Abstract
Background
There are no Indian studies correlating the CT pulmonary embolism index (Qanadli) with right ventricular function and outcome. In the present study we aimed to study the clinical manifestations of patients presenting with acute pulmonary thromboembolism and correlate the radiographic features with echocardiographic features and outcome.
Methods
Thirty five patients presenting with symptomatic acute pulmonary thromboembolism in between 2011 and 2013 were studied for clinical, radiological and echocardiographic features and outcome (in-hospital & 1 month follow up).
Results
The mean duration of presentation after onset of symptoms was 5.7 ± 3.7 days. Right ventricular dysfunction was observed in 11 (31.4%) patients. Out of 35 patients in whom CT pulmonary angiogram performed, 14 patients had Qanadli PE index >60% of whom 11 (78.6%) patients had right ventricular dysfunction. None had right ventricular dysfunction when PE index was <60% (p < 0.001). There was significant correlation between pulmonary vascular obstruction index and right ventricular dysfunction (p < 0.0001). Nine (25.7%) patients were thrombolysed with Streptokinase. Total mortality including in-hospital and 1 month follow up was 11.4% (4 patients). The mortality in patients with PE index >60% was 21.4% and was nil with <60% (p = 0.02). The mortality in patients with right ventricular dysfunction was 27.2% and was nil without right ventricular dysfunction (p = 0.0075).
Conclusion
A PE index which was shown to be a strong independent predictor of right ventricular dysfunction in PE, correlating linearly with different variables associated with higher morbidity and mortality, enabling accurate risk stratification and selection of patients for more aggressive treatment.
Keywords: Qanadli PE index, Acute pulmonary embolism, RV dysfunction
1. Introduction
Pulmonary embolism (PE) is a common problem, yet it is often difficult to diagnose. No diagnostic test for PTE has utility unless it is considered in the differential diagnosis. Therefore, clinicians must remain vigilant to detect PE. Pulmonary embolism ranges from asymptomatic, incidentally discovered emboli to massive embolism causing immediate death.
The occurrence of Venous thrombosis (VT) is a result of interplay between environmental and genetic risk factors.1 Important environmental risk factors are advancing age, male sex and obesity, precipitated by surgery, trauma, cancer, immobilization (surgical or medical causes), pregnancy and use of exogenous hormones.2,3 Genetic risk factors increase the risk of VT during risk periods and of idiopathic VT i.e. VT occurring in the absence of environmental triggers.4,5
If pulmonary embolism is suspected, a careful assessment of the patient's history, physical examination, and known risk factors need to be done. Electrocardiographic (ECG) abnormalities like unexplained tachycardia and manifestations of acute cor pulmonale, such as an S1, Q3, T3 pattern, right bundle branch block, P-wave pulmonale, or right axis deviation, are more common with massive embolism than with smaller emboli, but these findings are also nonspecific.6
The chest radiograph is usually nondiagnostic, but it may help in making an alternative diagnosis. An unexplained change in arterial oxygen saturation should raise suspicion because patients with acute pulmonary embolism are usually manifested with hypoxemia.6
Many modalities of imaging studies have been used in the diagnosis of acute pulmonary embolism, including ventilation–perfusion scanning, contrast-enhanced computed tomographic (CT) arteriography, magnetic resonance imaging (MRI), and standard pulmonary arteriography. Ultrasonography, CT venography, MRI, and standard venography imaging are used for detecting deep venous thrombosis as a surrogate for acute pulmonary embolism.
The multidetector CT arteriography with very thin sections, and low scanning times, has the highest sensitivity and specificity for detecting emboli in the main, lobar, or segmental pulmonary arteries.7
Risk stratification in acute PE may allow some aggressive therapeutic approaches to be undertaken so that we can reduce mortality from ∼30% to <10%. Computed Tomographic pulmonary angiography findings are usually reported either positive or negative even in present era with little or no quantitative description about the amount of thrombus present. The prognosis of a patient with a single isolated subsegmental embolus varies widely when compared to a patient with a saddle embolus. The clot burden expressed in the form of an index may have important prognostic and therapeutic implications and may even provide a reproducible standard for measuring response to management.8
Various clot burden scores have been described. Among them Qanadli PE index is simple and easily calculated, which was derived from the amount and location of the thrombus on CT images on the basis of a study.8 The index is defined as the product of N × D, where N is the value of the proximal clot site, equal to the number of segmental branches arising distally, and D is the degree of obstruction, defined as 1 for partial obstruction and 2 for total obstruction.
The presence of embolus in a segmental artery was scored 1 point. This value was multiplied by a factor D, defined as 1 for a partially occlusive embolus and 2 for a completely occlusive embolus. Thus, a totally occlusive thrombus in a segmental artery was assigned a value of 2. A clot in a proximal artery that gives rise to segmental arteries was scored as though each segmental artery had an embolus with the same degree of obstruction as the proximal embolus. Sub-segmental emboli were scored as a partial obstruction of the segmental artery. Accordingly, the maximum obstruction score for each patient was 40 (20 for each lung), and this score was converted into a percentage.
Echocardiography can aid in deciding the management strategy by providing findings that strongly suggest hemodynamically significant pulmonary embolism.9 In patients without pre-existing cardiopulmonary disease, for the rise in pulmonary pressures there must be occlusion of at least 25–30% of the pulmonary vasculature and 50–75% before RV failure ensues.6 Even patients who may initially appear clinically stable, can develop hypotension and shock rapidly once the RV begins to fail due to high afterload. The physiologic reaction to the vasoreactive substances released in response to the event and the cardiopulmonary status of the patient at baseline can determine the hemodynamic response to an acute PE episode.6
The right ventricular dysfunction is defined as presence of right ventricular hypokinesis, interpreted by a qualitative evaluation of the right ventricular wall motion.10 Acute right ventricular dysfunction can be considered when 1 or more of the following findings present: right ventricular dilation (end-diastolic diameter >30 mm or right ventricular–left ventricular end-diastolic diameter ratio >1 in 4-chamber view); or paradoxical septal systolic movements or pulmonary hypertension (defined as Doppler pulmonary acceleration time <90 ms or presence of a right ventricular-atrial gradient >30 mm Hg). In addition, absence of right ventricular wall hypertrophy (free wall thickness, >7 mm) is needed.11
The prognosis of PE correlates directly with the degree of hemodynamic compromise and symptomatic or asymptomatic RV dysfunction. Cardiogenic shock usually occurs in less than 5% of patients with PE. The mortality of patients with cardiogenic shock ranges from 25 to 40%, and is as high as 65–95% in patients requiring mechanical ventilation and cardiopulmonary resuscitation.12 The patients with massive PEs (defined as >60% occlusion of the pulmonary vasculature) who do not have clinical cardiogenic shock, have a much lower mortality than those patients with shock.12 Although massive PEs account for nearly 50% of the mortality in pulmonary embolism the remaining fatalities occur in patients with submassive or recurrent pulmonary emboli due to presence of other comorbidities like malignancy or other cardiopulmonary diseases.
There are few studies done in India evaluating the usefulness of the Qanadli PE index in assessing impact of clot burden on hemodynamic status, right ventricular function, survival rates and management of patients presenting with acute PE. Hence we attempted to study the clinical utility of the index.
2. Materials and methods
A total of 52 patients with clinical suspicion of acute pulmonary thromboembolism who presented to the Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati from January 2011 to December 2012 were screened in this prospective study. Among them 35 patients in whom the diagnosis of pulmonary embolism was confirmed by CT pulmonary angiogram were included in the study.
In all the patients, detailed history was obtained regarding the presenting complaints, mode of onset of symptoms and duration followed by a thorough physical examination. Electrocardiogram and chest roentgenogram were taken for all patients.
All patients were subjected to CT pulmonary angiography with intravenous contrast by 128 slice SEIMENS SOMATOM Definition AS+ computed tomographic machine. CT pulmonary obstruction index calculated by number of segmental or branch pulmonary arteries involved and expressed in percentage of obstruction.
Echocardiogram was performed using PHILIPS IE33 echocardiography machine (Netherlands). In all patients right atrial and right and left ventricular sizes were measured. Estimation of tricuspid regurgitation jet velocity measured in apical four chamber view by continuous wave Doppler. Right ventricular function was assessed using TAPSE (tricuspid annular plane systolic excursion).
Patients eligible for thrombolysis were treated with streptokinase. All patients were treated with intravenous unfractionated heparin/low molecular weight heparin, and oral anticoagulation was started simultaneously. Supportive management was given as needed i.e. ionotropes and mechanical ventilation.
The outcome of in-hospital stay was analyzed in terms of occurrence of death, and need for assisted mechanical ventilation by Kaplan–Meier survival curve. The Receiver operating curve was used for the cut-off value of PE index. The association between categorical variables and the outcome was evaluated by χ2 test and Fisher's exact test. All statistical analysis was carried out by Statistical Package for Social Sciences (SPSS) software, version 20.0, SPSS Inc., Chicago, USA.
3. Results
The mean age of the patients was 47.1 (±12.4) years and ranged from 25 years to 75 years. Among the patients 20 were males and 15 were females. The Mean duration of presentation after onset of symptoms was 5.7 days (±SD 3.7 days). The baseline clinical characteristics are given in Table 1.
Table 1.
Baseline clinical characteristics.
| Present study (n = 35) | Number (%) |
|---|---|
| Dyspnea | 33 (94.3) |
| Cough | 16 (45.7) |
| Chest pain | 11 (31.4) |
| Hemoptysis | 02 (5.7) |
| Leg swelling | 13 (37.1) |
| Palpitations | 09 (25.7) |
| Tachypnea (>20 & min) | 22 (62.8) |
| Rales (crackles) | 09 (25.7) |
| Tachycardia (>100/min) | 24 (68.6) |
| Increased pulmonary component of second sound | 11 (31.4) |
| Deep venous thrombosis | 09 (25.7) |
| Diaphoresis | 09 (25.7) |
| Temperature >38.5 °C | 02 (5.7) |
| Systolic blood pressure <90 mmHg | 09 (25.7) |
| Homan's sign | 04 (11.4) |
| Right ventricular lift | 03 (8.6) |
| Pleural friction rub | 03 (8.6) |
| Third heart sound | 05 (14.3) |
| Cyanosis | 01 (2.8) |
The majority of patients with PE, 34 of 35 (97.1%), were in sinus rhythm while atrial fibrillation occurred in 1 of 35 (2.8%). S1Q3T3 pattern was present in 18 patients (51.4%). Nonspecific abnormalities of the ST segment or T-wave were the most common ECG abnormalities and one or both occurred in 15 of 35 (42.8%) patients with PE. P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block occurred in 6% of patients with PE.
Echocardiogram showed dilated right atrium in 25 patients (71.4%) and both right atrial and right ventricular dilatation (RV/LV > 1) in 20 patients (57.4%). Pulmonary artery hypertension of moderate to severe degree was evident in 20 patients (57.4%). Right ventricular dysfunction observed in 11 patients (31.4%). Presence of thrombus in pulmonary artery, right atrium and right ventricle was evident in 6 (17.1%), 3 (8.6%) and 2 patients (5.7%) respectively.
Preexisting cardiac abnormalities were seen in few patients. Severe left ventricular dysfunction was present in one patient. One patient had preexisting rheumatic heart disease in the form of moderate mitral stenosis. The echocardiogram was normal in 2 patients (5.7%).
CT pulmonary angiography showed thrombus in main or branch PA in 21 (60.0%) patients. Among these 14 (40.0%) had massive burden of thrombus. Remaining 14 (40.0%) patients had thrombus in segmental branches. The clot burden was quantitated by Qanadli PE index. The PE index was >60% in 14 patients and <60% in 21 patients. The mean PE index was 51.8% (±17.3).
Out of 35 patients, 14 had PE index >60%. Among the patients with PE index >60%, 11 patients had right ventricular dysfunction. None had right ventricular dysfunction when PE index was <60% (p < 0.001) (Figs. 1 and 4).
Fig. 1.
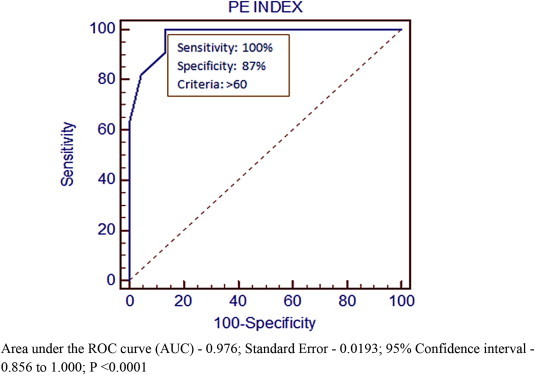
ROC curve for RV dysfunction and Qanadli PE index.
Fig. 4.
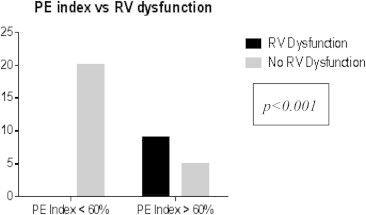
Chi-square test for PE index vs RV dysfunction.
Mean duration of hospital stay was 7.5 days (range 5–13 days). Total 9 patients (25.7%) were thrombolysed. Among these 4 patients (11.4%) required mechanical ventilation. Two patients recovered with mechanical ventilator support and other 2 patients couldn't.
Total mortality including in-hospital and 1 month follow up was 11.4% (4 patients). Among these, 3 patients who had PE index >60% and right ventricular dysfunction died in hospital during the initial admission. Two patients who died during the initial admission had pre-existing congestive heart failure due to cardiomyopathy, and the third patient had lung malignancy. One patient who had ovarian malignancy died after one month due to bleeding complication.
The mortality in patients with PE index >60% was 21.4% (p < 0.05) (Fig. 5) The mortality in patients with right ventricular dysfunction was 27.2% (p < 0.05) (Fig. 6) Regression analysis and Kaplan–Meier survival analysis showed PE index as independent predictor of mortality (Table 2, Fig. 7).
Fig. 5.
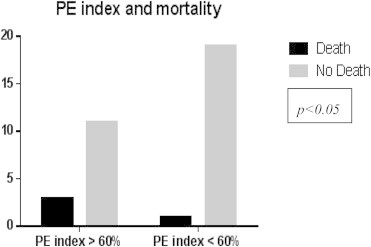
Chi-square test for PE index vs mortality.
Fig. 6.
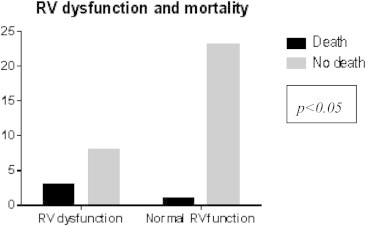
Chi-square test for RV dysfunction vs mortality.
Table 2.
Regression analysis showed PEI is an independent predictor of mortality.
| Independent variables | Coefficient | Std. error | T | p-value |
|---|---|---|---|---|
| (Constant) | 0.08305 | |||
| Age | 0.0003351 | 0.003652 | 0.0918 | 0.9276 |
| Sex | 0.07455 | 0.08805 | 0.847 | 0.4049 |
| Cardiac | −0.2630 | 0.1673 | −1.572 | 0.1281 |
| Cancer | 1.0788 | 0.1871 | 5.765 | <0.0001 |
| RVD | 0.3247 | 0.1416 | 2.294 | 0.0301 |
| PE index | 0.32987 | 0.13702 | 2.307 | 0.0312 |
Fig. 7.
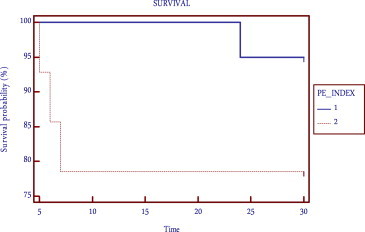
Kaplan–Meier survival curve for PE index.
4. Discussion
The present study was aimed at studying the clinical profile, radiographic features and outcome in patients with acute PE in India.
The clinical examination of the patients in the present study showed tachypnea and tachycardia as most common clinical signs. Hypotension and tachycardia as signs of cardiogenic shock were present in one fourth of patients which helped in the management of the patients, because both signs correlated well with the severity of pulmonary vascular obstruction in CT angiography (Figs. 2 and 3).
Fig. 2.
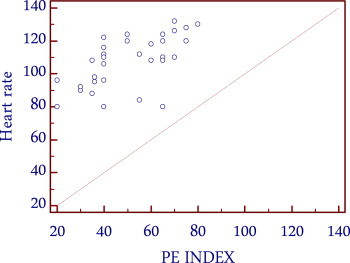
Correlation between heart rate and PE index.
Fig. 3.
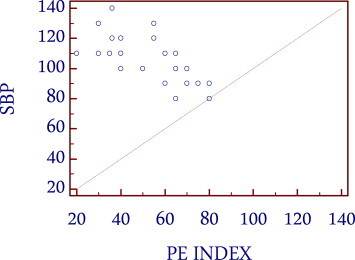
Correlation between systolic blood pressure and PE index.
There are several electrocardiographic (ECG) abnormalities associated with PE, but none are highly specific or sensitive. In patients with RV failure, the ECG may show sinus tachycardia, signs of RV strain, repolarization abnormalities, or ischemia including a complete or incomplete right bundle branch block, a right ward axis (>90°), an S1Q3T3 pattern, a Qr in lead V1, ST elevation in V1, or precordial T-wave inversions. The most frequent abnormalities were sinus tachycardia and nonspecific changes of the ST segment or T-wave. Abnormalities of the ST segment or T-wave previously have been reported in more than 40% of patients with PE who had no pre-existing cardiac or pulmonary disease.6,13 In the present study it was 42.8% of patients. While the electrocardiogram cannot be used as a diagnostic tool for PE, it may suggest the presence of underlying RV dysfunction and/or reveal important alternative diagnoses (such as myocardial infarction) in patients presenting with hypotension.
In patients with acute PE, the presence of RV dysfunction affords an increased mortality. In the present study the right ventricular dysfunction showed a significant correlation to mortality (p < 0.05) (Fig. 6). Six studies, including a total of 1773 patients, showed that patients with right ventricular dysfunction had at least a 2-fold higher risk of dying in the short term, as compared with patients without right ventricular dysfunction.14 The absolute difference ranged from 4% to 18%.13 This increase is supported by the multivariate analysis in the study by Ribeiro et al, which showed a relative risk of 6.0.15 In a prospective study by Grifoni Solivotto et al of 209 patients with documented PE, TTE was found to have a 100% negative predictive value for predicting PE related death on the basis of RV dysfunction, but a positive predictive value was only 5%.11 Thus, transthoracic echo is useful for early detection of RV dysfunction in normotensive patients who are at higher risk for hemodynamic decompensation, but with a low specificity (61%).
A pulmonary vascular obstruction of >30% has been shown to correlate with the presence of RV dysfunction on echo.13 The present study also showed good correlation between pulmonary vascular obstruction index and right ventricular dysfunction (p < 0.0001) (Fig. 4).
CT pulmonary angiography (CTPA) has dramatically improved the quality of imaging of the pulmonary vasculature in the last decade and has changed the diagnostic approach to suspected acute pulmonary embolism. The average sensitivity and specificity for detecting pulmonary embolism has been shown to be 88% and 92–96%, respectively.16 However, the interpretation of CTPA is mainly focused on the presence or absence of emboli and a rough estimation of their severity (massive, extensive, or isolated). A few published articles confront the challenge of quantifying pulmonary arterial tree obstruction by developing an obstruction index. In our study, the obstruction index proposed by Qanadli et al8 was used because of the simplicity of applying it in routine clinical practice.
The mean value of the pulmonary artery obstruction index in the present study was 51.8% (±17.3) which is higher than that described by other investigators (Metafratzi ZM et.al17 39% ± 27%, Qanadli et al8 29% ± 17%). A significant correlation also exists between the index of Qanadli et al and right ventricle dilatation. ROC curve analysis (Fig. 1) determined a cut-off value for Qanadli PEI8 of 60% for the presence of signs of RVD which is more than that obtained by Qanadli et al8 (40%), and similar to that of Wu et al18 (60%). A CT obstruction of 60% or greater will identify more than 90% of patients with right ventricular dilatation. The present study shows 78% of patients with right ventricular dysfunction had an index of >60%.
The PE index may have important prognostic value, since patients with a pulmonary vascular obstruction of more than 60% tended to have a poor clinical outcome (Table 2). In the study by Wu AS et.al, 83% of the patients with a PE index of higher than 60% died, while 52 of 53 (98%) patients with an index of less than 60% lived.18 In our study only 21.4% of patients died with PE index more than 60% (Fig. 5). But 19 of 20 (95%) patients with PE index less than 60% survived (Fig. 7). This finding, if valid, would allow the stratification of a patient's risk of death and might help identify patients who would benefit from more aggressive treatment strategies, such as thrombolysis.
Management of patients with acute PE is dictated by the presence or absence of hemodynamic compromise. Patients presenting with PE in cardiogenic shock should immediately receive vasopressor support, anticoagulation, and then be considered for thrombolysis and/or embolectomy where available. In our study all 9 patients (26%) with hemodynamic instability were thrombolysed.
Meta-analyses of lytic therapy trials have shown that the majority of benefit from thrombolytic therapy is limited to PE patients with hemodynamic instability. While normotensive patients with evidence of RV dysfunction and PE index >60% have increased mortality and thrombolytic therapy has been shown to improve RV dysfunction, definitive data demonstrating a significant morbidity and/or mortality benefit from lytic therapy in this population is lacking. Regression analysis and Kaplan–Meier survival analysis showed that PE index as an independent predictor of mortality (Table 2, Fig. 7). Further studies are needed to determine which hemodynamically stable patients will benefit from lytic therapy in the setting of RV dysfunction and high PE index.
Death strongly associated with cancer, age, and cardiovascular disease.19 In our study 4 patients died of which 2 patients had malignancy and other 2 had prior left ventricular dysfunction.
The main limitation of the study was a small sample size, so the statistical significance of PE index, right ventricular dysfunction and mortality may not be comparable to the larger studies.
In conclusion early diagnosis and risk stratification will reduce the mortality. A PE index which was shown to be a strong independent predictor of right ventricular dysfunction in PE, correlating linearly with different variables associated with higher morbidity and mortality, enabling accurate risk stratification and selection of patients for more aggressive treatment.
Conflicts of interest
All authors have none to declare.
References
- 1.Rosendaal F.R. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–1173. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 2.Beasley R., Raymond N., Hill S., Nowitz M., Hughes R. eThrombosis: the 21st century variant of venous thromboembolism associated with immobility. Eur Respir J. 2003;21:374–376. doi: 10.1183/09031936.03.00039403. [DOI] [PubMed] [Google Scholar]
- 3.Bick R.L. Coagulation abnormalities in malignancy: a review. Semin Thromb Hemost. 1992;18:353–372. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg J.S., Wells P.S., Brill-Edwards P. Antiphospholipid antibodies and venous thromboembolism. Blood. 1995;86:3685–3691. [PubMed] [Google Scholar]
- 5.Tapson V.F., Carroll B.A., Davidson B.L. The diagnostic approach to acute venous thromboembolism: clinical practice guideline. Am J Respir Crit Care Med. 1999;160:1043–1066. doi: 10.1164/ajrccm.160.3.16030. [DOI] [PubMed] [Google Scholar]
- 6.Stein P.D., Terrin M.L., Hales C.A. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100:598–603. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 7.Schoepf U., Holzknecht N., Helmberger T.K. Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology. 2002;222:483–490. doi: 10.1148/radiol.2222001802. [DOI] [PubMed] [Google Scholar]
- 8.Qanadli S.D., El Hajjam M., Vieillard-Baron A. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415–1420. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber S.Z., Visani L., De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 10.Kasper W., Konstantinides S., Geibel A., Tiede N., Krause T., Just H. Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart. 1997;77:346–349. doi: 10.1136/hrt.77.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grifoni S., Olivotto I., Pieralli F. Long-term clinical outcome of patients with pulmonary embolism with or without right ventricular dysfunction. Thromb Haemost. 2001;86 [Google Scholar]
- 12.Douketis J.D. Prognosis in pulmonary embolism. Curr Opin Pulm Med. 2001;7:354–359. doi: 10.1097/00063198-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues B., Correia H., Figueiredo A. Clot burden score in the evaluation of right ventricular dysfunction in acute pulmonary embolism: quantifying the cause and clarifying the consequences. Rev Port Cardiol. 2012 Nov;31:687–695. doi: 10.1016/j.repc.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Rozkovec A., Mantanes P., Oakley C.M. Factors that influence the outcome of primary pulmonary hypertension. Br Heart J. 1986;55:449–458. doi: 10.1136/hrt.55.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro A., Lindmarker P., Juhlin-Dannfelt A. Echocardiography Doppler in pulmonary embolism: RV dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479–487. doi: 10.1016/s0002-8703(97)70085-1. [DOI] [PubMed] [Google Scholar]
- 16.Remy-Jardin M., Remy J., Wattinne I., Giraud F. Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique—comparison with pulmonary angiography. Radiology. 1992;185:381–387. doi: 10.1148/radiology.185.2.1410342. [DOI] [PubMed] [Google Scholar]
- 17.Metafratzi Z.M., Vassiliou M.P., Maglaras G.C. Acute pulmonary embolism: correlation of CT pulmonary artery obstruction index with blood gas values. AJR Am J Roentgenol. 2006 Jan;186:213–219. doi: 10.2214/AJR.04.1320. [DOI] [PubMed] [Google Scholar]
- 18.Wu A.S., Pezzullo J.A., Cronan J.J., Hou D.D., Mayo-Smith W.W. CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome – initial experience. Radiology. 2004;235:831–835. doi: 10.1148/radiol.2303030083. [DOI] [PubMed] [Google Scholar]
- 19.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003 Jun 17;107(23 suppl 1):4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]


