha +effector/memory CD4+ lymphocytes in humans. J. Clin. Invest. 2010;120:1636–1644. doi: 10.1172/JCI38506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, Hersperger AR, et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8+ T Cells. PLoS Pathog. 2010;6:e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoji A, Rinaldo CR., Jr. Human CD8+ T cells specific for influenza A virus M1 display broad expression of maturation-associated phenotypic markers and chemokine receptors. Immunology. 2005;115:239–245. doi: 10.1111/j.1365-2567.2005.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardelid P, Andrews NJ, Hoschler K, Stanford E, Baguelin M, Waight PA, Zambon M, et al. Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol. Assess. 2010;14:115–192. doi: 10.3310/hta14550-03. [DOI] [PubMed] [Google Scholar]
- 39.Terajima M, Cruz J, Leporati AM, Orphin L, Babon JAB, Co MDT, Pazoles P, et al. Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients. J. Virol. 2008;82:9283–9287. doi: 10.1128/JVI.01047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodewes R, Kreijtz JHCM, Hillaire M, Geelhoed-Mieras MM, Fouchier RAM, Osterhaus ADME, Rimmelzwaan GF. Vaccination with whole inactivated virus vaccine affects the induction of heterosubtypic immunity against influenza A/H5N1 and immunodominance of virus specific CD8+ T cell responses in mice. J. Gen. Virol. 2010;91:1743–1753. doi: 10.1099/vir.0.020784-0. [DOI] [PubMed] [Google Scholar]
- 41.Laurie KL, Carolan LA, Middleton D, Lowther S, Kelso A, Barr IG. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A (H1N1) pandemic influenza virus in a ferret model. J. Infect. Dis. 2010;202:1011–1020. doi: 10.1086/656188. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 43.Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–972. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 44.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 2004;34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 46.Scheible K, Zhang G, Baer J, Azadniv M, Lambert K, Pryhuber G, Treanor JJ, et al. CD8+ T cell immunity to 2009 pandemic and seasonal H1N1 influenza viruses. Vaccine. 2011;29:2159–2168. doi: 10.1016/j.vaccine.2010.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 48.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crotty S, Ahmed R. Immunological memory in humans. Semin. Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 51.Hogan RJ, Cauley LS, Ely KH, Cookenham T, Roberts AD, Brennan JW, Monard S, et al. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J. Immunol. 2002;169:4976–4981. doi: 10.4049/jimmunol.169.9.4976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.