Abstract
Objective
Recent hypertension guidelines recommend initiation of treatment with a fixed dose combination of two drugs for more effective and quicker blood pressure control. Few of these have been assessed for efficacy and acceptability. This study examines the short term blood pressure control and acceptability of perindopril, with or without its fixed dose combinations (FDC) with amlodipine and Indapamide in younger patients.
Methods
In a multicentre prospective observational study, patients with stage 1 hypertension were prescribed perindopril 4 mg per day. Those with stage 2 or 3 hypertension were prescribed a single tablet per day of 4 mg perindopril and 5 mg amlodipine (COVERSYL AM), or 4 mg perindopril and 1.25 mg indapamide (COVERSYL PLUS)for 45 days. The primary outcomes were the frequency of patients achieving blood pressure control and the adverse effect of pedal edema.
Results
Of 426 patients, with a mean age of 45 years, distributed throughout India, and an average (SD) baseline systolic/diastolic blood pressure of 157.2 (13.5)/98.6 (7.4), 303 (71.1%) achieved blood pressure control. Mean (SD) SBP/DBP decreased from baseline by 26.9 (12.6), and DBP by 15.4 (7.2) mm Hg. Few patients discontinued treatment, and the frequency of cough that interfered with sleep and ankle edema was low.
Conclusion
In patients requiring combination antihypertensive treatment, the regimen of perindopril alone or its FDC with Indapamide or amlodipine reduces blood pressure effectively, resulting in high rates of blood pressure control over the short term, with a low frequency of side effects including cough and pedal edema.
Keywords: Hypertension, FDC, Indapamide, Amlodipine
1. Introduction
The presence of hypertension increases the risk of cardiovascular mortality,1 and its treatment reduces this risk.2 The evidence, however, limited to older patients, and the benefit to those who are younger is not clearly established.3
The importance of younger patients with hypertension in countries such as India, is emphasized by cross-sectional survey data, which showed that the average age of patients with stage 1 and 2 was about 49 years.4 Hence, there is a need to assess blood pressure lowering efficacy, acceptability, and cardiovascular risk reduction with drug treatment regimens in younger patients with hypertension. Recent guidelines recommend initiating treatment with long acting once daily formulations of an angiotensin converting enzyme inhibitor (ACEI) in patients below 55 years, with the addition, if required, of a calcium channel blocker (CCB), or thiazide like diuretic such as Indapamide.3 In this respect, The ACEI perindopril has been shown to reduce the risk of cardiovascular outcomes in combination with both amlodipine5 and indapamide6 in hypertensive patients.
The objective of this multicenter, prospective, observational study was to assess short term blood pressure control and acceptability of perindopril, with or without its fixed dose combinations (FDC) with amlodipine and Indapamide in younger patients.
2. Patients and methods
2.1. Selection of study investigators
Of 468 Indian cities with a population of over 1 million in 2007, 40 were randomly selected. Primary care physicians in these cities, and the metropolitan cities of Bombay, Calcutta, Delhi, and Chennai were identified by their membership of the Association of Physicians of India, and invited to participate in the study.
2.2. Selection of patients
Each investigator selected consecutive outpatients between 40 and 56 years of either sex, with untreated essential hypertension as suggested by European guidelines6 [Stage 1 hypertension was a systolic blood pressure (SBP)/diastolic blood pressure (DBP) equal to or greater than 140/90, but less than 160/100 mmHg; stage 2, equal to or greater than 160/100, but less than 180/110 mmHg; and stage 3, equal to or greater than 180/110, mmHg]. Patients with secondary hypertension, any chronic disease other than diabetes, a contraindication to the use of perindopril, amlodipine or indapamide SR (Natrilix SR) and who were pregnant or lactating were excluded.
2.3. Study design, assessments, and follow up (Fig. 1)
Fig. 1.
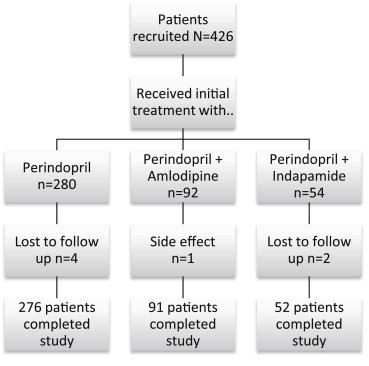
Study flow chart.
After giving their written informed consent, patients were assessed at baseline (Table 1) for demographic, clinical, prior treatment, and biochemical characteristics. Patients with stage 1 hypertension were prescribed perindopril 4 mg per day. Those with stage 2 or 3 hypertension were prescribed an FDC of 4 mg perindopril and 5 mg amlodipine (COVERSYL AM), or 4 mg perindopril and 1.25 mg indapamide (COVERSYL PLUS), to be taken with breakfast for 30 days. Treatment of associated disease was allowed at the discretion of the local physician. Patients were followed up and reassessed on the 30th, 60th and 90th day of the study. At each follow up visit, blood pressure was measured, and patients were asked open ended questions about side effects since the previous visit. All blood pressure measurements were made under guideline recommended conditions.3 Patients with BP under control (systolic BP < 140, and diastolic BP < 90 mm Hg) continued the same treatment prescribed at baseline. Among patients not achieving BP control on the 30th day of treatment, for those with stage 1 hypertension, perindopril 4 mg was withdrawn and replaced with 1 FDC tablet of COVERSYL AM or COVERSY PLUS per day at breakfast for the next 30 days. Patients uncontrolled on COVERSYL PLUS or COVERSYL AM were withdrawn from the study. All medications were required to be purchased by patients from the market, as they could not be provided within the resources of study.
Table 1.
Baseline characteristics of treatment naïve patients with hypertension.
| n = 426 | |
|---|---|
| Age, years | 45.2 ± 6.5 |
| Male sex | 289 (67.8) |
| Cardiovascular risk | |
| Current smoker | 90 (21.3) |
| Weight, kg | 70.3 ± 10.2 |
| Left ventricular hypertrophy | 12 (2.8) |
| Diabetes | 60 (14.1) |
| History of myocardial infarction | 4 (1.0) |
| History of stroke | 4 (1.0) |
| Family history of hypertension | 80 (18.8) |
| Systolic blood pressure mmHg | 157.2 ± 13.5 |
| Diastolic blood pressure mmHg | 98.6 ± 7.4 |
| Stage 1 hypertension | 280 (65.7) |
| Stage 2 hypertension | 116 (27.2) |
| Stage 3 hypertension | 30 (7.0) |
| Fasting plasma glucose mg/dl | 109.8 ± 60.4 |
| Total cholesterol mg/dl | 205.3 ± 118.1 |
| LDL cholesterol mg/dl | Not assessed |
| HDL cholesterol mg/dl | 47.5 ± 24.7 |
| Serum creatinine mg/dl | Not assessed |
Plus minus values are means ± standard deviation. All other values are numbers of patients followed by percentages of the group in parenthesis.
2.4. Statistical analysis
The primary outcomes were the mean changes in blood pressure from baseline after receiving the study treatment, and the number of patients achieving blood pressure control (SBP less than 140 and DBP less than 90 mmHg, including those with diabetes) on an intention to treat basis. Other outcomes were frequency of side effects reported by the patient and adherence with the study treatment. Significance was defined as a p value of less than 0.05.
3. Results
Primary care physicians (233), distributed throughout India (from 40 cities), recruited 426 patients with untreated essential hypertension. Baseline characteristics are given in Table 1. The average age of patients was 45.2 ± 6.5, median age 46.0, and 75th percentile, 50 years; 67.8% were men; stage 1 hypertension was present in 65.7%; stage 2 in 27.2%, and stage 3 in 7%. Diabetes was present in 14.1%, and 1% gave a history of myocardial infarction or stroke.
3.1. Blood pressure response to treatment
For all patients taken together, mean (SD) SBP decreased by 26.9 (12.6), and DBP by 15.4 (7.2) mm Hg. As shown in Fig. 2, In patients with stage 1 hypertension who received initial monotherapy with perindopril, followed, if required to achieve blood pressure control, with its FDC with amlodipine or indapamide, mean (95% Confidence interval, CI) baseline SBP decreased by 21.4 (20.4–22.3), and baseline DBP by 12.4 (11.7–13.0) mmHg after 90 days of treatment.
Fig. 2.
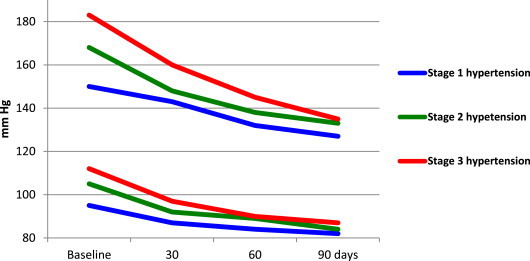
Blood pressure response to treatment with perindopril its fixed dose combination with amlodipine or indapamide in untreated hypertension.
Among patients with stage 2 hypertension, initiation of treatment with the FDC of perindopril and amlodipine (in 73 patients) decreased baseline SBP by 35.2 (31.7–38.7) and DBP by 20.6 (19.2–22.1) mmHg, while initial treatment with the FDC of perindopril and indapamide (in 40 patients) decreased baseline SBP by 35.7 (32.8–38.7) and DBP by 19.9 (17.6–22.1) mmHg.
In stage 3 hypertension, initiation of treatment with the FDC of perindopril and amlodipine (in 17 patients) decreased baseline SBP by 46.1 (40.1–52.3) and DBP by 23.8 (19.9–27.7) mmHg, while initial treatment with the FDC of perindopril and indapamide (in 12 patients) decreased baseline SBP by 41.8 (32.6–50.9) and DBP by 26.1 (21.4–30.7) mmHg.
3.1.1. Blood pressure control
Blood pressure control was achieved, on an intention-to-treat basis, among 280 patients with stage 1 hypertension, in 185 (66.1%) with perindopril monotherapy; in an additional 18 (6.4%) with its indapamide FDC; and in an additional 12 (4.3%) with its amlodipine FCD. Among 116 patients with stage 2 hypertension, control was attained in 25 out of 41 (55.6%) with the FDC of perindopril and indapamide, and in 43 out of 75 (57.3%) with the FDC of perindopril and amlodipine. Of the 30 patients with stage 3 hypertension, control was reached in 3 out of 12 (25.0%) with the FDC of perindopril and indapamide, and in 5 out of 18 (27.8%) with the FDC of perindopril and amlodipine. Overall, 303 (71.1%) patients achieved blood pressure control.
3.2. Withdrawals cough and other adverse effects
Seven patients (1.6%) discontinued treatment. These were, among patients who received initial perindopril monotherapy, 4 (all lost to follow up); of those who received the FDC of perindopril and amlodipine, 1 (due to cough); and among those who received the FDC of perindopril and indapamide, 2 lost to follow up.
Dry cough that interfered with sleep of patients was reported, among those who received perindopril monotherapy, in 10/280 (3.6%); of those taking the FDC of perindopril and indapamide, in 1/140 (1.8%); and among those who received the FDC of perindopril and amlodipine, in 4/93 (4.3%). Cough led to discontinuation of treatment in 1/10 (0.1%). Of the other adverse effects occurring at a frequency of more than 1, ankle edema developed in 3/94 (3.2%) patients treated with the FDC of perindopril and amlodipine.
4. Discussion
This was a study on untreated, relatively young hypertensive patients (75% were below the age of 50 years). The frequency of diabetes, and cardiovascular risk factors was low, and more than two thirds presented with early hypertension. On an intention to treat basis, over a 3 month period, treatment of grade 1 hypertension with perindopril monotherapy, decreased blood pressure sufficiently to control 66% of patients. A further 10% could be controlled by the FDC of perindopril with indapamide or amlodipine. Initiating treatment with these FDC formulations was effective in controlling about 50% of patients with stage 2, and 25% of patients with stage 3 hypertension. Few patients discontinued treatment, and the frequency of cough that interfered with sleep and ankle edema was low.
Hypertension is a major risk factor of cardiovascular mortality,1 and treatment to reduce blood pressure has been shown to lower the frequency of coronary events.2 However, this evidence is based on drug trials that recruited mostly older patients. The impact of blood pressure lowering treatment on both the extent of blood pressure decrease, and reduction of cardiovascular risk in younger patients is uncertain.3 In the absence of such evidence, the recent NICE guidelines recommend that since younger patients tend to have higher renin levels, an ACEI may be appropriate to initiate treatment in patients below 55 years. If blood pressure control is not achieved, the addition of a calcium channel blocker (CCB), or alternatively a thiazide like diuretic such as Indapamide, has been suggested. The guidelines also recommend the use of long acting, once daily drug formulations to enhance compliance with medication,3 and these study results appear to be in consonance with the NICE guidelines.
A search of the literature suggests that only a few studies have assessed the effect of ACEI on the surrogate end point of blood pressure reduction in younger patients.7–9 In comparison to full dose captopril,9 our result with the lowest dose of perindopril (4 mg) or its FDC with 1.5 mg indapamide or 5 mg amlodipine, decreased blood pressure to a greater degree (by 16/5 mm Hg), achieved a greater frequency of blood pressure control (by 15%), with fewer withdrawals because of adverse effects (by 3.5%).
Although the FDCs of perindopril with Indapamide and amlodipine have not as yet been shown to reduce cardiovascular risk in hypertension, recent studies have assessed the two combinations with each agent given individually. In the ASCOT study on high risk hypertensive patients, treatment with amlodipine and perindopril for 5.5 years significantly reduced all cause mortality, cardiovascular events, and stroke.5 The HYVET study on elderly hypertensive patients, showed that treatment with Indapamide and perindopril for 2 years significantly reduced risk of all cause and cardiovascular mortality, stroke, and heart failure.6 The antihypertensive efficacy and acceptability of an ACEI, alone, or in an FDC with a CCB or Indapamide among younger patients has not previously been assessed, and the present study is the first to report such results.
This study has limitations. The antihypertensive treatments were not compared with other similar formulations using a randomized protocol. The results are over the short term, and the possible long term benefit was not assessed.
However, the strengths of this study are that it is on younger patients, seen in day-to-day practice, and representing the heterogeneity of India. This may serve to clear the doubt among Indian physicians that ACEIs have poor antihypertensive efficacy with the reassurances that the incidence of cough may be low with Perindopril.
The results of this study suggest that in younger patients requiring guideline recommended antihypertensive treatment, the regimen of perindopril alone or its FDC with Indapamide or amlodipine reduces blood pressure effectively, resulting in high rates of blood pressure control over the short term, with a low frequency of side effects including cough and pedal edema. Such treatment favors long term compliance and could help increase the frequency of blood pressure control among younger patients.
Conflicts of interest
All authors have none to declare.
Acknowledgment
Authors are thankful to Serdia Pharmaceuticals (India) Pvt. Ltd., the manufacturers of Coversyl AM and Coversyl Plus for providing organizational support to conduct the study. The authors would also like to express gratitude to the all the participating sites.
References
- 1.Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood pressure lowering regimens on major cardiovascular events: results of prospectively designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 2.Collins R., Peto R. Antihypertensive drug therapy: effects on stroke and coronary heart disease. In: Swales J.D., editor. Textbook of Hypertension. Blackwell Scientific Publications; London: 1994. pp. 1156–1164. [Google Scholar]
- 3.NICE Guidelines. 2011. [Google Scholar]
- 4.Mohan V., Deepa M., Farooq S., Datta M., Deepa R. Prevalence, awareness and control of hypertension in Chennai – The Chennai Urban Rural Epidemiology Study. JAPI. 2007;55:326–333. [PubMed] [Google Scholar]
- 5.Hansson L., Zanchetti A., Carruthers S.G. Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 6.ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. June 11 2007 Published online. [Google Scholar]
- 7.Deary A.J., Schumann A.L., Murfet H., Haydock S.F., Foo R.S., Brown M.J. Double-blind, placebo-controlled crossover comparison of five classes of antihypertensive drugs. J Hypertens. 2002;20:771–777. doi: 10.1097/00004872-200204000-00037. [DOI] [PubMed] [Google Scholar]
- 8.Dickerson J.E., Hingorani A.D., Ashby M.J., Palmer C.R., Brown M.J. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet. 1999;353:2008–2013. doi: 10.1016/s0140-6736(98)07614-4. [DOI] [PubMed] [Google Scholar]
- 9.Materson B.J., Reda D.J., Cushman W.C. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive. N Engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]


