Abstract
This is a report on a 10-year-old child with anomalous origin of left coronary artery (LCA) from pulmonary artery (ALCAPA), severe pulmonary hypertension (PH), old myocardial infarction and poor intercoronary collateralization. It discusses the echocardiographic pitfalls in this particular setting and introduces a new echocardiographic view (posterior pulmonary cusp view) for visualization of the anomalous origin of LCA from the posterior pulmonary cusp (PC) in patients with ALCAPA from the PC of the pulmonary artery. We describe three echocardiographic pitfalls that can mislead the echocardiographer and two helpful hints that guide the clinician to the correct diagnosis.
The survival of this child shows that limited size of left ventricular myocardial infarction and severe mitral regurgitation in early infancy can result in a life-saving pulmonary hypertension which preserves viability and function of left ventricle despite lack of intercoronary collateral arteries. After one year follow-up, she is doing well on medical treatment.
Keywords: ALCAPA, Severe pulmonary hypertension, Posterior pulmonary cusp view
1. Introduction
Anomalous origin of left coronary artery from pulmonary artery (ALCAPA) can present as endocardial fibroelastosis, myocardial infarction, mitral regurgitation, dilated cardiomyopathy, ventricular fibrillation or sudden death. Pulmonary hypertension usually develops in the setting of abundant intercoronary collateral arteries.1–6 Computerized tomography (CT) angiographic and echocardiographic features of this anomaly are already described.7,8
However, in ALCAPA with severe pulmonary hypertension (PH) and absence of adequate collateralization between right coronary artery (RCA) and left coronary artery (LCA), none of the diagnostic clues such as dilated RCA or retrograde flow into pulmonary artery (PA) may be present. Furthermore, close proximity of abnormal LCA to the aorta can mislead the echocardiographer and add to the diagnostic challenge. This is a report on a 10-year-old child with ALCAPA, severe PH, inadequate intercoronary collaterals and normal size RCA. The aim of this study is to introduce a novel echocardiographic view for visualization of posterior pulmonary cusp and to explain how to avoid the diagnostic pitfalls during echocardiographic examination in these particular subset of patients.
2. Case report
A 10-year-old girl was referred to our outpatient clinic with the diagnosis of chronic dilated cardiomyopathy since infancy. On physical examination, she was in no visible distress, pink, underweight, with normal neurologic and motor development and in New York Heart Association (NYHA) functional class II. On cardiac examination, pulses were normal. S1 was normal, S2 was loud and single and a grade 3/6 holosystolic murmur at left sternal border with radiation to the auxiliary area was audible. On CXR, cardiomegaly and all Kerley lines of A, B and C were visible. Electrocardiogram showed normal sinus rhythm, QRS axis of 0°, left ventricular hypertrophy with strain pattern with very high R voltages in V5 and V6 and pathologic Q in a VL and V5 and V6. Echocardiography revealed huge left atrium (LA), enlarged left ventricle (LV), LV ejection fraction of 55%, severe mitral regurgitation and moderate tricuspid regurgitation with a pressure gradient of 75 mmHg. Normal-size right and left coronary arteries seemed to arise from aorta. Papillary muscles were hyperechogenic (Movie Clips 1 and 2). She was admitted for further diagnostic evaluation and right and left heart catheterization was performed. The patient was intubated and received 100% oxygen during the procedure. On fluoroscopy, bilateral double shadow (density) was evident due to massive LA enlargement. Attempt to enter LA through a possible probe-patent foramen ovale, for direct measurement of LA pressure, failed. Systolic, early diastolic and end –diastolic pressures of the right ventricle (RV) were 100, 5 and 25 mmHg, respectively. Pulmonary arterial oxygen saturation and pressures were 84% and 90/63 (mean 75) mmHg. LV systolic pressure was 100, LV early diastolic pressure was 25 and LV end-diastolic pressure was 42 mmHg. Aortic pressure was 97/66 (mean = 79) mmHg. Pulmonary capillary wedge pressure was 40–42 mmHg. Cardiac output was calculated 5.5 L/min/m2 and pulmonary vascular resistance was 6.6 Woods unit. Pulmonary artery pressure at the baseline and after 100% oxygen were the same. Noteworthy, no pulmonary complaint or respiratory sign, secondary to airway compression by the huge LA, was present, neither on physical examination nor on CT scan.
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.ihj.2014.10.409.
The following are the supplementary data related to this article:
Shows massively dilated LA and dilated LV in four-chamber view.
Indicates how the echocardiogram can be misleading. In this movie, it seems factitiously that LCA arises from the aorta.
Aortic root angiography in 45° LAO and 45° RAO views showed no definite left coronary artery. There was no retrograde flow into the main pulmonary artery at levophase. Attempts to perform a selective right coronary angiography were failed because of lack of appropriate catheter. A 256-slice multi-detector computerized tomographic (CT) scan showed anomalous origin of LCA from the posterior pulmonary sinus. Thallium 201 single-photon emission computed tomography (SPECT) myocardial perfusion scan demonstrated sufficient viable myocardium throughout LV myocardium with mixed component of infarcted and viable myocardium in the apical and anteroseptal segment. Right ventricle was visualized in both phases. The patient was referred to the pediatric cardiac surgeon for implantation of LCA onto the aorta and mitral annuloplasty. However, the parents did not give consent for operation. She is doing relatively well on medical treatment, one year after diagnosis.
3. Discussion
We introduced a 10-year-old child with undiagnosed ALCAPA, followed for a long time with the diagnosis of dilated cardiomyopathy and very tricky echocardiographic findings. The echocardiographic findings were misleading. Thus, we decided to review the echocardiographic study to find the source of our diagnostic error. We measured longitudinal systolic strain and strain rate using the X-Strain software (MyLab 60; Esaote, Genova, Italy) by two-dimensional speckle tracking echocardiography. We compared the echocardiographic and CT angiographies images. This reflective review yielded four important findings.
3.1. Novel echocardiographic view: Posterior pulmonary cusp view
We looked to find a view to best profile the posterior cusp of the pulmonary valve. The patient underwent another repeated echocardiographic examination. The view proposed by Jureidini et al8 was obtained which failed to show the origin of LCA from the posterior pulmonary cusp. We planned and obtained a new echocardiogarphic view, guided by CT angiographic information that showed the anomalous LCA arising from the posterior pulmonary cusp. This new “posterior pulmonary cusp view” clearly visualized the origin of the abnormal LCA from the posterior cusp of the PA. To obtain this so-called “posterior pulmonary cusp view”; three simple steps were done: 1-A standard parasternal long-axis view was obtained. 2-From this parasternal long-axis view, the pulmonary valve was visualized by tilting the probe cephalad to visualize the pulmonary valve. 3-Then, while focused on the two right and left pulmonary cusps, the probe was gently tilted posteriorly in a very fine manner until the posterior cusp and the origin of the anomalous LCA appeared (Video Clip 3).
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.ihj.2014.10.409.
The following is the supplementary data related to this article:
Shows how to obtain the posterior pulmonary cusp view and also demonstrates the structures seen in this view.
3.2. Three sources of echocardiographic diagnostic error
The anomalous LCA was extremely close to the aorta, this misleading anatomy deceived the echocardiographer to conclude that left coronary artery is arising normally from aorta. The severe PH added to this challenge, because neither any clear retrograde flow could be seen into the RCA, nor any dilatation of this vessel was observed (Fig. 1F and G). Thus, the echocardiographer should be aware of the following three misleading findings in the setting of ALAPA, severe PH and no significant intercoronary collateral arteries:
-
1
RCA is not dilated.
-
2
No retrograde flow may be seen into RCA.
-
3
LCA may seem to arise from aorta because of extreme proximity of the anomalous LCA to the aortic cusp.
Fig. 1.
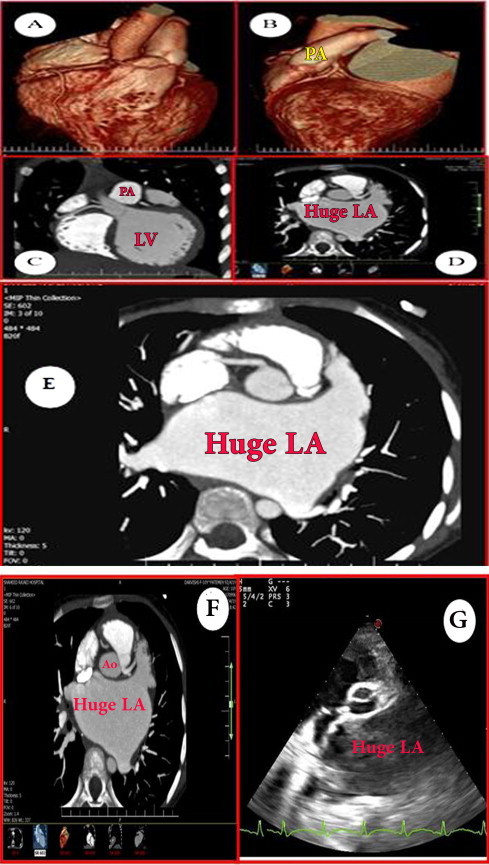
Image obtained by 256-multi-slice coronary CT angiography. A and C show RCA and conus artery arise separately from the aorta. B and D indicate anomalous origin of LCA from the posterior cusp of the pulmonary artery. In D, note the very close proximity of LCA to the aorta. This close proximity, here called the “proximity error”, misleads the echocardiographer. E shows that the anomalous LCA arises from the posterior cusp (see text); note that RCA is not enlarged, indicative of poor or no intercoronary collateralization. F and G clearly show how the proximity of the anomalous LCA to the aorta can produce misleading images on echocardiogram.
3.3. Two indirect diagnostic hints
We found two very helpful indirect echocardiographic hints. These clues are hyperechogenicity of papillary muscles and regional decrease in longitudinal and transverse peak systolic strain and strain rate of LV in the apical septal, mid-septal and mid-lateral segments compared with other walls.9,10,11
3.4. Speculations on pathophysiology of PH in this case
Courand et al reported 6 cases of late presentation of ALCAPA during a 36 years period. All patients aged more than 40 years and, in contrast to this case, all had plentiful intercoronary collaterals and dilation of RCA.12 Iriat et al showed that measurement of 2D strain is helpful in detection of subclinical ischemia in a 13-year-old patient with ALCAPA. Pulmonary artery pressure was not mentioned in their report.12 Pathophysiologically, patients with ALCAPA either have the infantile type and die early because of poor intercoronary collateralization or have the adult type with adequate collateralization which enables them to survive beyond infancy13. This case, cannot be included in neither of these two former groups. She probably developed a limited-size of myocardial infarction in early infancy leading to severe mitral regurgitation, but with no detrimental effect on myocardial function. This severe MR, caused early post-capillary pulmonary hypertension,14 which was life-saving for this child, despite lack of adequate intercoronary collateral arteries.
In summary, this report introduces a new echocardiographic view to visualize the origin of LCA from the posterior cusp of pulmonary artery in patients with ALCAPA from the posterior cusp of pulmonary artery. The echocardiographer should not get deceived by seeing two seemingly normal coronary arteries arising from aorta in challenging cases with severe PH. Proximity of anomalous LCA to the aortic wall can easily mislead the echocardiographer.
Conflicts of interest
The author has none to declare.
Acknowledgment
This case was introduced on Congenital Cardiovascular Interventional Study Consortium (CCISC) and pediheartnet listserve in June 2013. The author is most grateful to all the colleagues who so kindly shared their invaluable comments and advice on this case.
References
- 1.Gebhard C., Stahli B.E., Greutmann M. Reduced left ventricular compacta thickness: a novel echocardiographic criterion for non-compaction cardiomyopathy. J Am Soc Echocardiogr. 2012;25:1050–1057. doi: 10.1016/j.echo.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Clavier T., Hardy J.B., Devellenne C. Anomalous origin of the left coronary artery from the pulmonary artery presenting as sudden death in a young woman. Am J Respir Crit Care Med. 2013; 15;187:1394–1395. doi: 10.1164/rccm.201211-2023LE. [DOI] [PubMed] [Google Scholar]
- 3.Uysal F., Bostan O.M., Semizel E., Signak I.S., Asut E., Cil E. Congenital anomalies of coronary arteries in children: the evaluation of 22 patients. Pediatr Cardiol. 2014;35:778–784. doi: 10.1007/s00246-013-0852-8. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J., Ding W., Xiao Y. Anomalous origin of the left coronary artery from the pulmonary artery in children: 15 years experience. Pediatr Cardiol. 2011;32:24–31. doi: 10.1007/s00246-010-9798-2. [DOI] [PubMed] [Google Scholar]
- 5.Kristensen T., Kofoed K.F., Helqvist S., Helvind M., Søndergaard L. Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) presenting with ventricular fibrillation in an adult: a case report. J Cardiothorac Surg. 2008;26:33. doi: 10.1186/1749-8090-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta A., Shah S., Misri A., Suresh P.V., Maheshwari S. Severe mitral regurgitation: a misleading presentation. Indian Heart J. 2009;61:303–305. [PubMed] [Google Scholar]
- 7.Ripley D.P., Gosling O.E., Harries S. Multimodality imaging in Bland-White-Garland Syndrome in an adult with a left Dominant coronary artery System. Congenit Heart Dis. 2014;9(4):E110–E112. doi: 10.1111/chd.12092. [DOI] [PubMed] [Google Scholar]
- 8.Oncel G., Oncel D. Anomalous origin of the left coronary artery from the pulmonary artery: diagnosis with CT angiography. J Clin Imaging Sci. 2013;30:4. doi: 10.4103/2156-7514.106618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varghese M., Kothari S. The caveats in the diagnosis of anomalous origin of left coronary artery from pulmonary artery (ALCAPA) Images Paediatr Cardiol. 2010;12(3):3–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Carasso S., Rakowski H., Witte K.K. Left ventricular strain patterns in dilated cardiomyopathy predict response to cardiac resynchronization therapy: timing is not everything. J Am Soc Echocardiogr. 2009;22:242–250. doi: 10.1016/j.echo.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Marcus K.A., Mavinkurve-Groothuis A.M., Barends M. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr. 2011;24:625–636. doi: 10.1016/j.echo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Courand P.Y., Bozio A., Ninet J. Focus on echocardiographic and Doppler analysis of coronary artery abnormal origin from the pulmonary trunk with mild myocardial dysfunction. Echocardiography. 2013;30:829–836. doi: 10.1111/echo.12124. [DOI] [PubMed] [Google Scholar]
- 13.Iriart X., Jalal Z., Derval N. Two-dimensional strain as a marker of subclinical anterior ischaemia in anomaly of left coronary artery arising from pulmonary artery. Eur J Echocardiogr. 2009;10:732–735. doi: 10.1093/ejechocard/jep070. [DOI] [PubMed] [Google Scholar]
- 14.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013 24;62(25 Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shows massively dilated LA and dilated LV in four-chamber view.
Indicates how the echocardiogram can be misleading. In this movie, it seems factitiously that LCA arises from the aorta.
Shows how to obtain the posterior pulmonary cusp view and also demonstrates the structures seen in this view.


