Abstract
Differentiated HL-60 is an effector cell widely used for the opsonophagocytic-killing assay (OPKA) to measure efficacy of pneumococcal vaccines. We investigated the correlation between phenotypic expression of immunoreceptors and phagocytic ability of HL-60 cells differentiated with N,N-dimethylformamide (DMF), all-trans retinoic acid (ATRA), or 1α, 25-dihydroxyvitamin D3 (VitD3) for 5 days. Phenotypic change was examined by flow cytometry with specific antibodies to CD11c, CD14, CD18, CD32, and CD64. Apoptosis was determined by flow cytometry using 7-aminoactinomycin D. Function was evaluated by a standard OPKA against serotype 19F and chemiluminescence-based respiratory burst assay. The expression of CD11c and CD14 gradually increased upon exposure to all three agents, while CD14 expression increased abruptly after VitD3. The expression of CD18, CD32, and CD64 increased during differentiation with all three agents. Apoptosis remained less than 10% until day 3 but increased after differentiation by DMF or ATRA. Differentiation with ATRA or VitD3 increased the respiratory burst after day 4. DMF differentiation showed a high OPKA titer at day 1 which sustained thereafter while ATRA or VitD3-differentiated cells gradually increased. Pearson analysis between the phenotypic changes and OPKA titers suggests that CD11c might be a useful differentiation marker for HL-60 cells for use in pneumococcal OPKA.
Graphical Abstract
Keywords: Opsonophagocytic-Killing Assay, Streptococcus pneumonia, HL-60, Differentiation
INTRODUCTION
Streptococcus pneumoniae is a significant pathogen for young children and the elderly worldwide (1, 2). Antibiotic treatment is becoming less effective because of an increase in multidrug-resistant S. pneumoniae (3) and patients end up with serious sequelae despite effective antibiotic treatment (4). Therefore an effective pneumococcal vaccine is highly desirable (5). Currently, several pneumococcal conjugate vaccines more effective than the 23-valent polysaccharide (PS) vaccine are under development (6, 7, 8).
In evaluating pneumococcal vaccines, an enzyme-linked immunosorbent assay (ELISA) is commonly used as the measure of vaccine efficacy by quantitating antibodies to serotype specific S. pneumoniae PS in sera (9). However, the ELISA assays for antibodies to pneumococcal PS were not always specific and cross-reactivity of antibody binding to several serotypes was often observed (10, 11). Opsonophagocytic killing assay (OPKA) is an in vitro surrogate assay to test protective efficacy of the pneumococcal vaccines and is often used to complement the ELISA results (12).
For OPKA, granulocytes differentiated from HL-60 have been used as effector cells to lessen the effort of isolating fresh granulocytes from human peripheral blood (12). HL-60 cells can be differentiated into granulocytes by N,N-dimethylformamide (DMF) (13, 14) dimethylsulfoxide (DMSO) (15, 16, 17), all-trans retinoic acid (ATRA) (16, 17, 18) or granulocyte colony stimulating factor (G-CSF) (19) and into monocytes or macrophage-like cells by phorbol 12-myristate 13-acetate (PMA) or 1α,25-dihydroxyvitamin D3 (VitD3) (17, 20, 21, 22). Differentiated HL-60 cells change the expression of surface markers and the ability of OPKA activity (16, 17, 23) but the relationship between phenotypic expression and function of differentiated HL-60 cells has not been fully characterized.
In the present study, we investigated the correlation between phenotypic and functional changes that occur during differentiation of HL-60 cells with DMF, ATRA, and VitD3 over time. This data would be useful in optimizing the differentiation protocol of HL-60 cells for use in OPKA to S. pneumoniae.
MATERIALS AND METHODS
Cell culture and differentiation condition
The HL-60 cells (ATCC, CCL-240, Rockville, MD) were grown in RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Bio Whittaker, Walkersville, MD, USA), 0.2% sodium bicarbonate and appropriate antibiotics in 5% CO2-humidified incubator at 37℃ and the culture medium was changed every 3-4 days. The HL-60 cells at final concentrations of 5×105 cells/mL were induced to differentiate by incubation with 100 mM DMF (Fisher Scientific, Pittsburgh, PA, USA), 1 µM ATRA (Sigma, St. Louis, MO, USA), or 100 nM VitD3 (Sigma) for 5 days. These were optimized conditions for differentiation of HL-60 cell into granulocytes or monocytes in our and others' previous studies (12, 17, 18, 22). Triplicates of phenotypic and functional analyses were performed before and on day 1, 2, 3, 4, and 5 after each differentiation induction.
Flow cytometric analysis of phenotypic expression
Each ten microliters of phycoerythrin (PE)-conjugated mouse anti-human CD64, CD32, CD14, and CD11c (mouse IgG1, Serotec, Oxford, England), PE-conjugated rat anti-human CD18 (rat IgG2b, Serotec), and PE-conjugated mouse IgG1 (Serotec) and PE-conjugated rat IgG2b (Serotec), as isotype controls, were added to 1×106 differentiated HL-60 cells and incubated at room temperature for 15 min. Then the cells were centrifuged at 300 ×g for 5 min and suspended in 1 mL of phosphate-buffered saline (PBS, pH 7.4). The cells were centrifuged again and washed twice as such. The cells were resuspended in 5 mL of PBS with 0.5% paraformaldehyde and the fluorescence intensities were measured with FACS Calibur (Beckton-Dickinson, San Jose, CA, USA).
Opsonophagocytic-killing assay
S. pneumoniae serotype 19F (ATCC) was grown in Todd-Hewitt broth (Difco, Detroit, MI, USA) with 0.5% yeast extract to the log phase, aliquoted in 15% glycerol and frozen at -70℃ for further use. The recovery rate and dilution factor of bacteria were assessed. The same frozen lot was used for OPKA throughout the entire investigation. HL-60 cells before and day 1, 2, 3, 4, or 5 of differentiation by DMF, ATRA or VitD3 were used for OPKA. HL-60 cells were diluted to 1×107 cells/mL in Hanks' buffer supplemented with 0.1% gelatin and 10% FCS. Intravenous immunoglobulin (IVIG, Green Cross, Yongin, Korea) was also diluted in the same buffer. Ten microliters of pneumococci solution containing 2,000 colony forming unit (CFU) and 40 µL of serial 1:3 dilutions of IVIG were placed in a well of 96-well micro -titer plate. After 30 min incubation at room temperature, 40 µL of HL-60 suspension (4×105 per well) and 10 µL of baby rabbit complement (Accurate Chemical, Westbury, NY, USA) were added to the well. The mixture was incubated for 1 hr at 37℃ with shaking. Ten microliters of the reaction mixture was plated in a blood agar plate. The plates were incubated at 37℃ in 5% CO2 for 12-18 hr. CFU was determined by counting bacterial colonies in the plates. OPKA activity is defined by % killing as follows.
% Killing = (CFU in the absence of HL-60 - CFU in the presence of HL-60)×100/CFU in the absence of HL-60
Measurement of respiratory burst using a chemoiluminescence assay
A reaction mixture was prepared containing 50 mM luminol (5-Amino-2, 3-dihydro-1, 4-phthalazinedione, Sigma) and 500 nM PMA in 800 µL of PBS and was stabilized in a luminometer vial at 37℃ for 5 min. HL-60 cells (5×105 cells/200 µL) were added to the mixture and luminescent intensity was measured at 37℃ for 2 hr with a luminometer (Multi-biolumat LB9505C, Berthold, Germany). The tests were repeated three times and the mean values of the maximum amounts were calculated.
Analysis of apoptotic cell death
HL-60 cells were stained with 20 µg/mL of 7-amino-actinomycin D (7-AAD, Sigma) and analyzed by flow cytometry. Briefly, 7-AAD was solubilized in small volume of acetone and diluted in PBS to adjust the concentration to 200 µg/mL. Fifty microliters of the 7-AAD solution was added to 450 µL of cell suspension to adjust the final concentration into 20 µg/mL and cultured for 20 min at room temperature. Flow cytometric analysis was carried out to analyze apoptotic cells, which are moderately stained by the 7-AAD.
Statistical analysis
The phenotypic expressions of differentiated HL-60 cells were expressed as means±SD and were statistically analyzed by two-tailed non-paired t-test. Correlations between expression of CD64, CD32, CD14, or CD11b/CD18 and OPKA activity were analyzed by Pearson correlation analysis. P value of <0.05 was considered statistically significant.
RESULTS
Phenotypic expression of immune receptors on the differentiated HL-60 cells
CD11c was not expressed on the undifferentiated HL-60 cells but a gradual increase in its expression was observed on the cells differentiated with DMF, ATRA, or VitD3 up to day 5 (Table 1). There was no expression of CD14 on the HL-60 cells before the induction of differentiation and it increased significantly by DMF on day 3 and by ATRA on day 4, respectively. Meanwhile, VitD3 showed the highest induction of CD14 expression on day 2 and a gradual decrease thereafter. The expression of CD18 was already high (76.5%) before the induction and increased a little bit further by DMF on day 3, by ATRA on day 1, and by VitD3 on day 2. On the 4th and 5th day, there were differences in the expression of CD18 on the cells differentiated with the 3 agents and DMF produced the highest expression. The expression of CD64 on HL-60 cells was 98.5% before differentiation and it sustained over 95% through day 1, 2, 3, 4, and 5 after addition of DMF, ATRA, or VitD3. The expression of CD32 on HL-60 cells before the induction of differentiation was 39.2% and on day 1 the expression increased significantly by all three inducers and it remained constant until the fifth day.
Table 1.
Phenotypic expression of immune receptors on the differentiated HL-60 cells
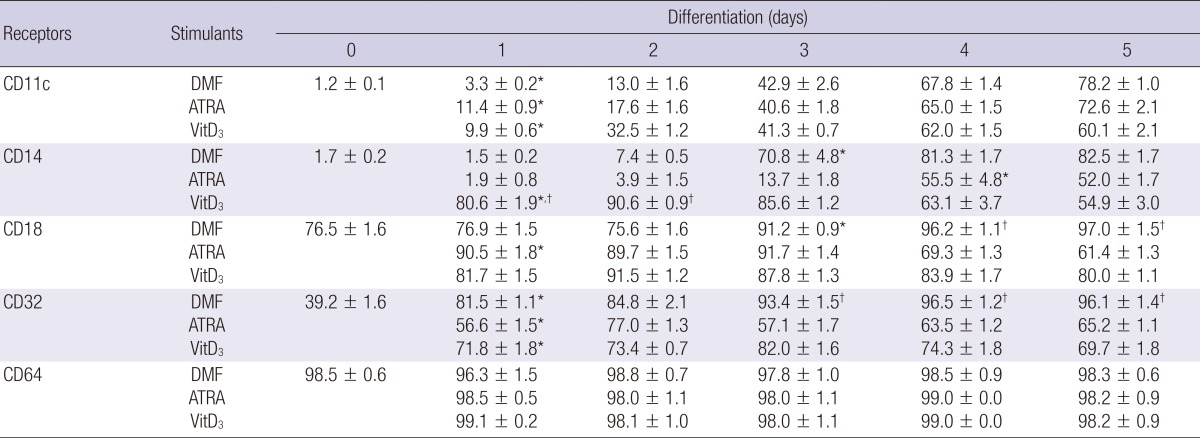
Numbers in the Table are the geometric mean±SD of fluorescence intensity. *P<0.05 (vs. Day 0); †P<0.05 (DMF vs. ATRA or VitD3).
Change in apoptotic cell population during differentiation
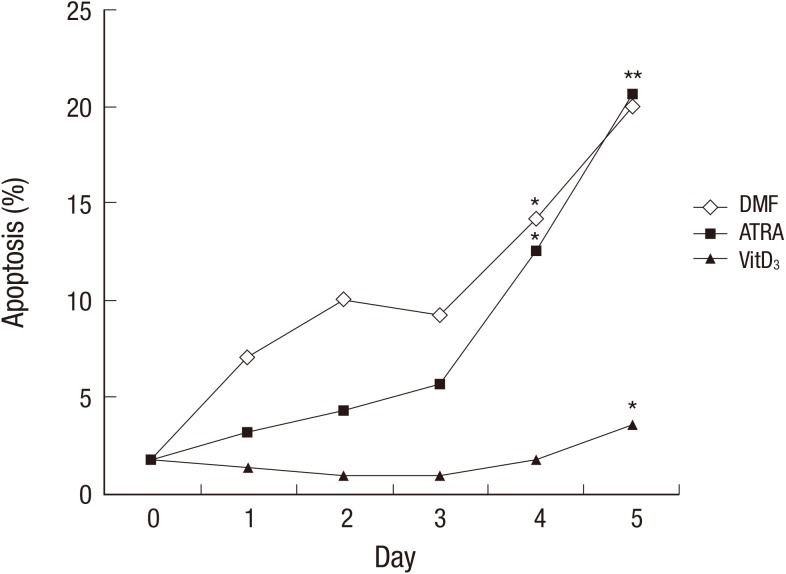
Cells stop proliferation during differentiation and begin to die at a certain time point after the end of differentiation. In order to examine the change in dead cell populations during differentiation, apoptotic cells were enumerated using 7-AAD, a fluorescent dye intercalating into DNA of dead cell. Dead cell fraction of HL-60 cells before the differentiation was 1.8% and it remained less than 10% for 3 days when the cells were differentiated with DMF or ATRA. However, apoptotic cells increased on day 4 and 5 post DMF or ATRA treatment. On the contrary to DMF and ATRA, VitD3 showed no increase of dead cells until day 4 with a small increase in the dead cells (3.5%) on day 5 (Fig. 1).
Fig. 1.
Analysis of apoptotic cell death during HL-60 differentiation. HL-60 cells were incubated with or without DMF (0.8%), ATRA (1 µM), or VitD3 (20 µg/mL) for the indicated time period. Cells were stained with 20 µg/mL of 7-AAD and percentage of apoptotic cell populations were determined by analyzing fluorescence intensity of moderately stained cells with flow cytometry. *Indicates P < 0.05 when the values were compared with that of the day 0. DMF, dimethylformamide; ATRA, all-trans retinoic acid; VitD3, vitamin D3; 7-ADD, 7-aminoactinomycin D.
Respiratory burst of the differentiated HL-60 cells
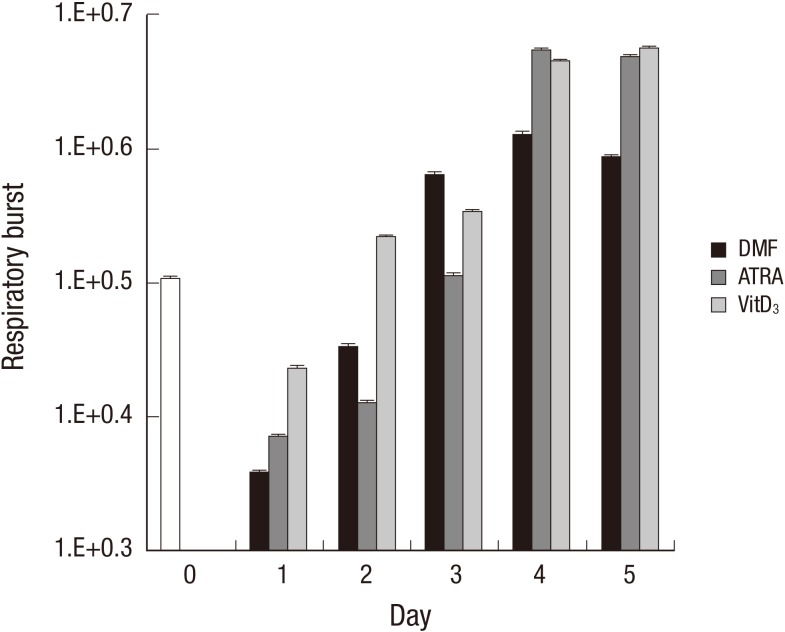
During phagocytosis, a metabolic process known as the respiratory burst occurs in the phagocytes producing a number of reactive oxygen species that are extremely toxic to the microorganisms. Respiratory burst of the differentiated HL-60 cells was determined by the chemiluminescence assay. The production of reactive oxygen species was immediately down-regulated at the early stage of differentiation (up to 2 days after induction). Then, DMF showed a robust induction of the respiratory burst on day 3 and the induction reached its maximum on day 4. ATRA and VitD3 showed similar patterns with DMF in the kinetics of the respiratory burst but produced 10 times higher intensities of the fluorescence on day 4 and 5 compared to DMF (Fig. 2).
Fig. 2.
Analysis of respiratory burst during HL-60 differentiation. HL-60 cells were incubated with or without DMF (0.8%), ATRA (1 µM), or VitD3 (20 µg/mL) for the indicated time period. The HL-60 cells were incubated with 50 mM luminal and 500 nM PMA for 2 hr. Chemiluminescence intensity was examined with a luminometer. One of the three similar results is shown. DMF, dimethylformamide; ATRA, all-trans retinoic acid; VitD3, vitamin D3; 7-ADD, 7-aminoactinomycin D.
Correlation between the phenotypic expression patterns and OPKA titers
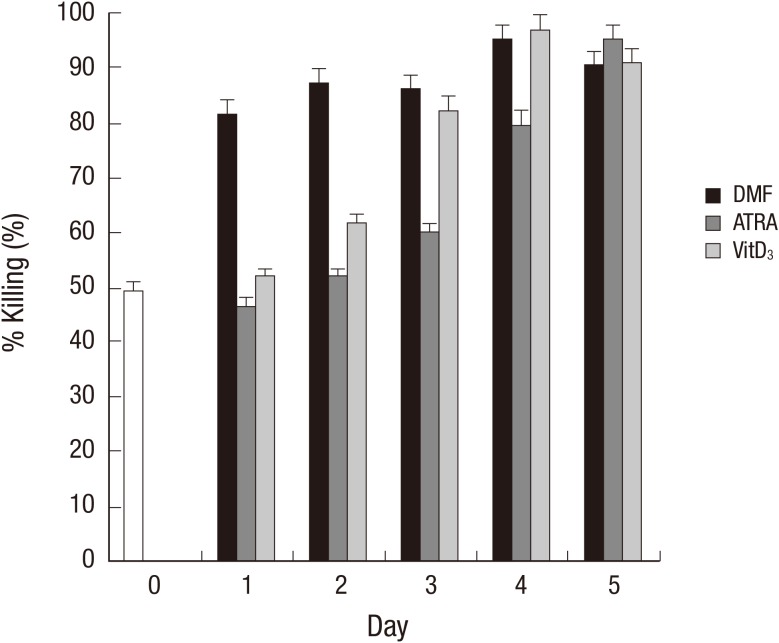
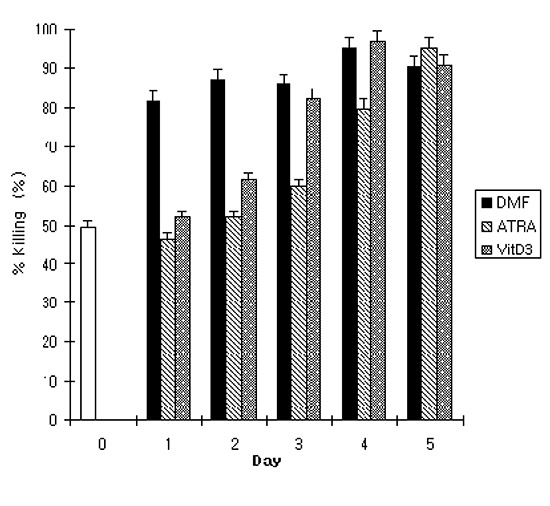
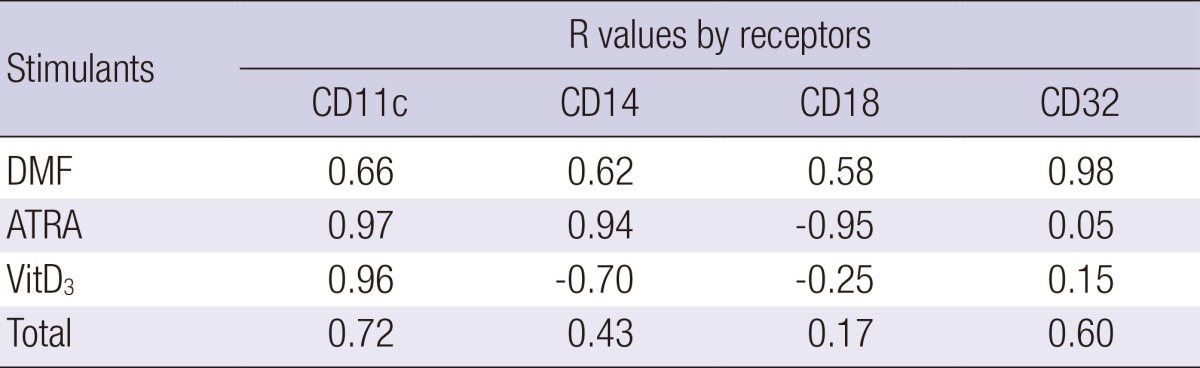
To further investigate the correlation between phenotypic change of the cells and OPKA activity during differentiation, a standard OPKA was performed using serotype 19F of S. pneumoniae. Undifferentiated HL-60 cells showed 49.7% of OPKA activity as determined by percent killing. With DMF, OPKA activity increased significantly on day 1 and remained constant till day 5. On the contrary, ATRA and VitD3 gradually increased the OPKA activity as the differentiation continued. At the end of differentiation, there was no significant difference in OPKA activity among the 3 agents and the titer reached approximately 90% (Fig. 3). Pearson correlation analysis demonstrated that changes in CD11c expression of the differentiated HL-60 cells correlated well with OPKA activity in all 3 agents (Table 2). With DMF, CD32 expression had a strong correlation with the OPKA activity and the expression of other markers such as CD14, CD11c, and CD18 demonstrated a moderate correlation with the OPKA activity compared with other agents. CD14 showed a strong correlation with the OPKA activity on the differentiation of HL-60 cells induced by ATRA treatment.
Fig. 3.
Opsonophagocytic-killing assay activity of HL-60 cells during differentiation. HL-60 cells were incubated with or without DMF (0.8%), ATRA (1 µM), or VitD3 (20 µg/mL) for the indicated time period. The HL-60 cells were applied to a conventional pneumococcal OPKA against serotype 19F as described in the Materials and Methods. DMF, dimethylformamide; ATRA, all-trans retinoic acid; VitD3, vitamin D3; 7-ADD, 7-aminoactinomycin D.
Table 2.
Correlation between the phenotypic expression of immune receptors and opsonophagocytic-killing assay titer of the differentiated HL-60
DISCUSSION
Having originated from a leukemia cell with karyotypic abnormalities, HL-60 cells can be differentiated into phagocytes to display phenotypic and functional characteristics commonly associated with normal peripheral blood granulocytes including OPKA activity and respiratory burst. The current study was performed to identify the correlation between phenotypic expression of HL-60 cells and the OPKA activity during differentiation induced by DMF, ATRA, and VitD3 and demonstrated that CD11c expression correlates well with OPKA activity in HL-60 cells differentiated with all three agents. When HL-60 cells are differentiated with DMF, CD32 expression also correlates with OPKA activity. These results suggest that CD11c might be a useful marker for confirming differentiation of HL-60 cells and CD32 can be used as a marker for DMF-differentiated cells to achieve qualification of HL-60 cells as an effector cell for the pneumococcal OPKA.
Phagocytosis involves two processes: 1) attachment of opsonized bacteria to the receptors of phagocytes and 2) subsequent engulfment and digestion of target microbes. Since recognition and attachment of opsonized bacteria are mediated through various adhesion molecules expressed on phagocytic cells, CD11c could play an important role in this process by forming adhesions such as CD11c/CD18 complex (C3b receptor, CR4). CD11c contributes to the adhesive properties of granulocytes and mediates adsorptive endocytosis on granulocytes by binding to a complement component C3b (24). Expression of CD11c on the differentiated HL-60 cells was significantly increased on day 1 in all three groups and increased continuously until the 5th day with rates ranging between 60%-80%, and there was no significant difference among the three agents in the expression of CD11c. These results are in agreement with Miller et al. (25). Along with the CD11c, CD11b also participates in the cell-to-cell interaction by forming CD11b/CD18 complex (CR3, iC3b receptor, Mac-1) which is a part of integral family of adhesion molecules. Previously, we reported that the expression of CD11b correlated well with OPKA activity as well (26). Although the HL-60 cells have been shown to express only the FcγRIIa-R131 allotype, which has a lower affinity for IgG2, these cells are still capable of phagocytosis of opsonized pneumococci in the presence of complements (27). Thus, the C3b receptor containing CD11b appears to play a critical role in the opsonophagocytosis of S. pneumoniae. On the other hand, CD18 was expressed on the 76.5% of HL-60 cells before differentiation, which signifies that it is already expressed in the early stage of myelopoiesis.
OPKA measures bacterial uptake and killing by phagocytes in the presence of antibody and complement (24). Our results demonstrate that cells differentiated with DMF for 1-5 day(s) yielded comparable OPKA activity. However it took 3 days with VitD3 and 4 days with ATRA to reach sufficient opsonophagocytic-killing, respectively. On day 5 there was no difference in OPKA titers among all three inducer groups. The results were comparable to similar studies in which HL-60 cells differentiated by DMSO or ATRA were assessed for OPKA activity (26, 28). It is also notable that HL-60 cells are able to be used for OPKA in just 2 days post DMF treatment in the view of the fact that DMF can differentiate HL-60 cells into effector cells earlier than ATRA or VitD3.
Since Martinez et al. (29) reported that the in vitro differentiated HL-60 cells showed similar effectiveness in the phagocytosis of labeled pneumococci to granulocytes isolated from multiple donors, enormous efforts have been done to improve the efficacy of phagocytes used for OPKA. However, there are difficulties that remain to be solved. Appropriate differentiation condition should derive HL-60 cells into the cells capable of successfully mimicking genuine granulocytes circulating through the body and playing a role in phagocytosis of foreign microorganisms. It may be worthwhile to examine the differentiation of HL-60 cells with a combination of various inducers in order to improve the effectiveness of cells for a better bioassay. For instance, HL-60 cells differentiate into functional granulocytes better when they are exposed to a mixture of ATRA, VitD3 and G-CSF (30). Next, as HL-60 cells are differentiated, they cease proliferation and begin to express new genes and molecules and undergo morphologic changes, then begin to die mainly via apoptosis. It might be useful to find a condition to make the differentiated cells survive longer without changing their functions and phenotypic expression even after the end of differentiation. One promising approach would be to generate a transfectant ectopically expressing anti-apoptotic genes to prevent cells from apoptotic cell death. Simultaneously, a technology for cryo-preservation would be developed to preserve the differentiated effecter cells till the time to use.
Despite the instability of HL60 culture and the variability of the phagocytic functions of the differentiated HL-60 cells, HL-60 is widely used in surrogate pneumococcal OPKA from its simple differentiation process, availability of HL-60 cell line, and difficulties in obtaining human granulocytes through a routine phlebotomy and complicated isolation procedures from blood (31, 32, 33). However, to make the OPKA more reliable, procedures for differentiation of HL-60 cell into effective phagocytes should be optimized and further standardized. Therefore, this study will provide the basis to identifying the correlation between phenotypic expression pattern and OPKA activity and to suggest the markers that should be examined to confirm differentiation into relevant effector HL-60 cells.
Footnotes
This study was supported by the grant from the Ministry of Food and Drug Safety (11172MFDS360) to KHK.
The authors have no conflicts of interest to disclose.
Conceived and designed the experiments: Kim KH, Seoh JY. Performed the experiments: Kim KH. Analyzed the data: Kim KH, Cho SJ. Contributed reagents/materials/analysis tools: Kim KH, Seoh JY. Wrote the first draft of the manuscript: Kim KH, Seoh JY, Cho SJ. Wrote the paper: Kim KH, Seoh JY, Cho SJ. ICMJE criteria for authorship read and met: Kim KH, Seoh JY, Cho SJ. Agree with manuscript results and conclusions: Kim KH, Seoh JY, Cho SJ.
References
- 1.Centers for Disease Control (CDC) Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1989;38:64–68. 73–76. [PubMed] [Google Scholar]
- 2.Fedson DS, Musher DM. Pneumococcal polysaccharide vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th ed. Philadelphia: Pa: Saunders; 2004. [Google Scholar]
- 3.Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, Lefkowitz L, Cieslak PR, Cetron M, Zell ER, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 4.Tan TQ. Antibiotic resistant infections due to Streptococcus pneumoniae: impact on therapeutic options and clinical outcome. Curr Opin Infect Dis. 2003;16:271–277. doi: 10.1097/00001432-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Siber GR. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 6.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Lee LH, Lee CJ, Frasch CE. Development and evaluation of pneumococcal conjugate vaccines: clinical trials and control tests. Crit Rev Microbiol. 2002;28:27–41. doi: 10.1080/1040-840291046678. [DOI] [PubMed] [Google Scholar]
- 8.Puumalainen T, Zeta-Capeding MR, Käyhty H, Lucero MG, Auranen K, Leroy O, Nohynek H. Antibody response to an eleven valent diphtheria- and tetanus-conjugated pneumococcal conjugate vaccine in Filipino infants. Pediatr Infect Dis J. 2002;21:309–314. doi: 10.1097/00006454-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514–519. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin RT, White AC, Anderson CA, Carlone GM, Klein DL, Treanor J. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine. 1998;16:1761–1767. doi: 10.1016/s0264-410x(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Sun Y, Frasch C, Concepcion N, Nahm MH. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin Diagn Lab Immunol. 1999;6:519–524. doi: 10.1128/cdli.6.4.519-524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graziano RF, Ball ED, Fanger MW. The expression and modulation of human myeloid-specific antigens during differentiation of the HL-60 cell line. Blood. 1983;61:1215–1221. [PubMed] [Google Scholar]
- 14.Hassan HT, Rees JK. Triple combination of retinoic acid, low concentration of cytarabine and dimethylformamide induces differentiation of human acute myeloid leukaemic blasts. Chemotherapy. 1990;36:51–57. doi: 10.1159/000238748. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson JP, Jones EA. Biosynthesis of the human C3b/C4b receptor during differentiation of the HL-60 cell line. Identification and characterization of a precursor molecule. J Clin Invest. 1984;74:1649–1657. doi: 10.1172/JCI111581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trayner ID, Bustorff T, Etches AE, Mufti GJ, Foss Y, Farzaneh F. Changes in antigen expression on differentiating HL60 cells treated with dimethylsulphoxide, all-trans retinoic acid, α1,25-dihydroxyvitamin D3 or 12-O-tetradecanoyl phorbol-13-acetate. Leuk Res. 1998;22:537–547. doi: 10.1016/s0145-2126(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 18.Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanayasu-Toyoda T, Yamaguchi T, Uchida E, Hayakawa T. Commitment of neutrophilic differentiation and proliferation of HL-60 cells coincides with expression of transferrin receptor. Effect of granulocyte colony stimulating factor on differentiation and proliferation. J Biol Chem. 1999;274:25471–25480. doi: 10.1074/jbc.274.36.25471. [DOI] [PubMed] [Google Scholar]
- 20.Drayson MT, Michell RH, Durham J, Brown G. Cell proliferation and CD11b expression are controlled independently during HL60 cell differentiation initiated by 1,25 α-dihydroxyvitamin D(3) or all-trans-retinoic acid. Exp Cell Res. 2001;266:126–134. doi: 10.1006/excr.2001.5200. [DOI] [PubMed] [Google Scholar]
- 21.Murao S, Gemmell MA, Callaham MF, Anderson NL, Huberman E. Control of macrophage cell differentiation in human promyelocytic HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and phorbol-12-myristate-13-acetate. Cancer Res. 1983;43:4989–4996. [PubMed] [Google Scholar]
- 22.Zinzar S, Ohnuma T, Holland JF. Effects of simultaneous and sequential exposure to granulocytic and monocytic inducers on the choice of differentiation pathway in HL-60 promyelocytic leukemia cells. Leuk Res. 1989;13:23–30. doi: 10.1016/0145-2126(89)90027-1. [DOI] [PubMed] [Google Scholar]
- 23.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 25.Miller LJ, Schwarting R, Springer TA. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986;137:2891–2900. [PubMed] [Google Scholar]
- 26.Kim HS, Kim KH, Kim GH, Seoh JY. Fcγ Receptor and Mac-1 Expression and Functional Differentiation of HL-60 Cells by All-trans Retinoic Acid. J Korean Pediatr Soc. 1999;42:462–471. [Google Scholar]
- 27.Jansen WT, Breukels MA, Snippe H, Sanders LA, Verheul AF, Rijkers GT. Fcγ receptor polymorphisms determine the magnitude of in vitro phagocytosis of Streptococcus pneumoniae mediated by pneumococcal conjugate sera. J Infect Dis. 1999;180:888–891. doi: 10.1086/314920. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Kim KH, Kim GH, Seoh JY. Functional differentiation of HL-60 cells by dimethylsulfoxide and phorbol 12-myristate 13-acetate. J Korean Pediatr Soc. 1999;42:355–363. [Google Scholar]
- 29.Martinez JE, Romero-Steiner S, Pilishvili T, Barnard S, Schinsky J, Goldblatt D, Carlone GM. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin Diagn Lab Immunol. 1999;6:581–586. doi: 10.1128/cdli.6.4.581-586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleck RA, Athwal H, Bygraves JA, Hockley DJ, Feavers IM, Stacey GN. Optimization of nb-4 and hl-60 differentiation for use in opsonophagocytosis assays. In Vitro Cell Dev Biol Anim. 2003;39:235–242. doi: 10.1290/1543-706X(2003)039<0235:OONAHD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Esposito AL, Clark CA, Poirier WJ. An assessment of the factors contributing to the killing of type 3 Streptococcus pneumoniae by human polymorphonuclear leukocytes in vitro. APMIS. 1990;98:111–121. [PubMed] [Google Scholar]
- 32.Lortan JE, Kaniuk AS, Monteil MA. Relationship of in vitro phagocytosis of serotype 14 Streptococcus pneumoniae to specific class and IgG subclass antibody levels in healthy adults. Clin Exp Immunol. 1993;91:54–57. doi: 10.1111/j.1365-2249.1993.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obaro SK, Henderson DC, Monteil MA. Defective antibody-mediated opsonisation of S. pneumoniae in high risk patients detected by flow cytometry. Immunol Lett. 1996;49:83–89. doi: 10.1016/0165-2478(95)02487-5. [DOI] [PubMed] [Google Scholar]