Abstract
Biofilm formation is often associated with increased Candida resistance toward antifungal agents. Therefore, the current study aimed to assess the incidence of biofilm formation among Candida isolates and to investigate the effect of high doses of fluconazole {FLC}, voriconazole {VOC} and amphotericin B {AMB}, singly and in combination on mature biofilms. Moreover, it aimed to assess the expression of selected genes (CDR1, KRE1 and SKN1) responsible for Candida biofilm resistance. The study included 49 patients; samples were collected from the King Khalid Hospital, Riyadh, Saudi Arabia. Isolates were prepared for biofilm formation and quantification using 0.4% (w/v) crystal violet. Minimum Inhibitory concentration (MIC) and fractional inhibitory concentration (FIC) were conducted by the broth microdilution method. Biofilm eradication was evaluated using counting, XTT stain intensity and observed under the inverted microscope. Selected genes were evaluated in Candida biofilms under the effect of antifungal exposure using QPCR. The major isolates were Candida albicans (65.3%) followed by Candida tropicalis and Candida glabrata. 77.6% of the strains were biofilm formers. AMB showed susceptibility in 87.8% of isolates, followed by VOC (77.6%) and FLC (67.3%). MIC50 and MIC90 were (0.03, 0.125), (0.5, 8), (2, >128) μg/ml for AMB, VOC and FLC, respectively. 34.7% and 18.4% of the isolates were antagonistic to AMB/FLC and AMB/VOC, respectively. Mature biofilms of ten selected isolates were found resistant to FLC (1000 μg/ml). VOR and AMB concentration required to inhibit biofilm formation was 16–250 fold higher than the MIC for planktonic cells. Isolates showed significant reduction with antifungal combination when compared with the untreated controls (p value ⩽ 0.01), or using fluconazole alone (p value ⩽ 0.05). High doses of the antifungals were employed to assess the effect on the persisters’ selected gene expression. Marked over expression of SKN1 and to a lesser extent KRE1 was noticed among the mature biofilms treated with AMB alone or in combination after 1 h of exposure, and SKN1 expression was even more sharply induced after 24 h. No statistically significant over expression of CDR1 was observed in biofilms after exposure to high doses of FLC, VOC or any of the combinations used.
Keywords: Candida spp., Biofilm, Antifungal, CDR1, SKN1, KRE1
1. Introduction
Candida species are the most common cause of fungal infections. Candida induced infections range from non-life-threatening mucocutaneous illnesses to invasive processes that may involve virtually any organ. The growing frequency of hospital acquired Candida especially bloodstream infections is due to the increased use of immunosuppressive therapy in cancer and transplant patients, which leads to breakdown of the barrier between the gut and bloodstream (Nucci and Anaissie, 2001).
Candida cells, as proven in many studies, are able to adhere to and colonize surfaces of medical devices, such as central venous catheters, orthopedic prostheses, intrauterine devices and prosthetic joints and valves, among others, resulting in the development of a biofilm (Douglas and Cobbs, 1992; Raad et al., 1993; Tunney et al., 1999). Infections due to the presence of fungal biofilms are a major clinical concern as these structures are characterized by increased resistance to antifungal therapy (Ramage et al., 2006).
Various antifungal agents are used to treat these infections, including azoles and polyenes (Pappas et al., 2004). Fluconazole (FLC) as well as voriconazole (VOC), approved in 2002, belong to the tiazoles, they interfere with ergosterol biosynthesis by binding to lanosterol 14-α demethylase (Richardson, 1990). The latter enzyme is crucial for ergosterol production, and inhibition of its activity which causes disruption of the cell membrane leading to growth inhibition of the fungus (Kelly et al., 1993). Amphotericin B (AMB) is a member of the polyene family (Warnock, 1991). This molecule binds to ergosterol and forms pores resulting in a disorganized membrane with increased permeability. In addition, AMB induces cell damage by generating lethal reactive oxygen species (Brajtburg et al., 1990).
The progression of drug resistance within Candida biofilms has been associated with a parallel increase in the maturation process (Sardi et al., 2011). Furthermore, some studies have shown that biofilms of Candida develop statically in the presence of a minimal matrix and exhibit the same level of resistance to antifungal treatment; as cells grown in shaker and exhibiting large amounts of matrix (Seneviratne et al., 2008; Sardi et al., 2011). However, several molecular mechanisms of resistance to antifungal agents in Candida have been described. In particular, these include the increased efflux of antifungal agents due to the overexpression of efflux genes, CDR1 and CDR2 (the family of ABC membrane transport proteins – the ATP binding cassette) (Sardi et al., 2011). Moreover, changes in β-1,6-glucan biosynthesis have also been proposed as a resistance mechanism against AMB (Gale, 1986). SKN1 and KRE1, two genes involved in β-1,6-glucan biosynthesis (Mio et al., 1997), were found to be differentially expressed after in vitro exposure to antifungal treatment (Liu et al., 2005).
A combined action of different mechanisms is believed to contribute to increased resistance, especially in the presence of persisters in the biofilm, which are able to tolerate high concentrations of antimycotics (Seneviratne et al., 2008). Interestingly, these persisters are not mutants but rather phenotypical variants of wild type cells (LaFleur et al., 2006). Until now, the molecular basis of persistence in Candida species biofilms is not fully understood (Lewis, 2010).
Most of the published studies examining the transcriptional expression of drug-resistance genes in Candida spp. have been confined to the planktonic mode of growth, and few data are available for the biofilm mode (Sanglard et al., 1995; White, 1997; Lopez-Ribot et al., 1998; Franz et al., 1998, 1999; Perea et al., 2001; Holmes et al., 2008). Therefore, the current study aimed to assess the incidence of biofilm formation among Candida clinical isolates and to investigate the effect of high doses of commonly used antifungal drugs (fluconazole, voriconazole and amphotericin B, singly and in combination) on Candida mature (48 h) biofilms. Moreover, it aimed to assess the expression of selected genes (CDR1, KRE1 and SKN1) of those known to be responsible for resistance in Candida biofilms under the effect of antifungal exposure.
2. Subjects and methods
2.1. Fungal isolates
The current study included 49 Candida isolates; they were collected from the Microbiology Laboratory, King Khalid Hospital, KSU, Riyadh, SA, between December 2010 and May 2012 from various clinical samples. Clinical data were collected including; sex, age, site of sample as well as departments involved. Further processing of the isolates was performed in the Microbiology laboratories of the College of Pharmacy, King Saud University.
2.2. Confirming the identity of the strains
All isolates of Candida spp. were identified using standard procedures based on the germ tube test, the formation of characteristic colonies on CHROM agar Candida (CHROMagar Microbiology, Paris, France), growth features, and the fermentation and assimilation profiles on API 20C (BioMerieux). Isolates were maintained in 18% (v/v) glycerol at −80 °C. For further analysis, strains were first transferred from stock cultures onto YEPD plates (1% yeast extract, 2% Bacto peptone, 2% d-glucose, 1.5% agar) and incubated at 37 °C. These fresh cultures were further prepared for biofilm quantification, Minimum Inhibitory concentration (MIC) and biofilm eradication as well as QPCR. Candida albicans (C. albicans) ATCC 90028 and Candida parapsilosis ATCC 96142 served as controls in each experiment.
2.3. Biofilm formation and evaluation
A loopful of actively growing cells was transferred to a sterile Yeast Nitrogen Base (YNB) broth (Difco Laboratories) containing 0.9% d-glucose. After incubation at 37 °C for 24 h with shaking, the cells were centrifuged and washed twice with 0.5 ml phosphate buffer saline (PBS) by vortexing and centrifuging at 5000g for 5 min. The washed cells were then resuspended in 1 ml YNB broth. Optical density of cells was determined for each suspension (Ultraspec 2100 Pro spectrophotometer, BioChrom) and adjusted to a final OD 600 value of 1 with YNB broth (2.5–5 × 106CFU). Thereafter, 100 μl of the suspensions was deposited in 96-well plates and incubated at 37 °C for 48 h without shaking. Biofilm was stained with 0.4% (w/v) crystal violet and quantified at 590 nm after solubilization with 95% ethanol. The experiment was performed in triplicates. OD at 590 nm of the blanks, which only contained Yeast Nitrogen Base broth without inoculums, were subtracted from the values of each well of the samples (Baillie and Douglas, 1998, 1999; Ramage et al., 2001).
2.3.1. Biofilm calculation
The optical density (OD) of each strain was obtained by the arithmetic mean of the absorbance of three wells and this value was compared with the mean absorbance of negative controls (ODnc). The following classification was used for the determination of biofilm formation: no biofilm production (ODs = ODnc), weak biofilm production (ODnc < ODs ⩾ 2ODnc), moderate biofilm production (2ODnc < ODs ⩾ 4ODnc) and strong biofilm production (4ODnc < ODs) (Rodrigues et al., 2010).
2.4. MIC of the Candida strains and biofilm treatment
2.4.1. Susceptibility testing
MICs of amphotericin B (AMB), fluconazole (FLC) and voriconazole (VOC), were determined for all isolates using the microdilution broth procedure recommended by the National Committee for Clinical Laboratory Standards (2002) [recently known as Clinical and Laboratory Standards Institute, CLSI]. MIC testing of AMB, FLC and VOC was performed in the following concentrations, 0.06–16 μg/ml, 0.06–128 μg/ml and 0.004–32 μg/ml, respectively, using RPMI 1640 broth with l-glutamine, without sodium bicarbonate (Sigam, St. Louis, MO, USA) and buffered with morpholine propanesulfonic acid (MOPS) (Sigma, St. Louis, MO, USA). An inoculum of 0.5–2.5 × 103 CFU per ml was employed from each yield and incubated at 35 °C for 48 h, and MIC end points were calculated.
2.4.2. In vitro activity of antifungal combinations against planktonic cells
Chequerboard tests were conducted against all Candida isolates (Moody, 1991). The drugs used in combinations were AMB/FLC and AMB/VOC at the concentrations described above MICs of individual drugs determined by visual readings correspond to either a complete (100% for amphotericin B) or a prominent decrease in turbidity (50% for fluconazole and voriconazol) compared with the growth control. However, the MICs of drug combinations corresponded to prominent (50%) growth inhibition for both dual treatments.
2.4.3. Interpretation of drug combination interaction
Drug combination interaction was classified on the basis of the fractional inhibitory concentration index (FICi) (LaFleur et al., 2006). The FICi was calculated by the formula: FICi = (Ac/Aa) + (Bc/Ba), where Ac and Bc are the MICs of antifungal drugs in combination, and Aa and Ba are the MICs of antifungal drugs A and B alone. The interaction was defined as synergistic if the FICi was <1, FICi = 1 is additive, while, indifferent if the FICi was >1 and ⩽4, and antagonistic if the FICi was >4.0.
2.4.4. In vitro activity of antifungal treatment against Candida biofilms
The effects of AMB, FLC and VOC alone, and their antifungal combinations were tested on two groups of selected candida isolates. The first group contains 5 strong biofilm producers (3 C. albicans, 1 Candida glabrata and 1 Candida tropicalis) while the second one contained 5 weak biofilms forming Candida species (3 C. albicans, 1 C. glabrata and 1 C. tropicalis). Biofilms were assessed using the microdilution method and the biofilms formed were detected by XTT-based colorimetric assay (Lewis, 2010). The amount of colorimetric change was measured using a microplate reader at A 490 nm (model EL340, Mandel Scientific) after 1 and 24 h time period of antifungal exposure. The changes were also observed by the inverted microscope at the same time period. Microtitre wells containing only YNB broth but no microbe were used as negative controls.
Moreover, 0.5 cm2 disks were cut from catheters, sterilized and placed in 24-well tissue culture plates. Cell suspension (80 μl) was applied to the surface of each disk, and the disks were incubated for 1 h at 37 °C for adherence. Non-adherent organisms were removed by gentle washing with 0.15 M PBS (5 ml), and the disks were then submerged in 1 ml of yeast nitrogen base medium containing 50 mM glucose and incubated for 48 h at 37 °C (mature biofilm). Catheters were then divided into 6 groups as follows: gp 1 untreated organisms as control, gp 2 treated with FLC alone, gp 3 treated with VOC alone, gp 4 treated with AMB alone, gp 5 treated with AMB/FLC and gp 6 treated with AMB/VOC. The concentration of the antimicrobials used was in very high doses, FLC (1000 μg/ml), VOC (500 μg/ml) and AMB (32 μg/ml) and reduced to half in the combination form. These concentrations were selected because they resulted in the survival of only a small fraction of cells of each strain. After 1 h and 24 h of incubation at 35 °C, half the disks of each group were subjected to sonication (ultrasonic processor XL, NY, USA) and counting was conducted (Hawser and Douglas, 1994).
2.5. Real time PCR
2.5.1. Preparation of settling cells for extraction
They were prepared as mentioned above, on the surface of catheter disks. Thereafter, settling cells were removed by sonication and centrifugation and harvested at 1 and 24 h time periods for extraction.
2.5.2. RNA extraction cDNA formation by reverse transcriptase
Total RNA from biofilm populations was isolated using the RiboPure yeast kit (Ambion, Inc.) according to the manufacturer’s instructions. RNA concentrations and purity were determined by measuring the absorbance at 260 and 280 nm (ND-1000 spectrophotometer, NanoDrop Technologies). Equal amounts of RNA (3 μg in 20 μl reactions) were reverse transcribed with oligo (dT) primers using the Superscript reverse transcriptase II (Invitrogen).
2.5.3. Quantitative PCR (QPCR) using the listed primers
Sequences of C. albicans genes were downloaded from the Candida Genome Database using Omiga (version 2.0) software (Table 1). Primer3 software (65) was used to design primers for quantitative RT-PCR. The optimal conditions for choosing efficient primers included: biasing the primers toward the 3′ end of the gene, restricting the amplicon size to between 50 and 200 base pairs, maintaining the GC content of the primers at a range of 40–60%, and keeping the difference in melting temperature between the two primers below 1 °C.
Table 1.
The selected genes chosen for transcript analysis using quantitative RT-PCR.
| Gene symbol | Orientation | Sequence (5′–3′) | Concentration (nM) |
|---|---|---|---|
| ACT1 | Forward | TTTCATCTTCTGTATCAGAGGAACTTATTT | 300 |
| Reverse | ATGGGATGAATCATCAAACAAG AG | 300 | |
| EFB1 | Forward | GAAGGCTGCTAAAGGTCCAAA | 300 |
| Reverse | ACCCCAAGTCAAACCTTCCA | 300 | |
| CDR1 | Forward | CAGCAACCATGGGTCAATTATG | 300 |
| Reverse | GTAGCCAAATTGGCAGCATTATC | 300 | |
| KRE1 | Forward | CCTTGCGGCAGATAAAACGT | 300 |
| Reverse | GCATCAGTACCTGTGACCCATACT | 300 | |
| SKN1 | Forward | CCCTGAAATTGATGCATTGGA | 300 |
| Reverse | CATAAGGAGCAACTTGTAATGATTGAG | 300 | |
Two housekeeping genes were employed in the current investigation, the ACT1, actin producing gene, and EFB1, translation elongation factor EF-1 beta gene. The studied genes included CDR1, ABC membrane transporter involved in active efflux of a variety of components including antifungal agents, KRE1 gene expected producer of glycosyl phosphatidyl inositol, an anchor protein with a role in β-1,6-glucan biosynthesis, and SKN1 N-glycosylated type II membrane protein producer with a predicted role in β-1,6-glucan biosynthesis.
2.5.4. Quantitative RT-PCR protocol
Each PCR cocktail contained the following: 1× 20 mM Tris–HCl (pH 8.3) buffer, 50 mM KCl, 3 mM MgCl2, 0.3 μM forward primer, 0.3 μM reverse primer, 1× additive reagent (0.2 mg/ml bovine serum albumin, 150 mM trehalose, and 0.2% Tween-20), 0.25× SYBR green, 1.5 μl FastStart Taq polymerase, a 200 μM concentration of each deoxynucleoside triphosphate, 2 μl cDNA, and H2O to bring the final volume to 25 μl (LightCycler® FastStart DNA MasterPLUS SYBR Green I, Roche). The cDNA was amplified according to the following steps: (i)95 °C for 2 min, (ii) 95 °C for 12 s, (iii) 58–63 °C for 15 s, (iv) 72 °C for 20 s, and (v) 80 °C for 6 s to detect SYBR green (nonspecific products melt at 80 °C and therefore were not detected). Steps ii–v were repeated for 40 cycles, and at the end of the last cycle the temperature was increased at 0.2 °C/s from 60 to 95 °C to produce a melting curve. Smart Cycler software calculated the corresponding cycle threshold (CT) values for a threshold fluorescence of 30.
All samples had RNA obtained from three independent experiments. To avoid variation between runs, samples of all populations were run simultaneously for each gene. Relative quantification of gene expression was performed using the comparative CT method (sequence detection systems chemistry guide; Light Cycler® version 2, Software 4.1, Roche). The most stable housekeeping genes, EFB1 and ACT1 (Beggs et al., 2004), across the sample states were chosen using the statistical technique described by Vandesompele et al. (2002). The internal normalization factor was the geometric mean of the stable housekeeping genes (Vandesompele et al., 2002). The propagated standard error of a set of triplicate samples was estimated using the differential equation of Gauss as described by Muller et al. (2002).
2.6. . Statistical analysis
Each experiment was performed in duplicate, and repeated at least three times on different days. For the growth inhibition assay the arithmetic mean and standard error obtained on three different occasions were calculated. Significance of difference (P ⩽ 0.05) was assessed using the Mann–Whitney U-test.
3. Results
The current study included 49 candida isolates, collected from different clinical specimens in the King Khalid Hospital, Microbiology Laboratory, Riyadh, Saudi Arabia. Ages of the patients included in the study ranged between 1 and 93 years, with a mean of 47.5 ± 22.6 years. There were 39 females representing 79.6% of the cases and 10 males representing 20.4%. The most frequent specimens were vaginal discharge representing 40.8% (20 samples) followed by respiratory tract infection in 28.6% (14 samples), urinary tract infection in 24.5% and blood borne infections (BBI) only in 6.1% of the patients.
The most isolated organism was C. albicans (65.3%) followed by C. tropicalis and C. glabrata found in 16.3% each, as well as, Candida krusei which was found only in one case representing 2%. In the present investigation, 77.6% of strains were biofilm formers (47.4% strong, 21% moderate, and 31.5% weak biofilm formers). There were no significant differences (p value > 0.05) found relating biofilm formation to the site of sample collection (Fig. 1). Moreover, there was no detected relationship between the degree of biofilm formation and the ability of the organisms to show resistance or type of species examined (Fig. 2).
Figure 1.
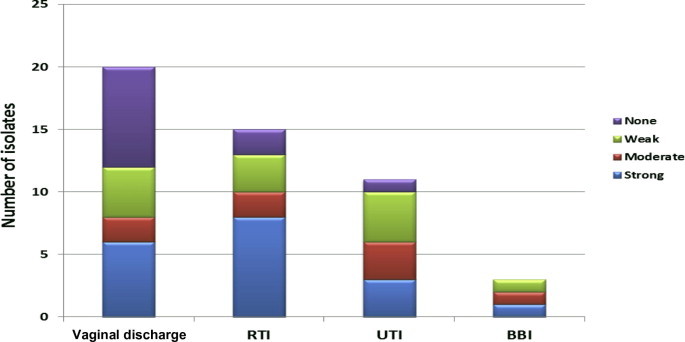
Distribution of isolates’ biofilm strength among different sample sites.
Figure 2.
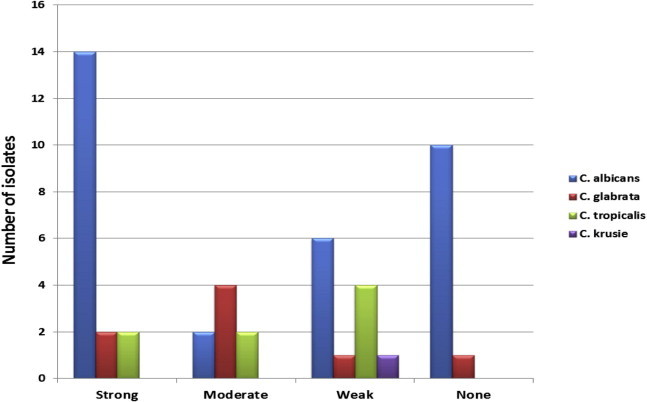
Distribution of isolates’ biofilm strength among different Candida species examined.
As assessed by the broth microdilution method, the most active antifungal tested was AMB, with sensitivity of 87.8% of the examined yields, followed by VOC in 77.6%, while, the least active was FLC found active in 67.3%. They exhibited MIC50 and MIC90 of 0.03, 0.125 μg/ml for AMB, 0.5, 8 μg/ml in VOC and 2, ⩾128 μg/ml for FLC, respectively (Tables 2 and 3). Moreover, C. albicans isolates showed lower rate of resistance than the Non- C. albicans isolates (P ⩽ 0.05).
Table 2.
Antifungal susceptibility results (percent) of Candida isolates.
| C. albicans | C. tropicalis | C. glabrata | C. krusei | Total | ||
|---|---|---|---|---|---|---|
| (n = 32) | (n = 8) (%) | (n = 8) (%) | (n = 1) (%) | (n = 49) (%) | ||
| FLC | R | 5 (15.6%) | 5 (62.5%) | 3 (37.5%) | 1 (100%) | 14 (28.6%) |
| I | 1 (3.1%) | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 2 (4.1%) | |
| S | 26 (81.3%)⁎ | 3 (37.5%) | 4 (50%) | 0 (0.0%) | 33 (67.3%) | |
| VOC | R | 3 (9.4%) | 2 (25%) | 3 (37.5%) | 1 (100%) | 9 (18.3%) |
| I | 0 (0.0%) | 1 (12.5%) | 1 (12.5%) | 0 (0.0%) | 2 (4.1%) | |
| S | 29 (90.6%)⁎ | 5 (62.5%) | 4 (50%) | 0 (0.0%) | 38 (77.6%) | |
| AMB | R | 1 (3.1%) | 1 (12.5) | 1 (2.5) | 0 (0.0) | 3 (6.1) |
| I | 0 (0.0%) | 1 (12.5) | 1 (25) | 0 (0.0) | 3 (6.1) | |
| S | 31 (96.9%)⁎ | 7 (75) | 6 (62.5) | 1 (100) | 43 (87.8) | |
p value ⩽ 0.05.
Table 3.
MICs of the selected antifungals among the tested isolates.
| Antifungals (μg/ml) | MIC50 | MIC90 | MIC range | MIC mean |
|---|---|---|---|---|
| FLC | 2 | ⩾128 | 0.125–⩾128 | 14.67 |
| VOC | 0.5 | 8 | 0.03–⩾16 | 0.298 |
| AMB | 0.03 | 0.125 | 0.03–⩾8 | 0.25 |
In addition, chequerboard study for the drug combination was conducted to all isolates and FICi values were calculated. Seventeen isolates (34.7%) showed antagonistic reaction with AMB/FLC combination (FIC ranged from 5 to 44). On the other hand, 18.4% of studied strains showed antagonistic reaction with AMB/VOC combination at FIC range of 4.2–18.5. Both combinations were most antagonistic against C. albicans isolates rather than the Non-C. albicans strains. The synergism was more evident with AMB/VOC combination than with AMB/FLC (32.7% vs 12.2%, respectively) (Table 4, Fig. 3).
Table 4.
Fractional inhibitory concentration (FIC) indices of AMB/FLC and AMB/VOC combinations against candida isolates.
| Combined antibiotics tested | FIC indices |
|||
|---|---|---|---|---|
| Synergistic reaction | Additive reaction | Indifferent reaction | Antagonistic reaction | |
| (FIC < 1) | (FIC = 1) | (1 > FIC ⩽ 4) | (FIC > 4) | |
| % (No) FIC range | % (No) FIC | % (No) FIC range | % (No) FIC range | |
| AMB/FLC | 12.2% (6) | 43.5% (20) | 12.2% (6) | 34.7% (17) |
| (0.1–0.4) | (1) | (1.4–1.7) | (5–44) | |
| AMB/VOC | 32.7% (16) | 36.7% (18) | 12.2% (6) | 18.4% (9) |
| (0.04–0.4) | (1) | (1.2–1.8) | (4.2–18.5) | |
Figure 3.
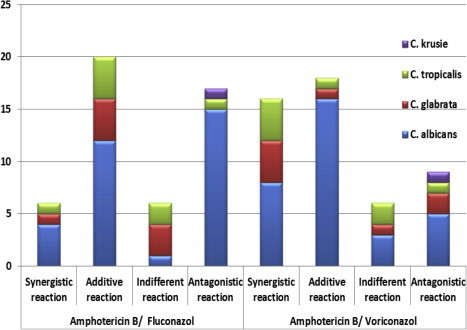
Fractional inhibitory concentration (FIC) indices among the studied strains.
The mature biofilms from the ten selected isolates were found resistant to FLC and no significant inhibition was obtained even at very high concentration (i.e. 1000 μg/ml). Also, VOC and AMB concentration required to inhibit biofilm formation was 16–250 fold higher than the MIC for planktonic. Interestingly, when high antifungal concentrations were administered simultaneously in combinations; FLC (500 μg/ml), VOC (250 μg/ml) and AMB (16 μg/ml) showed significant reduction when compared with the untreated controls (p value ⩽ 0.01). Furthermore, they revealed to be considerably more effective than using FLC alone (p value ⩽ 0.05). Nevertheless, though reduction was more in combination forms, they did not show significance when compared to either VOC or AMB when used alone. These results were also confirmed by using XTT assay and observing biofilms under the inverted microscope (Figs. 4 and 5 and Table 5).
Figure 4.
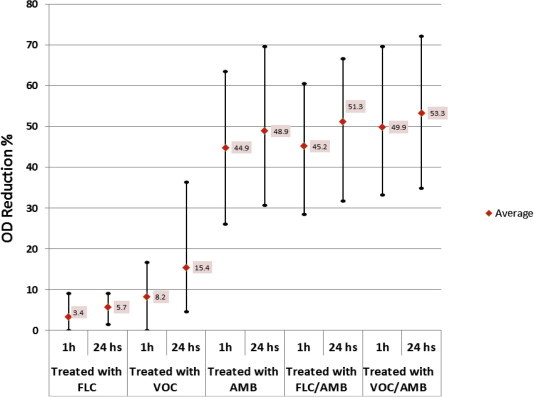
Reduction Chart of ten selected isolates at 1 and 24 time periods per hour. The values are expressed in maximum, minimum and average % of biofilm reduction by XTT assay.
Figure 5.
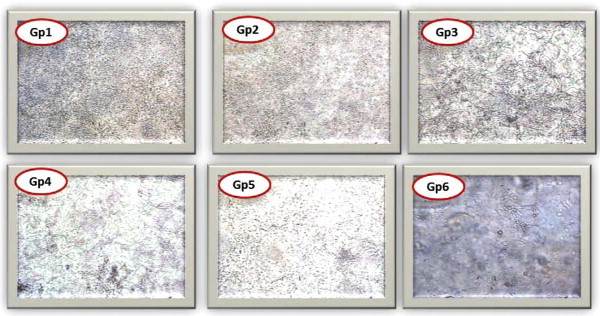
Candida spp. under inverted microscope × 200. Gp1: control strain after 48 h, Gp2: effect of FLC alone on settling cells after 24 h exposure, Gp3: effect of VOC alone, Gp4: effect of AMB alone, Gp5: effect of AMB/FLC, Gp6: effect of AMB/VOC.
Table 5.
Average number of sessile organisms (after 48 h – mature biofilm) among the ten selected Candida isolates (CFU/disk) before and after treatment.
| Sample No | Untreated disks (gp1) | Treated with FLC(gp 2) |
Treated with VOC (gp 3) |
Treated with AMB (gp 4) |
Treated with FLU/AMB (gp 5) |
Treated with VOC/AMB (gp 6) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| After 48 h | 1 h | 24 h | 1 h | 24 h | 1 h⁎ | 24 h⁎,a | 1 h⁎ | 24 h⁎⁎,a | 1 h⁎⁎,a | 24 h⁎⁎,a | |
| 5 | 3.3 ± 0.3 | 3 ± 0.3 | 3 ± 0.6 | 3 ± 0.5 | 2.1 ± 0.3 | 1.2 ± 0.6 | 1 ± 0.5 | 1.3 ± 0.3 | 1.2 ± 0.6 | 1 ± 0.5 | 1 ± 0.9 |
| 2 | 3.6 ± 0.4 | 3.6 ± 0.5 | 3.5 ± 0.4 | 3.2 ± 0.7 | 3.1 ± 0.4 | 1.6 ± 0.3 | 1.3 ± 0.3 | 1.7 ± 0.4 | 1.4 ± 0.5 | 1.3 ± 0.7 | 1.5 ± 0.3 |
| 36 | 3.6 ± 0.5 | 3.3 ± 0.4 | 3.3 ± 0.3 | 3 ± 0.3 | 3.1 ± 0.6 | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.6 ± 0.3 | 1.2 ± 0.6 | 1.4 ± 0.7 | 1 ± 0.5 |
| 4 | 3.8 ± 0.6 | 3.5 ± 0.3 | 3.5 ± 0.4 | 3.3 ± 0.2 | 3 ± 0.4 | 1.7 ± 0.4 | 1.5 ± 0.2 | 1.7 ± 0.2 | 1.3 ± 0.4 | 1.4 ± 0.2 | 1.2 ± 0.3 |
| 15 | 4.2 ± 0.9 | 4.1 ± 0.7 | 4 ± 0.4 | 3.9 ± 0.5 | 3.5 ± 0.4 | 2.1 ± 0.6 | 2 ± 0.9 | 2 ± 0.4 | 1.8 ± 0.4 | 2 ± 0.4 | 1.7 ± 0.4 |
| 6 | 5 ± 0.3 | 5 ± 0.3 | 4.6 ± 0.7 | 5 ± 0.2 | 4.5 ± 0.4 | 3.2 ± 0.4 | 3 ± 0.7 | 3.2 ± 0.4 | 3.1 ± 0.4 | 2.8 ± 0.7 | 2.9 ± 0.5 |
| 27 | 5.4 ± 0.3 | 5.3 ± 0.4 | 5.1 ± 0.4 | 5.1 ± 0.3 | 5 ± 0.6 | 3.4 ± 0.4 | 3.2 ± 0.4 | 3.4 ± 0.6 | 3 ± 0.4 | 3.2 ± 0.7 | 2.8 ± 0.3 |
| 18 | 6.3 ± 0.4 | 6.1 ± 0.3 | 6 ± 0.7 | 6 ± 0.2 | 5.8 ± 0.2 | 4.5 ± 0.5 | 4.3 ± 0.3 | 4.5 ± 0.4 | 4.3 ± 0.9 | 4.2 ± 0.4 | 4.1 ± 0.9 |
| 9 | 6.5 ± 0.3 | 6.4 ± 0.4 | 6.4 ± 0.4 | 6 ± 0.3 | 6.2 ± 0.4 | 4.8 ± 0.2 | 4.5 ± 0.4 | 4.2 ± 0.6 | 4 ± 0.2 | 4.2 ± 0.2 | 4 ± 0.7 |
| 10 | 6.5 ± 0.4 | 6.3 ± 0.2 | 6.2 ± 0.4 | 6 ± 0.6 | 5 ± 0.4 | 4 ± 0.2 | 3.8 ± 0.4 | 4 ± 0.7 | 3.5 ± 0.6 | 4.2 ± 0.4 | 3.8 ± 0.4 |
Statistically significant difference in settling organism’s CFU between untreated and treated biofilms.
Statistically significant difference in settling organism’s CFU between treated biofilms and FLC treated disks.
P ⩽ 0.05 compared with untreated controls.
P ⩽ 0.01 compared with untreated controls.
P ⩽ 0.05 compared with fluconazole treated disks in similar time periods.
High doses of the antifungals were employed to assess the effect on mature biofilms and the correlation of antifungal exposure time on the persisters’ selected gene expression.
Marked over expression of SKN1 was noticed among the mature biofilms treated with AMB after 1 h of exposure (p ⩽ 0.05), and its expression was even more sharply induced after 24 h (p ⩽ 0.01). Furthermore, KRE1 was also significantly upregulated after 1 h and 24 h time periods (p < 0.05). In the same context, both combinations exhibited similar SKN1 over expression, even steeper, though the dose of AMB used was reduced to half.
Surprisingly, no statistically significant over expression of CDR1 (p > 0.05), gene encoding efflux pumps, was observed in biofilms after exposure to high doses of FLC, VOC or any of the combinations used (Table 6).
Table 6.
Average gene expression levels of mature (48 h) candida biofilms after exposure to high doses of antifungal (FLC alone1000 μg/ml, VOC alone 500 μg/ml and AMB alone 32 μg/ml) and their combinations (AMB/FLC 16/500 μg/ml) and (AMB/VOC 16/250 μg/ml).
| Treated with FLC (gp 2) |
Treated with VOC (gp 3) |
Treated with AMB (gp 4) |
Treated with AMB/FLC (gp 5) |
Treated with AMB/VOC (gp 6) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | 1 h | 24 h | 1 h | 24 h | 1 h | 24 h | |
| CDR1 | 1 ± 0.4 | −1.6 ± 1 | 1.5 ± 0.5 | 1 ± 0.3 | ND | −1.3 ± 0.8 | 1.7 ± 0.3 | ND | 1.6 ± 1.2 | 1.2 ± 0.4 |
| KRE1 | 1.4 ± 0.4 | 1.9 ± 0.6 | 1.8 ± 0.4 | 2 ± 0.3 | 5.2 ± 2.2⁎ | 3.3 ± 1.3⁎ | 4.3 ± 1.3⁎ | 2.4 ± 1.7 | 5.3 ± 1.3⁎ | 3.3 ± 1.8 |
| SKN1 | 1.3 ± 0.3 | 3.3 ± 0.6 | 2.3 ± 0.6 | 3.7 ± 1.6 | 14.3 ± 3.4⁎ | 23.5 ± 6.2⁎⁎ | 9.3 ± 3.5⁎ | 33.3 ± 3.5⁎⁎ | 7.3 ± 1.3⁎ | 35.3 ± 2.4⁎⁎ |
Statistically significant difference in gene expression between biofilms treated with antifungal drugs and untreated biofilms.
Gene expression levels (fold up-or down regulation) were designated with the expression of a gene in biofilms antifungal treated compared to its expression in untreated biofilms.
p ⩽ 0.05.
p ⩽ 0.01.
4. Discussion
In the last two decades, Candida infections have increased disproportionately as a result of the increased number of compromised host populations, such as patients with AIDS, diabetes and various cancers, as well as organ-transplant recipients (Beck-Sagué and Jarvis, 1993; Wisplinghoff et al., 2004). The number of drug-resistant Candida strains has also increased dramatically owing to the increased use of antifungal agents. The expression of drug-resistance genes as a mechanism by which Candida acquires drug resistance is a well-known phenomenon in the planktonic mode of growth of this fungus. In the present study, we examined the regulation of set of genes’ transcription associated with drug resistance in Candida spp.-induced mature biofilms (after 48 h), and when mature biofilms were exposed to high dose of antifungals.
In the present study, 49 Candida isolates were collected from different clinical specimens. Females represented (79.6%) majority of cases; as, the most frequent specimens were vaginal discharge representing 40.8% (20 samples) of the cases. Most of these patients were diabetics (43.6%) admitted for complications of diabetes mellitus (DM), followed by pregnant females admitted for antenatal care (20.5%). This was consistent with Nyirjesy and Sobel (2013) who identified DM and pregnancy as important risk factors of Candida spp. genital infections and determining C. albicans as the predominant isolated pathogen in their study. Moreover, Emam et al. (2012) reported C. albicans as a predominant species isolated from pregnant women and non-pregnant women with vulvovaginitis.
The next prevalent isolates were respiratory tract infection isolated in 28.6% of cases (14 samples), urinary tract infection in 24.5% and blood borne infections (BBI) only in 6.1% of the patients. Moreover, the most isolated organism was C. albicans (65.3%) followed by C. tropicalis and C. glabrata found in 16.3% each. These results were comparable to a study done in Turkey by Ece et al. (2012), who found that C. albicans (38.6%) and C. tropicalis (13.9%) were the most prevalent isolates, however, the most prevalent specimens were from UTI unlike the present findings. The studies made in Saudi Arabia were few and addressing mostly invasive candidal infections, nevertheless, all of these investigations indicated the predominance of C. albicans and C. tropicalis (Al-Jasser and Elkhizzi, 2004; Al-Tawfiq, 2007; Mohamed and Al-Ahmadey, 2013).
The present findings provided evidence that 77.6% of the isolated strains were biofilm formers. There were no significant difference (p value > 0.05) found related to the site of sample collection (Fig. 1) or type of species examined (Fig. 2). The studies done in this context showed contradicting findings. While, Mohamed and Al-Ahmadey (2013) found that biofilm formation was more prevalent among Non-C. albicans clinical isolates, Kuhn et al. (2002a) results showed that C. albicans produce quantitatively more biofilm than other Candida spp. These contradictions may be due to the difference in sample size and methods of biofilm detection. Moreover, these studies found that BBI isolates were more biofilm formers than others; this was not evident in the current findings due to the small sample size of BBI isolates.
In vitro susceptibility results of 49 Candida isolates against amphotericin B, fluconazole and voriconazole was determined by the broth microdilution method. The most active antifungal tested was AMB with sensitivity of 87.8% of the examined yields, followed by VOC in 77.6%, while, the least active was FLC found active in 67.3%. They exhibited MIC50 and MIC90 of 0.03, 0.125 μg/ml for AMB, 0.5, 8 μg/ml in VOC and 2, ⩾128 μg/ml for FLC, respectively (Tables 2 and 3). Moreover, C. albicans showed lower rate of resistance than the Non-C. albicans isolates (P ⩽ 0.05). Similar findings were reached by several studies done in Saudi Arabia, Egypt and Kuwait, they found that AMB was the most active antifungal against candida isolates. Voriconazole, though similar in mode of action to fluconazole, showed activity against FLC resistant yields and could be used against them. Moreover, they found C. albicans to be more sensitive than the Non-C. albicans isolates, which was consistent with the present results (Mokaddas et al., 2007; Helmi, 2009; Mohamed and Al-Ahmadey, 2013). However, the rate of resistance was noticed to be raised in the current investigation, this may be explained by the widespread use of antifungal drugs that could differ from one facility to another, long-term use of suppressive azoles, and the use of short courses of antifungal drugs (Ng et al., 2000).
In addition, the data provided from chequerboard study showed antagonistic reaction in 34.7% of the isolates against AMB/FLC combination, while, 18.4% of studied strains showed antagonistic reaction with AMB/VOC. Both combinations revealed to be most antagonistic against C. albicans isolates rather than the Non-C. albicans strains. The synergism was more evident with AMB/VOC combination than with AMB/FLC (32.7% vs 12.2%, respectively) (Table 4, Fig. 3). This relatively high antagonistic effect between azoles and poleynes was demonstrated by many earlier studies (Petrou and Rogers, 1991; Martin et al., 1994; Lignell et al., 2011). Theoretically, the depletion of ergosterol in the cell membrane of fungi by fluconazole would antagonize the binding and consequent antifungal activity, of amphotericin B. However, this may be more evident on consequent administration of azoles followed by AMB rather than the simultaneous administration.
Antifungal combination approach against the planktonic form of Candida has been studied and was found promising; however, only limited studies are available on biofilms (Bachmann et al., 2003; Uppuluri et al., 2008; Tobudic et al., 2010a). Results obtained with planktonic forms not necessarily work on biofilm models. In the present study, we sought to determine whether the mature biofilms will be affected by high dose of antifungal agents and whether this anti-biofilm activity is dependent on exposure time alone and in combinations.
Thus, ten selected isolates’ mature biofilms formed on catheter disks were exposed to FLC at very high concentration (i.e. 1000 μg/ml). Also, VOR and AMB in concentration of 500 and 32 μg/ml, respectively, were employed. These results were also confirmed by using XTT assay and observing biofilms under the inverted microscope (Figs. 4 and 5 and Table 5). Treatment of mature biofilms for 1 and 24 h indicated the high resistance against FLC even after 24 h of exposure (p value ⩾ 0.05). VOC though had better results than FLC but it did not reach significance. Nevertheless, AMB showed significant results after 24 h of exposure (p value ⩽ 0.05). These results were comparable to the findings of several studies, which indicated that mature biofilms are tolerant to high doses of FLC and confirmed that the longer exposure time had no significant effect on their inhibition (Hawser and Douglas, 1995; Chandra et al., 2001; Ramage et al., 2002; Mukherjee et al., 2003; Nailis et al., 2010). Moreover, comparable results were found with the use of 32 μg/ml AMB on mature biofilms by these same studies, however, the rate of inhibition was found to reach 99% unlike the present study. This may be because of the difference in the age of the mature biofilms. Several studies had also confirmed the lack of voriconazole activity against pre-formed mature Candida biofilms, as reported by Kuhn et al. (2002b), Katragkou et al. (2008) and Valentin et al. (2012).
Interestingly, when high antifungal concentrations were administered simultaneously in combinations using half the doses used before [(FLC (500 μg/ml), VOC (250 μg/ml) and AMB (16 μg/ml)], they showed significant reduction when compared with the untreated controls (p value ⩽ 0.01). Moreover, they also revealed to be considerably more effective than using FLC alone (p value ⩽ 0.05). Nevertheless, though reduction was more in combination forms, they did not show significance when compared to either VOC or AMB when used alone. Similar findings were reached by other studies, though they were using a different azole, posaconazole, the authors demonstrated higher susceptibility when biofilms were treated with a combination of amphotericin B and posaconazole than when treated with single drugs (Martinez and Fries, 2010; Tobudic et al., 2010b).
Persister cells are now known as an important mechanism of resistance in chronic infections and they have gathered great attention recently in the context of fungal biofilms’ resistance (Bink et al., 2011; Ramage et al., 2012). In the current study, high doses of the antifungals were employed to assess the correlation of antifungal exposure time on the persisters’ selected gene expression. Marked over expression of SKN1 was noticed among the mature biofilms treated with AMB after 1 h of exposure, and its expression was even more sharply induced after 24 h. Furthermore, KRE1 was also significantly up-regulated after 1 h and 24 h time periods (p < 0.05) (Table 6). In the same context, both combinations exhibited similar SKN1 over expression, even steeper, though the dose of AMB used was reduced to half. Exposure of biofilms to high doses of AMB, alone or in combinations, resulted in the survival of a small number of very resistant cells, and these persisters seemed to exhibit high expression levels of SKN1 (and, to a lesser extent, KRE1). This was found consistent with the results of Nailis et al. (2010), who found AMB to induce similar outcomes in the persisters. On the other hand, Khot et al. (2006) demonstrated that SKN1 and KRE1 were highly up-regulated in biofilms in the absence of AMB. Overexpression of SKN1 and KRE1 suggests changes in cell wall maintenance, which, in turn, could explain the high resistance of biofilms toward AMB (Gale, 1986; Liu et al., 2005). Therefore, overexpression of both genes could be responsible for the resistance phenotype of persisters in the presence of high doses of AMB, alone or in combinations, on one hand, and also for the intrinsic resistance of biofilms in the absence of AMB on the other hand.
Surprisingly, no statistically significant over expression of CDR1, gene encoding efflux pumps was observed in biofilms after exposure to high doses of FLC, VOC or any of the combinations used (Table 6). Similar results were obtained by Nailis et al. (2010) after exposure of persisters to high doses of FLC. In the same context, comparable findings were noticed after treatment of planktonic cells with itraconazole (De Backer et al., 2001). However, when planktonic cells were exposed to FLC, upregulation of CDR1 was observed (Hernáez et al., 1998). This confirms the difference in gene expression between persisters in biofilm setting and planktonic cells.
5. Conclusion
To sum it up, the current study has demonstrated that azole/AMB combinations showed relatively high antagonistic reaction against Candida spp. in the planktonic model. However, these combinations were significantly effective against mature biofilms, in high doses, when compared with control strains (p value ⩽ 0.01) or the use of FLC alone (p value ⩽ 0.05). Treatment of biofilms with high doses of AMB, alone or in combination, resulted in upregulation of genes encoding proteins involved in β-1,6-glucan biosynthesis (SKN1, KRE1). Transcriptional changes in sessile Candida cells in the presence of AMB suggest upregulation of the associated biosynthetic pathways, thereby contributing to a resistant biofilm phenotype. Interestingly, though high resistance to azoles was quite obvious by Candida biofilms, exposure to azoles in high doses did not cause upregulation of genes encoding efflux pumps (CDR1) or even in the non-treated biofilm setting. Therefore, biofilms should be studied as a separate entity that differs from their planktonic models. Further research is recommended to provide more data about the molecular mechanisms underlying the biofilm resistance especially to azoles.
Acknowledgments
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University. We are grateful to Nasr Sinjilawi for providing useful advice concerning qPCR data analysis at the Molecular Laboratory, King Khalid Hospital, KSU.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Tawfiq A.J. Distribution and epidemiology of Candida species causing fungemia at a Saudi Arabian hospital 1996–2004. Int. J. Infect. Dis. 2007;11:239–244. doi: 10.1016/j.ijid.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Al-Jasser A.M., Elkhizzi N.A. Distribution of Candida species among bloodstream isolates. Saudi Med. J. 2004;25:566–569. [PubMed] [Google Scholar]
- Bachmann S.P., Ramage G., VandeWalle K., Patterson T.F., Wickes B.L., Lo’pez-Ribot J.L. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob Agents Chemother. 2003;47(11):3657–3659. doi: 10.1128/AAC.47.11.3657-3659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie G.S., Douglas L.J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 1998;42:1900–1905. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie G.S., Douglas L.J. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 1999;310:644–656. doi: 10.1016/s0076-6879(99)10050-8. [DOI] [PubMed] [Google Scholar]
- Beck-Sagué C.M., Jarvis W.R. National nosocomial infections surveillance system secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J. Infect. Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- Beggs K.T., Holmes A.R., Cannon R.D., Rich A.M. Detection of Candida albicans mRNA in archival histopathology samples by reverse transcription-PCR. J. Clin. Microbiol. 2004;42:2275–2278. doi: 10.1128/JCM.42.5.2275-2278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bink A., Vandenbosch D., Coenye T., Nelis H., Cammue B.P., Thevissen K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 2011;55(9):4033–4037. doi: 10.1128/AAC.00280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Powderly W.G., Kobayashi G.S., Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob. Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J., Kuhn D.M., Mukherjee P.K., Hoyer L.L., McCormick T., Ghannoum M.A. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 2001;183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer M.D., Ilyina T., Ma X.J., Vandonink S., Luyten W.H., Vanden Bossche H. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chemother. 2001;45(6):1660–1670. doi: 10.1128/AAC.45.6.1660-1670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J.L., Cobbs C.G. Prosthetic valve endocarditis. In: Kaye D., editor. Infective Endocarditis. second ed. Raven Press; New York: 1992. pp. 375–396. [Google Scholar]
- Ece G., Samlioglu P., Akkoclu G., Atalay S., Kose S. The evaluation of the distribution of yeast like fungi ‘Candida Species’ at a tertiary care center in Western Turkey. Int. J. Med. Sci. 2012;2012:9. doi: 10.7150/ijms.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emam S.M., Abo Elazm A.A., Morad A.W.A. Exoenzymes production and antifungal susceptibility of candida species isolated from pregnant women with vulvovaginitis. J. Am. Sci. 2012;8(12):1392–1399. [Google Scholar]
- Franz R., Kelly S.L., Lamb D.C., Kelly D.E., Ruhnke M., Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 1998;42(12):3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz R., Ruhnke M., Morschhäuser J. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses. 1999;42(7–8):453–458. doi: 10.1046/j.1439-0507.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- Gale E.F. Nature and development of phenotypic resistance to amphotericin B in Candida albicans. Adv. Microb. Physiol. 1986;27:277–320. doi: 10.1016/s0065-2911(08)60307-0. [DOI] [PubMed] [Google Scholar]
- Hawser S.P., Douglas L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994;62(3):915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawser S.P., Douglas L.J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 1995;39(9):2128–2131. doi: 10.1128/aac.39.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmi H. Bloodstream infections due to candida species and antifungal susceptibility profile. Egypt. J. Med. Microbiol. 2009;18(4):13–22. [Google Scholar]
- Hernáez M.L., Gil C., Pla J., Nombelas C. Induced expression of the Candida albicans multidrug resistance gene CDR1 in response to fluconazole and other antifungals. Yeast. 1998;14(6):517–526. doi: 10.1002/(SICI)1097-0061(19980430)14:6<517::AID-YEA250>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Holmes A.R., Lin Y.H., Niimi K., Lamping E., Keniya M., Niimi M., Tanabe K., Monk B.C., Cannon R.D. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 2008;52(11):3851–3862. doi: 10.1128/AAC.00463-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katragkou A., Chatzimoschou A., Simitsopoulou M., Dalakiouridou M., Diza-Mataftsi E., Tsantali C., Roilides E. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob. Agents Chemother. 2008;52(1):357–360. doi: 10.1128/AAC.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.L., Arnoldi A., Kelly D.E. Molecular genetic analysis of azole antifungal mode of action. Biochem. Soc. Trans. 1993;21(4):1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- Khot P.D., Suci P.A., Miller R.L., Nelson R.D., Tyler B.J. A small subpopulation of blastospores in Candida albicans biofilms exhibit resistance to amphotericin B associated with differential regulation of ergosterol and be‘ta-1,6-glucan pathway genes. Antimicrob. Agents Chemother. 2006;50(11):3708–3716. doi: 10.1128/AAC.00997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D.M., Chandra J., Ghannou M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70(2):878–888. doi: 10.1128/iai.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D.M., George T., Chandra J., Mukherjee P.K., Ghannoum M.A. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002;46(6):1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur M., Kumamoto C.A., Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006;50(11):3839–3846. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Persister cells. Annu. Rev. Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- Lignell A., Lowdin E., Cars O., Sanglard D., Sjolin J. Voriconazole-induced inhibition of the fungicidal activity of amphotericin B in Candida strains with reduced susceptibility to voriconazole: an effect not predicted by the MIC value alone. Antimicrob. Agents Chemother. 2011;55(4):1629–1637. doi: 10.1128/AAC.00791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.T., Lee R.E., Barker K.S., Lee R.E., Wei L., Homayouni R., Rogers P.D. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 2005;49(6):2226–2236. doi: 10.1128/AAC.49.6.2226-2236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ribot J.L., McAtee R.K., Lee L.N., Kirkpatrick W.R., White T.C., Sanglard D., Patterson T.F. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 1998;42(11):2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Maier F., Bhakdi S. Antagonistic effects of fluconazole and 5-fluorocytosine on candidacidal action of amphotericin B in human serum. Antimicrob. Agents Chemother. 1994;38(6):1331–1338. doi: 10.1128/aac.38.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L.R., Fries B.C. Fungal biofilms: relevance in the setting of human disease. Curr. Fungal Infect. Rep. 2010;4(4):266–275. doi: 10.1007/s12281-010-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio T., Yamada-Okabe T., Yabe T., Nakajima T., Arisawa M., Yamada-Okabe H. Isolation of the Candida albicans homologs of Saccharomyces cerevisiae KRE6 and SKN1: expression and physiological function. J. Bacteriol. 1997;179:2363–2372. doi: 10.1128/jb.179.7.2363-2372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S.A., Al-Ahmadey Z.Z. Biofilm formation and antifungal susceptibility of Candida isolates from various clinical specimens. Br. Microbiol. Res. J. 2013;3(4):590–601. [Google Scholar]
- Mokaddas E.M., Al-Sweih N.A., Khan Z.U. Species distribution and antifungal susceptibility of Candida bloodstream isolates in Kuwait: a 10-year study. J. Med. Microbiol. 2007;56:255–259. doi: 10.1099/jmm.0.46817-0. [DOI] [PubMed] [Google Scholar]
- Moody J.A. Synergism testing. Broth microdilution checkerboard and Broth microdilution methods. In: Isenberg H.D., editor. vol. 1. American Society for Microbiology; Washington, DC: 1991. (Clinical Microbiology Procedures Handbook). 5,18:1–28. [Google Scholar]
- Mukherjee P.K., Chandra J., Kuhn D.M., Ghannoum M.A. Mechanisms of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane esterols. Infect. Immun. 2003;71(8):4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P.Y., Janovjak H., Miserez A.R., Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Nailis H., Vandenbosch D., Deforce D., Nelis H.J., Coenye T. Transcriptional response to fluconazole and amphotericin B in Candida albicans biofilms. Res. Microbiol. 2010;161(4):284–292. doi: 10.1016/j.resmic.2010.02.004. [DOI] [PubMed] [Google Scholar]
- ∗∗∗National Committee for Clinical Laboratory Standards, 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Second Edition: Approved Standard M27–A2. NCCLS, Wayne, PA, USA.
- Ng K.P., Saw T.L., Na S.L., Soo-Hoo T.S. Systemic Candida infection of University Hospital 1997–1999: the distribution of Candida biotypes and antifungal susceptibility patterns. Mycopathologia. 2000;149(3):141–146. doi: 10.1023/a:1007283211220. [DOI] [PubMed] [Google Scholar]
- Nucci M., Anaissie E. Revisiting the source of candidemia: skin or gut? Clin. Infect. Dis. 2001;33(12):1959–1967. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- Nyirjesy P., Sobel J.D. Genital mycotic infections in patients with diabetes. Postgrad. Med. 2013;125(3):33–46. doi: 10.3810/pgm.2013.05.2650. [DOI] [PubMed] [Google Scholar]
- Pappas P.G., Rex J.H., Sobel J.D., Filler S.G., Dismukes W.E., Walsh T.J., Edwards J.E. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 2004;38(2):161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- Perea S., López-Ribot J.L., Kirkpatrick W.R., McAtee R.K., Santillán R.A., Martínez M., Calabrese D., Sanglard D., Patterson T.F. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus infected patients. Antimicrob. Agents Chemother. 2001;45(10):2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M.A., Rogers T.R. Interactions in vitro between polyenes and imidazoles against yeast. J. Antimicrob. Chemother. 1991;27(4):491–506. doi: 10.1093/jac/27.4.491. [DOI] [PubMed] [Google Scholar]
- Raad I., Costerton W., Sabharwal U., Sacilowski M., Anaissie E., Bodey G.P. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 1993;168(2):400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]
- Ramage G., Bachmann S., Patterson T.F., Wickes B.L., López-Ribot J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002;49(6):973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- Ramage G., Martínez J.P., López-Ribot J.L. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6(7):979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- Ramage G., Rajendran R., Sherry L., Williams C. Fungal biofilm resistance. Int. J. Microbiol. 2012;2012(528521):1–14. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G., VandeWalle K., Wickes B.L., Lopez-Ribot J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K. The discovery and profile of fluconazole. J. Chemother. 1990;2:51–54. doi: 10.1080/1120009x.1990.11738981. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.B., Santos L.R., Tagliari V.Z., Rizzo N.N., Trenhago G., Oliveira A.P., Goetz F., Nascimento V.P. Quantification of biofilm production on polystyrene by Listeria, Escherichia coli and Staphylococcus aureus isolated from a poultry slaughterhouse. Braz. J. Microbiol. 2010;41:1082–1085. doi: 10.1590/S1517-838220100004000029.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D., Kuchler K., Ischer F., Pagani J.L., Monod M., Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi J.C., Almeida A.M., Mendes Giannini M.J. New antimicrobial therapies used against fungi present in subgingival sites: a brief review. Arch. Oral Biol. 2011;56:951–959. doi: 10.1016/j.archoralbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Seneviratne C.J., Jin L., Samaranayake L.P. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008;14(7):582–590. doi: 10.1111/j.1601-0825.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- Tobudic S., Kratzer C., Lassnigg A., Graninger W., Presterl E. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 2010;65(2):271–274. doi: 10.1093/jac/dkp429. [DOI] [PubMed] [Google Scholar]
- Tobudic S., Lassnigg A., Kratzer C., Graninger W., Presterl E. Antifungal activity of amphotericin B, caspofungin and posaconazole on Candida albicans biofilms in intermediate and mature development phases. Mycoses. 2010;53(3):208–214. doi: 10.1111/j.1439-0507.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- Tunney M.M., Patrick S., Curran M.D., Ramage G., Anderson N., Davis R.I., Gorman S.P., Nixon J.R. Detection of prosthetic joint biofilm infection using immunological and molecular techniques. Methods Enzymol. 1999;310:566–576. doi: 10.1016/s0076-6879(99)10044-2. [DOI] [PubMed] [Google Scholar]
- Uppuluri P., Nett J., Heitman J., Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 2008;52(3):1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A., Canton E., Peman J., Martinez J.P. Voriconazole inhibits biofilm formation in different species of the genus Candida. J. Antimicrob. Chemother. 2012;67(10):2418–2423. doi: 10.1093/jac/dks242. [DOI] [PubMed] [Google Scholar]
- Vandesompele J.K., De Preter F., Pattyn B., Poppe N., Van Roy A., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034.1 – RESEARCH0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D.W. Amphotericin B: an introduction. J. Antimicrob. Chemother. 1991;28:27–38. doi: 10.1093/jac/28.suppl_b.27. [DOI] [PubMed] [Google Scholar]
- White T.C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]



