Abstract
The aims of this study were to assess the risk of tuberculosis (TB) and the status of latent tuberculosis infection (LTBI) in Korean patients with inflammatory bowel disease (IBD) receiving tumor necrosis factor (TNF)-α blockers. We reviewed medical records of 525 Korean IBD patients (365 TNF-α blocker naïve and 160 TNF-α blocker exposed) between January 2001 and December 2013. The crude incidence of TB was significantly higher in IBD patients receiving TNF-α blockers compared to TNF-α-blocker-naïve patients (3.1% vs. 0.3%, P=0.011). The mean incidence of TB per 1,000 patient-years was 1.84 for the overall IBD population, 4.89 for TNF-α blocker users, and 0.45 for TNF-α-blocker-naïve patients. The adjusted risk ratio of TB in IBD patients receiving TNF-α blocker was 11.7 (95% confidence interval, 1.36-101.3). Pulmonary TB was prevalent in patients treated with TNF-α blockers (80.0%, 4/5). LTBI was diagnosed in 17 (10.6%) patients, and none of the 17 LTBI patients experienced reactivation of TB during treatment with TNF-α blockers. Treatment with TNF-α blockers significantly increased the risk of TB in IBD patients in Korea. De novo pulmonary TB infection was more prevalent than reactivation of LTBI, suggesting an urgent need for specific recommendations regarding TB monitoring during TNF-α blocker therapy.
Graphical Abstract
Keywords: Tuberculosis, Latent Tuberculosis, Tumor Necrosis Factor-alpha, Inflammatory Bowel Disease
INTRODUCTION
The striking effectiveness of tumor necrosis factor (TNF)-α blockade has revolutionized the treatment of several chronic inflammatory diseases, including inflammatory bowel disease (IBD) (1). Today, a large number of IBD patients are being treated with immunomodulators or biologics, in addition to corticosteroids. This multimodal approach is associated with a significantly altered overall immune system and an increased risk of infectious adverse events. In particular, TNF-α blockers have been reported to reactivate tuberculosis (TB), which is normally sequestrated in a latent state within the confines of granulomas, and increase the risk of new TB infection (2,3,4).
Meanwhile, in the past two decades, the incidence and prevalence of IBD in Asia have increased significantly (5,6,7). Since the emergence of IBD in traditionally low prevalence regions such as Asia is only a recent finding, the risk of TB development in Asian IBD patients subjected to TNF-α blocker therapy has not been well documented compared to that in Western countries. Given that 59% of global TB cases originate from Asia, the lack of evaluation on the TB risk associated with TNF-α blocker therapy is concerning (8, 9).
Therefore, we conducted a longitudinal cohort study in Korea, where the annual incidence of TB is estimated to be 108 cases per 100,000 patients (10), to assess the risk of TB in IBD patients treated with TNF-α blockers. We also aimed to document how latent TB screening is performed and efficacy of the screening. Additionally, since TNF-α blockers are also widely used to treat rheumatoid arthritis (RA) (11), we compared the rate of TB development in IBD patients using TNF-α blockers to that of RA patients using TNF-α blockers.
MATERIALS AND METHODS
Study design and subjects
This was a single-center, retrospective, longitudinal cohort study of all IBD patients during the study period. TNF-α blockers have been used in Korea since 2001; thus, the study period was set from January 2001 to December 2013. Non-Korean patients were excluded. We reviewed medical records including the bacteriological examination results, radiologic data, results of tuberculin skin test (TST) and interferon-gamma releasing assay (IGRA), type of TNF-α blocker therapy and response to anti-TB treatment. Medical records of all patients with RA who were treated at our institution during the same study period were reviewed in an identical manner.
Diagnosis of active TB
The Korean Tuberculosis Surveillance System (KTBS), an internet-based reporting system, acts as a nation-wide data depository for consolidated information on TB in Korea (12). The KTBS defines a TB case as a person with either 1) typical symptoms of TB and bacterial confirmation, or 2) typical symptoms and radiological or histological findings, but without bacterial confirmation. Accordingly, in our study, the diagnosis of TB was made when Mycobacterium tuberculosis was identified in culture of any clinical specimen, or when M. tuberculosis DNA was detected in any clinical specimen by polymerase chain reaction (PCR) analysis. Patients with symptoms and signs of TB, and with a definitive response to anti-TB medication were also defined as TB cases, irrespective of bacterial confirmation. Only those TB cases that developed during the period of TNF-α blocker therapy, or within 3 months of TNF-α blocker therapy discontinuation, were thought to be relevant to TNF-α blocker use.
Screening and definition of LTBI
According to the 2011 Korean practice guidelines for the control and management of TB published by the Korean Academy of Tuberculosis and Respiratory Diseases, LTBI was diagnosed when either TST or IGRA test result was positive (13). Those patients without a history of TB treatment but with evidence of healed TB on a chest x-ray (CXR) were also considered as LTBI (13). The TST was performed on the volar side of the forearm, and according to the Mantoux method using a 2-tuberculin unit (TU) dose of purified protein derivative RT23 (Statens Serum Institut, Copenhagen, Denmark). The results were interpreted after 48 to 72 hr using the ballpoint method, and were considered positive if the transverse diameter of the induration was equal to or greater than 10 mm (13). The IGRA was performed using QuantiFERON®-TB Gold In-Tube test (QFT-GIT test; Cellestis Ltd., Carnegie, Victoria, Australia), and according to the manufacturer's instructions. The results were considered positive for an IFN-gamma response to the TB antigen that was significantly above the baseline IFN-gamma value of 0.35 IU/mL.
Statistics
The results are presented as means±standard deviation (SD) or medians (range). Differences between groups were assessed using a Student's t-test or one-way analysis of variance for continuous variables, and chi-square test for categorical variables, as appropriate. The relative risk of TB development in patients treated with TNF-α blockers was compared to that of TNF-α blocker naïve patients, calculated using binary logistic regression analysis. All data were analyzed using PASW® Statistics 18 (SPSS® Inc., Chicago, IL, USA). P value<0.05 was considered to statistically significant.
Ethics statement
The study was conducted according to the Declaration of Helsinki, and the protocol was approved by the institutional review board of Kyung Hee University Hospital (KMC IRB 1413-04). In light of the retrospective nature of the study, informed consent was waived.
RESULTS
Baseline demographic and disease characteristics
From January 2001 to December 2013, 525 IBD patients were considered eligible and enrolled. The study population consisted of 48.0% Crohn's disease and 52.0% ulcerative colitis. The proportion of male patients was 63.4% and the mean age was 33.4 yr, with mean disease duration of 69.4 months. A total of 160 patients (30.5%) underwent TNF-α blocker therapy and these patients were younger at IBD diagnosis, compared to TNF-α-blocker-naïve patients (P<0.001). Infliximab was the most frequently used TNF-α blocker (81.9%, 131/160). More patients in the TNF-α exposed group had been exposed azathiopurine/6-mercaptopurine (MP) compared to those in the TNF-α naïve group (97.5% vs. 55.0%), but the use of systemic steroids did not differ between the two. The detailed baseline characteristics are shown in Table 1.
Table 1.
Baseline demographics and disease characteristics of the study population
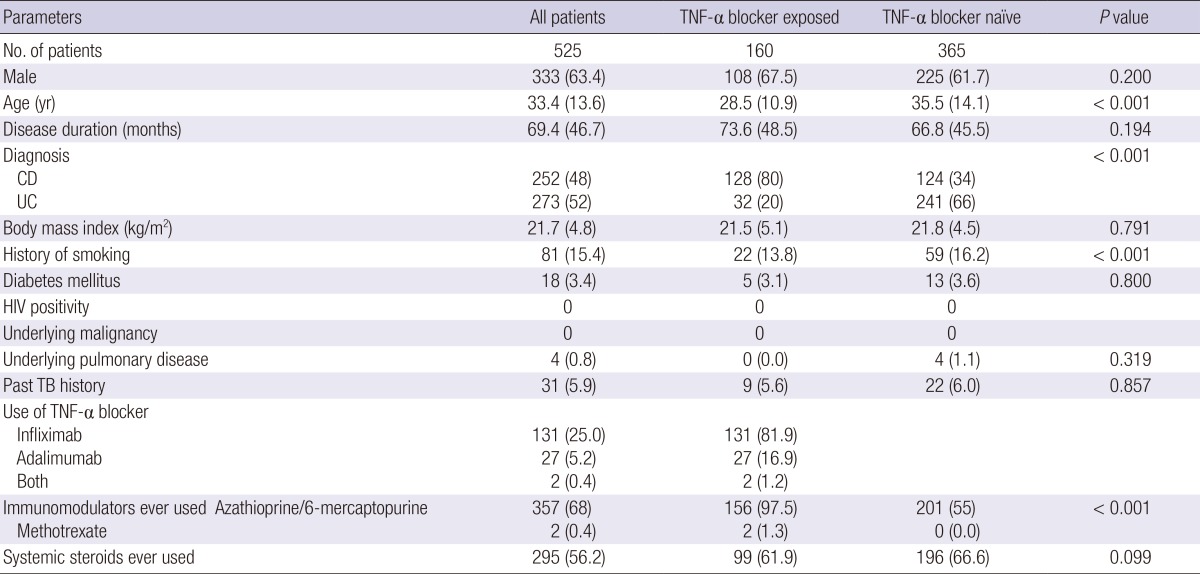
Data are expressed as means (standard deviation) or numbers (%). CD, Crohn's disease; HIV, human immunodeficiency virus; TB, tuberculosis; TNF-α, tumor necrosis factor-α; UC, ulcerative colitis.
Incidence and characteristics of newly developed TB
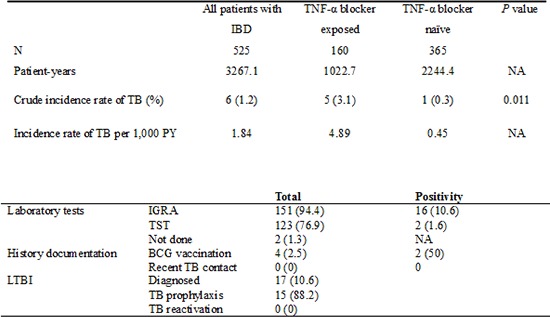
A total of six (1.1%, 6/525) patients developed TB during 3,267 patient-years follow-up. Thus, the overall incidence of TB in the IBD study cohort was 1.84 per 1,000 patient-years. Five TB cases developed in TNF-α blocker exposed patients (3.1%, 5/160; 4.89 per 1,000 patient-years) and one in TNF-α blocker naïve patients (0.3%, 1/365 patients; 0.45 per 1,000 patient-years) (P=0.011) (Table 2). Logistic regression analysis confirmed that TNF-α blocker therapy significantly increased the risk of TB development (odds ratio [OR] adjusted for age, sex, type of IBD, and disease duration, 11.7; 95% confidence interval [CI], 1.4-101.1).
Table 2.
Incidence of tuberculosis in patients with inflammatory bowel disease
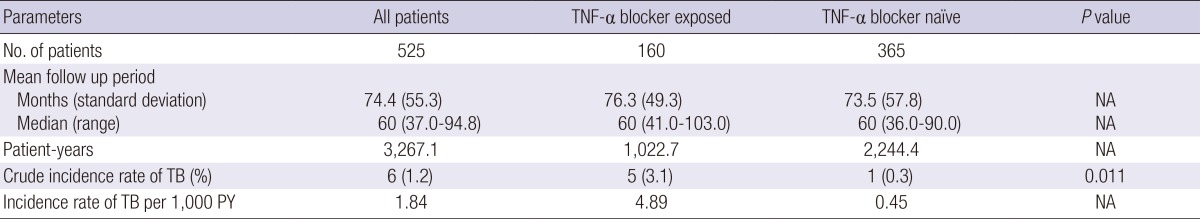
NA, not applicable; TB, tuberculosis; TNF-α, tumor necrosis factor-α; PY, patient-years.
Of the five patients who developed TB in association with TNF-α blocker therapy, four developed pulmonary infection; only one was extrapulmonary. These patients contracted TB within a median (range) of 24 months (2-44) from TNF-α blocker therapy initiation. The median (range) number of TNF-α blocker doses was 22.5 (3-29) (Table 3). All five TB cases in patients who had received TNF-α blockers were negative in LTBI screening, indicating de novo M. tuberculosis infection. Although most patients showed a favorable course of TB with standard first-line anti-TB medication, one patient developed multi-drug resistant TB (MDR-TB), and received second-line anti-TB medication for a prolonged period. One patient in the TNF-α-blocker naïve group had TB peritonitis with enteritis, which was diagnosed based on acid-fast bacilli (AFB) staining of a colonoscopic biopsy specimen. This patient was lost to subsequent follow-up, thus the clinical course and prognosis remain obscure.
Table 3.
Characteristics of newly-developed tuberculosis in a cohort of 525 patients with IBD
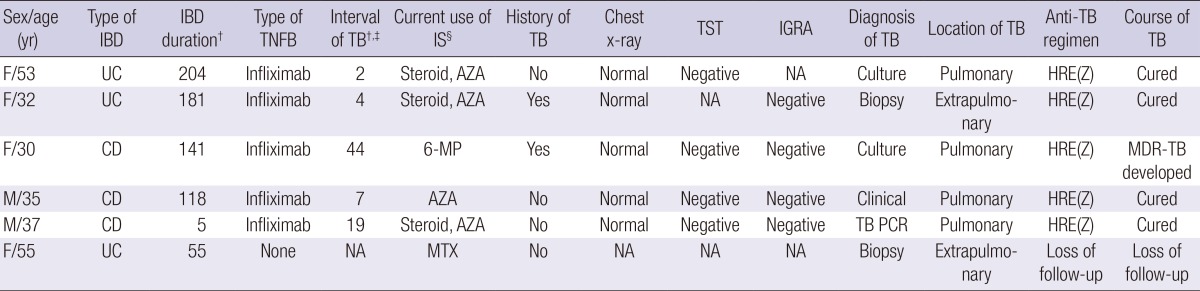
†Data are presented as months; ‡Interval between the first use of a TNF-α blocker and the development of TB; §Use of immunosuppressant within 3 months of TB development. AZA, azathioprine; CD, Crohn's disease; E, ethambutol; F, female; H, isoniazid; IGRA, interferon-γ releasing assay; IS, immunosuppressant; M, male; 6-MP, 6-mercaptopurine; MDR-TB, multi-drug resistant tuberculosis; MTX, methotrexate; NA, not available; PCR, polymerase chain reaction; R, rifampicin; TB, tuberculosis; TNFB, TNF-α blocker; TST, tuberculin skin test; UC, ulcerative colitis; Z, pyrazinamide.
Screening of LTBI in patients who underwent TNF-α blocker treatment
The LTBI screening rates were generally acceptable at 94.4% (151/160), 98.8% (158/160), and 76.9% (123/160) for IGRA, CXR, and TST, respectively. The positive rates were 10.6% (16/151), 1.9% (3/158), and 1.6% (2/123) for IGRA, CXR, and TST, respectively. IGRA results were "indeterminate" for 3 (2.0%) patients. However, the BCG vaccination history was only asked to 4 (2.5%) patients. The history of recent exposure to TB was never asked. LTBI was diagnosed in 17 (10.6%) patients (Table 4). LTBI was diagnosed by IGRA in 16 cases, TST in 2 case, and positive for both tests in 1 case. Out of 17 LTBI patients, 15 successfully completed standard 9-months prophylaxis with isoniazid (INH). However, two did not receive any treatment for LTBI due to physician's negligence in one and the patient's refusal in the other.
Table 4.
Summary of latent tuberculosis infection
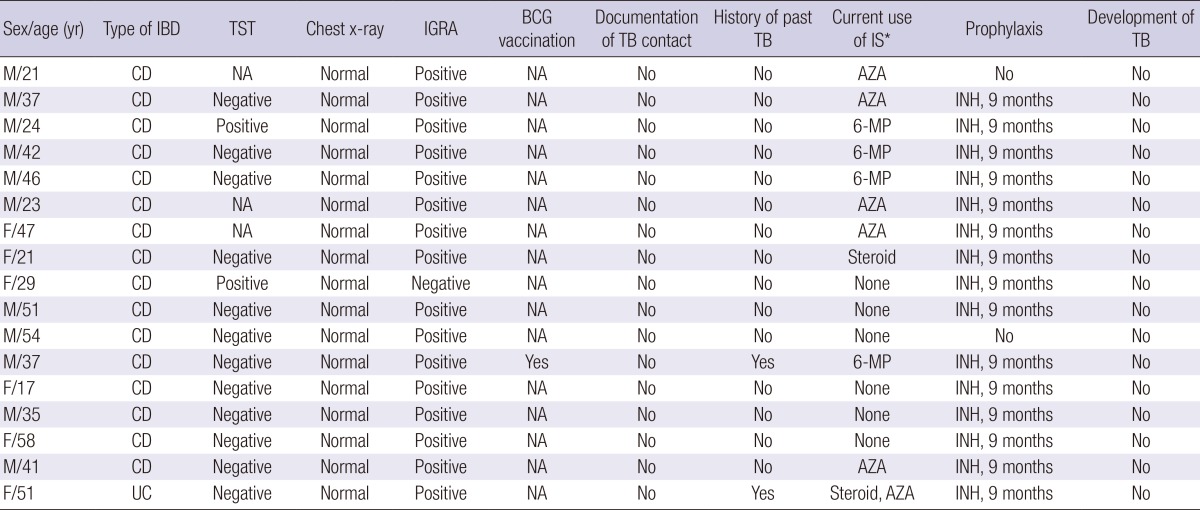
*Use of immunosuppressant within 3 months of TB development. AZA, azathioprine; BCG, Bacillus Calmette-Guerin; CD, Crohn's disease; F, female; IBD, inflammatory bowel disease; IGRA, interferon-γ releasing assay; INH, isoniazid; IS, immunosuppressant; M, male; 6-MP, 6-mercaptopurine; NA, not available; TB, tuberculosis; TST, tuberculin skin test; UC, ulcerative colitis.
Comparison of IBD and RA patients treated with TNF-α blockers
A total of 57 RA patients were treated with TNF-α blockers at our institution during the study period. As shown in Table 5, when compared with IBD patients using TNF-α blockers, RA patients treated with TNF-α blockers tended to be older and more likely to have other documented risk factors for TB, including chronic medical illness, coexistent pulmonary diseases, recent exposure to systemic steroids, and a history of past TB infection (all P<0.05). Furthermore, RA patients had a higher rate of LTBI (10.6% vs. 29.8%, P=0.001). The crude incidence of TB showed no statistically significant difference between the two groups (3.1% vs. 1.8%, P=1.00).
Table 5.
Comparison of crude TB incidence between inflammatory bowel disease patients treated with TNF-α blocker and rheumatoid arthritis patients treated with TNF-α blocker
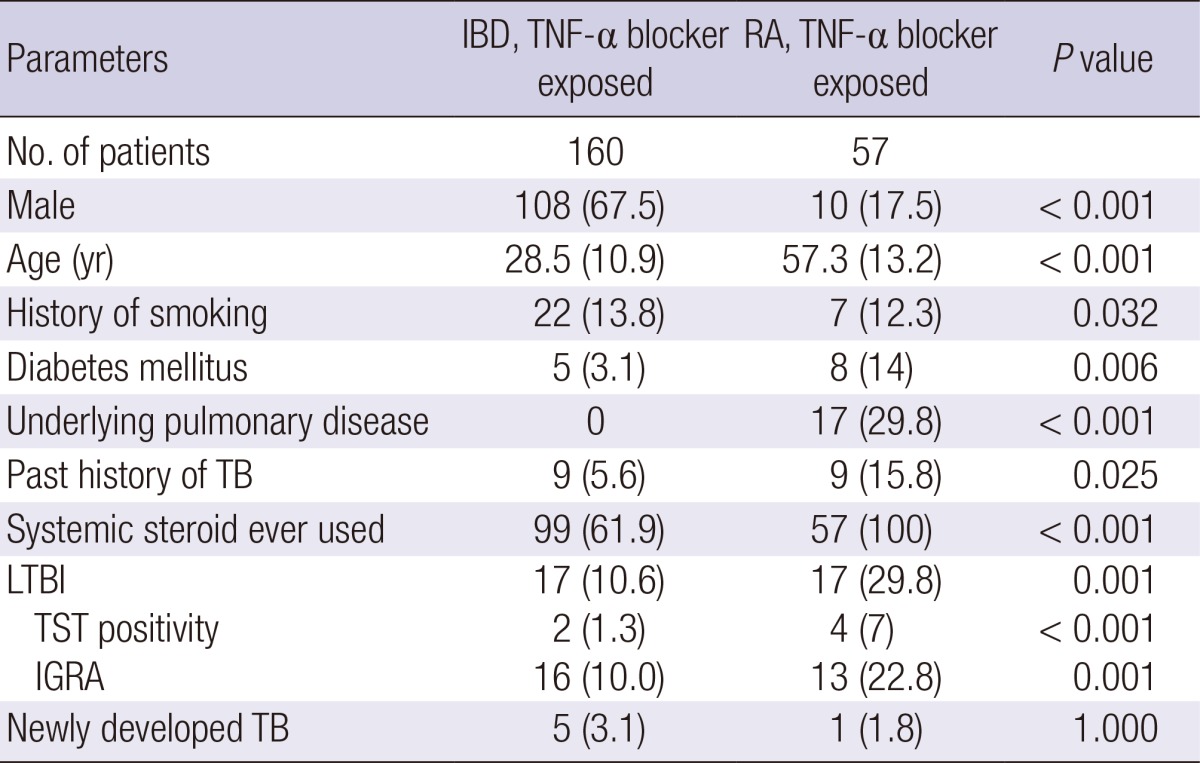
Data are expressed as means (standard deviation) or numbers (%). IBD, inflammatory bowel disease; IGRA, interferon-γ releasing assay; LTBI, latent tuberculosis infection; M, male; RA, rheumatoid arthritis; TB, tuberculosis; TNF-α, tumor necrosis factor-α; TST, tuberculin skin test.
DISCUSSION
Although the number of IBD patients in Asian countries is increasing, studies on the risk of TB in IBD patients are scarce, including those on the incidence of TB in IBD patients receiving TNF-α blockers. To our knowledge, this is the first detailed and structured report of TB incidence in a large number of IBD patients in Korea, a country with an intermediate TB burden. In the current study, five cases of newly developed TB were identified 2 to 44 months after initiation of TNF-α blocker therapy (crude incidence of 3.1%, 5/160). The mean incidence of TB per 1,000 patient-years was estimated to be 1.84 for overall IBD population, 4.89 for patients receiving TNF-α blockers, and 0.45 for TNF-α-blocker-naïve patients. Thus, treatment with TNF-α blockers significantly increased the risk of TB in IBD patients in a country with an intermediate TB burden (adjusted OR, 11.7; 95% CI, 1.4-101.1; P=0.011).
Our study not only indicates that the development of TB is a real problem in Asian IBD patients treated with TNF-α blockers, but also has several novel strengths compared to previous studies. First, interestingly, most of the patients receiving TNF-α blockers in our study contracted pulmonary TB (80.0%, 4/5). This finding contradicts previous reports that extrapulmonary or disseminated sites are more common in TB occurring with TNF-α blocker use (14, 15, 16). One explanation for this discrepancy is the difference in study cohort composition. Early reports have shown that more than 60% of RA patients treated with TNF-α blocker develop extrapulmonary or disseminated TB (17, 18). The higher prevalence of extrapulmonary or disseminated TB in previous studies appears to be due largely to the fact that more rheumatoid disease patients were evaluated, whereas the proportion and absolute number of IBD patients evaluated were small (14, 15). Another explanation is that the clinical site of TB infection differs according to the M. tuberculosis phylogenetic lineage (19). Click et al. (20) reported that compared with cases with East Asian lineage, exclusively extrapulmonary tuberculosis was more common in cases with Euro-American (OR, 1.1; 95% CI, 1.0-1.2), Indo-Oceanic (OR, 2.0; 95% CI, 1.8-2.2), and East-African Indian (OR, 3.6; 95% CI, 3.1-4.2) lineages. Therefore, our findings may not be coincidental, and the predominant sites of TB infection in IBD patients receiving TNF-α blocker therapy may indeed vary across the world.
Secondly, in the present study, five patients developed TB in association with TNF-α blocker use in spite of negative LTBI screening, indicating de novo TB infection is as problematic as TB reactivation. LTBI was diagnosed in 17 patients (10.6%), and among them 15 (88.2%) received TB prophylaxis. Fortunately, none of the 17 patients experienced TB reactivation, suggesting that standard chemoprophylaxis for LTBI is effective in practice. Our results call attention to the following two issues: 1) the need to revisit current guidelines for LTBI screening; and 2) the need to establish specific recommendations regarding monitoring of TB during TNF-α blocker therapy. Our domestic guidelines recommend three tests (TST alone, IGRA alone, TST in combination with IGRA) equally for LTBI diagnosis in immunocompromised patients (13). The problem with TST is that its ability to detect LTBI in immunocompromised patients is limited due to anergy to skin test antigens and the effects of immunosuppressive drugs (21). Because most IBD patients are on immunosuppressive drugs at the time of LTBI screening (64.7% of LTBI patients in our study), false negative results can occur. Likewise, in our study, TST was positive in only 2 (1.6%) patients with LTBI. This is even lower than previous domestic data, reporting the TST positivity in CD patients to be 10.0% (22). Such large difference (1.6% vs. 10.0%) is probably due to the subjective nature of the TST, which has been repeatedly pointed out as a limitation of the test (23). There are no gold standards to assess the sensitivity and specificity of TST (24), and inconsistency in the intradermal placement and/or in the reading of the results is inevitable. Another problem with TST is that in Korea, the national BCG vaccination program to newborns was established in the 1980s and is still in place. For this reason, some studies have reported particularly high TST positivity (37.0%) in Korean patients treated with TNF-α blockers, and such data inconsistency suggest that TST might not be the optimum method of detecting LTBI (25). Recent updates on the European Crohn's and Colitis Organisation (ECCO) guidelines acknowledged these shortcomings of TST, and now recommend booster TST for patients on immunomodulators with initially negative TST (26). Although the confounding effect of BCG vaccination is almost insignificant in adults above 30 yr of age, the guideline also recognized IGRA as the preferred screening method in BCG-immunized individuals (26). Not surprisingly, IGRA yielded a higher detection rate in our study and was positive in 16 of the 17 LTBI patients (94.1%). These results also correlate with recent changes in the United States guidelines on LTBI diagnosis, which now recommend replacement of TST with IGRA in all patients (27). In this regard, we suggest performing IGRA as the method of LTBI screening at baseline for IBD patients who are planned to undergo TNF-α blocker therapy in TB-endemic areas. We also believe repeating IGRA in cases of highly suspicious LBTI but with negative test results would be helpful.
Together with laboratory tests, the Korean national guidelines also recommend documenting the history of BCG vaccination, recent contact with active TB, and history of past TB, whether treated, inadequately treated or untreated, as part of LTBI screening (13). The importance of thorough history taking was also underscored in the recent ECCO guidelines, which stated that a diagnosis of LTBI should be considered when there is a history of recent exposure to the disease and positive initial TST (26). Unfortunately, at our center, documentation of BCG vaccination and recent TB exposure history was performed in only four (2.5%) and zero (0.0%) patients, respectively. Our results highlight that thorough history taking is rarely performed in clinical practice, resulting in possible neglect of those at risk of LTBI reactivation. Recognition of thorough history taking as an integral part of LTBI screening is critical. We believe that this issue should be a target of quality improvement for prevention of opportunistic infection in patients with IBD.
There is a need to monitor TB during TNF-α blocker therapy, and we suggest long-term monitoring of patients with a high index of suspicion for TB development because TB can occur as late as 44 months after TNF-α blocker therapy initiation as shown in our study. This is supported by previous studies reporting the risk of TB reactivation increases fivefold in the first 52 weeks of TNF-α blocker therapy (17, 23, 28). Abreu et al. (14) reported that TB can develop as late as 107 months after TNF-α blocker therapy initiation in a country with an intermediate TB burden, which further advocates our suggestion. In light of the higher percentage of pulmonary TB and the lack of classic symptoms of TB in immunocompromised patients, regular CXR follow-ups seem legitimate, although further studies are needed (3).
The risk of TB in Korean RA patients subjected to TNF-α blockers are well documented (11). While RA itself increases the TB risk by 8.9-folds compared to the general Korean population, the risk of TB is reportedly 30.1 times higher in those using infliximab (11). The baseline characteristics of RA patients in our study, such as the greater percentage of past TB infection, steroid exposure, and LTBI, suggest that these patients might be at greater risk of TB. Alarmingly, there was no difference in TB development between IBD patients using TNF-α blockers and RA patients using TNF-α blockers (3.1% vs. 1.8%, P=1.00). The shortcomings of these analyses include the relatively small number of RA patients receiving TNF-α blocker therapy (n=57) and only one case of TB occurrence. Nevertheless, our study calls immediate attention to the need for a nationwide multicenter study, assessing the risk of TB in IBD patients undergoing TNF-α blocker therapy compared with the general population.
There are other limitations to our study. This was a single referral medical center study; thus, the results cannot be readily extrapolated to the general population of IBD patients. Also, in this study, we did not attempt to identify predictive factors for TB development in association with TNF-α blocker therapy. Therefore, more systemized, multicenter studies are needed to corroborate our results. In addition, not all of the risk factors for mycobacterial infections were documented, and the TB prophylaxis rate and follow-up periods differed among the patients. In some cases, the follow-up period may have been too short to detect the development of TB. Lastly, the fact that BCG vaccination history and history of recent contact with active TB was so poorly documented could have acted as a bias.
In conclusion, treatment with TNF-α blockers significantly increases the risk of TB in IBD patients in an Asian country with an intermediate TB burden. De novo pulmonary TB infection is more prevalent than LTBI reactivation in the current study, suggesting an urgent need for specific recommendations regarding monitoring of TB during TNF-α blocker therapy. Given the established efficacy of LTBI prophylaxis prior to TNF-α blocker therapy initiation, we believe that more vigorous screening of LTBI is required for IBD patients. In addition, IGRA should be considered as the primary LTBI screening test in TB-endemic areas.
Footnotes
Funding: This work was supported by a grant from Kyung Hee University in 2014 (KHU-20140685).
The authors declare no conflicts of interest.
Conceived and designed the study: Byun JM, Lee CK, Kim HJ. Analyzed the data: Rhee SY, Byun JM, Lee CK. Contributed reagents/materials/analysis tools: Rhee SY, Kim JW, Shim JJ, Jang JY. Wrote the first draft of the manuscript: Byun JM, Lee CK. Wrote the paper: Byun JM, Lee CK, Kim HJ. ICMJE criteria for authorship read and met: Byun JM, Lee CK, Rhee SY, Kim HJ, Kim JW, Shim JJ, Jang JY. Agree with manuscript results and conclusions: Byun JM, Lee CK, Rhee SY, Kim HJ, Kim JW, Shim JJ, Jang JY. Enrolled patients: Lee CK, Kim HJ.
References
- 1.Dave M, Purohit T, Razonable R, Loftus EV., Jr Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 2014;20:196–212. doi: 10.1097/MIB.0b013e3182a827d2. [DOI] [PubMed] [Google Scholar]
- 2.Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford) 2005;44:714–720. doi: 10.1093/rheumatology/keh567. [DOI] [PubMed] [Google Scholar]
- 3.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 4.Lee SK, Kim SY, Kim EY, Jung JY, Park MS, Kim YS, Kim SK, Chang J, Kang YA. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565–571. doi: 10.1007/s00408-013-9481-5. [DOI] [PubMed] [Google Scholar]
- 5.Ng SC, Tsoi KK, Kamm MA, Xia B, Wu J, Chan FK, Sung JJ. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:1164–1176. doi: 10.1002/ibd.21845. [DOI] [PubMed] [Google Scholar]
- 6.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. 54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 7.Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 8.Debeuckelaere C, De Munter P, Van Bleyenbergh P, De Wever W, Van Assche G, Rutgeerts P, Vermeire S. Tuberculosis infection following anti-TNF therapy in inflammatory bowel disease, despite negative screening. J Crohns Colitis. 2014;8:550–557. doi: 10.1016/j.crohns.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- 10.Zumla A, George A, Sharma V, Herbert N Baroness Masham of Ilton. WHO's 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013;382:1765–1767. doi: 10.1016/S0140-6736(13)62078-4. [DOI] [PubMed] [Google Scholar]
- 11.Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, Kim TH, Jun JB, Yoo DH, Lee JT, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007;34:706–711. [PubMed] [Google Scholar]
- 12.Lew WJ, Lee EG, Bai JY, Kim HJ, Bai GH, Ahn DI, Lee JK, Kim SJ. An Internet-based surveillance system for tuberculosis in Korea. Int J Tuberc Lung Dis. 2006;10:1241–1247. [PubMed] [Google Scholar]
- 13.Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. Seoul: MEDrang Inc; 2011. [Google Scholar]
- 14.Abreu C, Magro F, Santos-Antunes J, Pilão A, Rodrigues-Pinto E, Bernardes J, Bernardo A, Magina S, Vilas-Boas F, Lopes S, et al. Tuberculosis in anti-TNF-alpha treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis. 2013;7:e486–e492. doi: 10.1016/j.crohns.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Chung KB, Lee EY, Im JP, Han SK, Yim JJ. Clinical characteristics and treatment responses of patients who developed tuberculosis following use of a tumor necrosis factor-alpha inhibitor. Korean J Intern Med. 2013;28:174–179. doi: 10.3904/kjim.2013.28.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jauregui-Amezaga A, Turon F, Ordás I, Gallego M, Feu F, Ricart E, Panés J. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013;7:208–212. doi: 10.1016/j.crohns.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 18.Baronnet L, Barnetche T, Kahn V, Lacoin C, Richez C, Schaeverbeke T. Incidence of tuberculosis in patients with rheumatoid arthritis. A systematic literature review. Joint Bone Spine. 2011;78:279–284. doi: 10.1016/j.jbspin.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328–337. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 20.Click ES, Moonan PK, Winston CA, Cowan LS, Oeltmann JE. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clin Infect Dis. 2012;54:211–219. doi: 10.1093/cid/cir788. [DOI] [PubMed] [Google Scholar]
- 21.Yun JW, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, Cha HS, Koh EM, Koh WJ. Diagnosis and treatment of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. J Korean Med Sci. 2007;22:779–783. doi: 10.3346/jkms.2007.22.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim TS. Diagnosis and treatment of latent tuberculosis infection due to initiation of anti-TNF therapy. Tuberc Respir Dis. 2014;76:261–268. doi: 10.4046/trd.2014.76.6.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qumseya BJ, Ananthakrishnan AN, Skaros S, Bonner M, Issa M, Zadvornova Y, Naik A, Perera L, Binion DG. QuantiFERON TB gold testing for tuberculosis screening in an inflammatory bowel disease cohort in the United States. Inflamm Bowel Dis. 2011;17:77–83. doi: 10.1002/ibd.21329. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhut M, Fidler K. Performance of Tuberculin Skin Test Measured against Interferon Gamma Release Assay as Reference Standard in Children. Tuberc Res Treat. 2014;2014:413459. doi: 10.1155/2014/413459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS, Kim CH, Hur M, Hyun IG, Park MJ, Song W, Park JY, Kang HJ, Lee KM. Clinical usefulness of T-SPOT.TB test for the diagnosis of tuberculosis. Korean J Lab Med. 2010;30:171–177. doi: 10.3343/kjlm.2010.30.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, et al. European Crohn's and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 28.Afif W, Loftus EV., Jr Safety profile of IBD therapeutics: infectious risks. Med Clin North Am. 2010;94:115–133. doi: 10.1016/j.mcna.2009.08.016. [DOI] [PubMed] [Google Scholar]


