Abstract
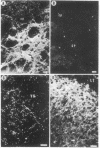
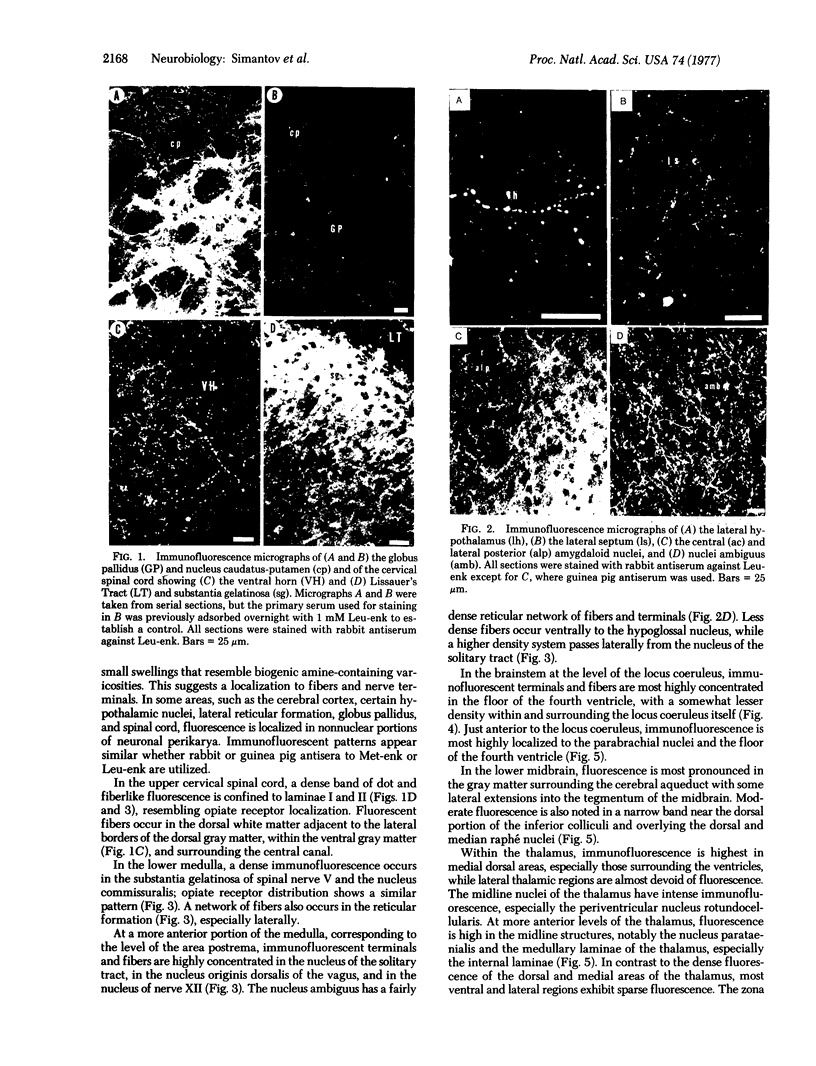
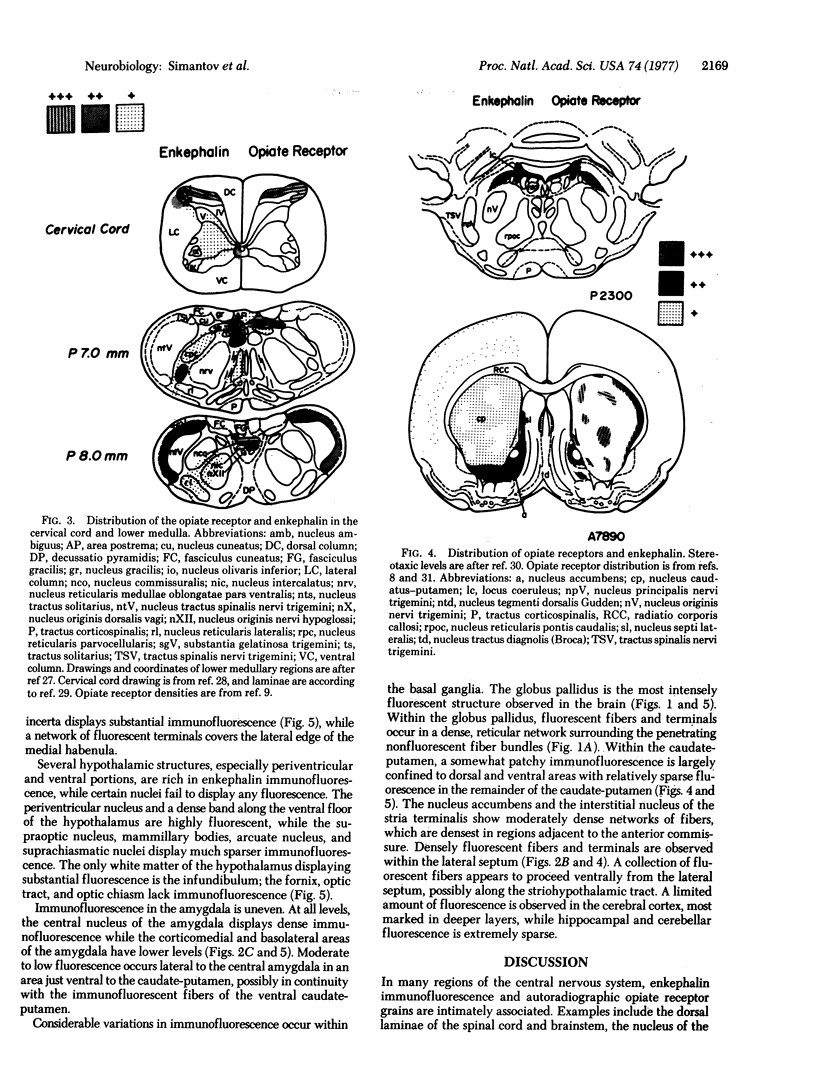
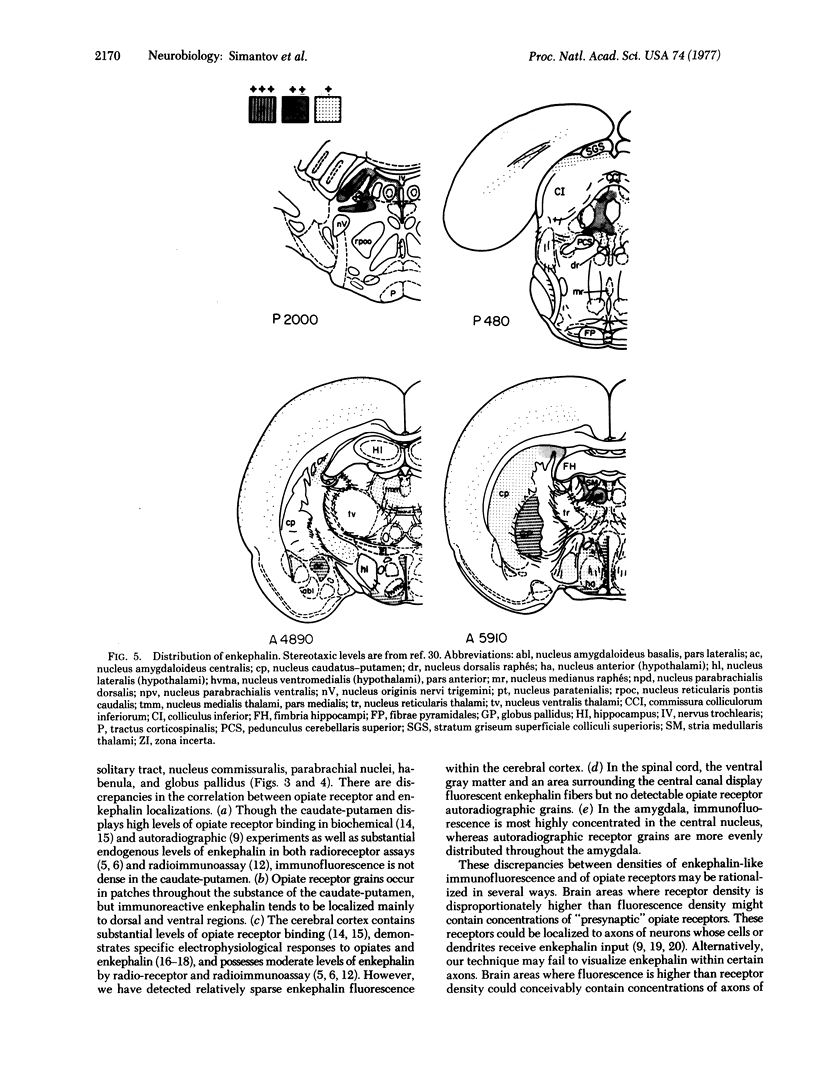
Using specific antisera to methionine-enkephalin and leucine-enkephalin, we have visualized apparent enkephalin-containing neuronal fibers and terminals throughout the central nervous system of the rat. Immunoreactive enkephalin displays sharply defined localizations. Regions of highest immunofluorescent density include the laminae I and II of the spinal cord, the substantia gelatinosa of the caudal nucleus of nerve V, the vagal nuclei of the medulla, the periventricular and periaqueductal areas of the upper medulla and midbrain, dorsomedial thalamic regions, specific hypothalamic nuclei, the basal ganglia, particularly the globus pallidus and the central nucleus of the amygdala, and the lateral septum. In certain regions enkephalin immunofluorescence corresponds closely with the distribution of autoradiographic opiate receptor grains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Mayer D. J., Liebeskind J. C. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976 Mar 5;191(4230):961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- Atweh S. F., Kuhar M. J. Autoradiographic localization of opiate receptors in rat brain. I. Spinal cord and lower medulla. Brain Res. 1977 Mar 18;124(1):53–67. doi: 10.1016/0006-8993(77)90863-0. [DOI] [PubMed] [Google Scholar]
- Elde R., Hökfelt T., Johansson O., Terenius L. Immunohistochemical studies using antibodies to leucine-enkephalin: initial observations on the nervous system of the rat. Neuroscience. 1976 Aug;1(4):349–351. doi: 10.1016/0306-4522(76)90063-4. [DOI] [PubMed] [Google Scholar]
- Frederickson R. C., Norris F. H. Enkephalin-induced depression of single neurons in brain areas with opiate receptors--antagonism by naloxone. Science. 1976 Oct 22;194(4263):440–442. doi: 10.1126/science.10625. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Opioid peptides endorphins in pituitary and brain. Science. 1976 Sep 17;193(4258):1081–1086. doi: 10.1126/science.959823. [DOI] [PubMed] [Google Scholar]
- Hill R. G., Pepper C. M., Mitchell J. F. Depression of nociceptive and other neurones in the brain by iontophoretically applied met-enkephalin. Nature. 1976 Aug 12;262(5569):604–606. doi: 10.1038/262604a0. [DOI] [PubMed] [Google Scholar]
- Hiller J. M., Pearson J., Simon E. J. Distribution of stereospecific binding of the potent narcotic analgesic etorphine in the human brain: predominance in the limbic system. Res Commun Chem Pathol Pharmacol. 1973 Nov;6(3):1052–1062. [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Jacquet Y. F., Lajtha A. Morphine action at central nervous system sites in rat: analgesia or hyperalgesia depending on site and dose. Science. 1973 Nov 2;182(4111):490–492. doi: 10.1126/science.182.4111.490. [DOI] [PubMed] [Google Scholar]
- Kraulis I., Foldes G., Traikov H., Dubrovsky B., Birmingham Distribution, metabolism and biological activity of deoxycorticosterone in the central nervous system. Brain Res. 1975 Apr 25;88(1):1–14. doi: 10.1016/0006-8993(75)90942-7. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J., Pert C. B., Snyder S. H. Regional distribution of opiate receptor binding in monkey and human brain. Nature. 1973 Oct 26;245(5426):447–450. doi: 10.1038/245447a0. [DOI] [PubMed] [Google Scholar]
- Lamotte C., Pert C. B., Snyder S. H. Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res. 1976 Aug 13;112(2):407–412. doi: 10.1016/0006-8993(76)90296-1. [DOI] [PubMed] [Google Scholar]
- Li C. H., Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Burgus R., Guillemin R. Isolation, primary structure, and synthesis of alpha-endorphin and gamma-endorphin, two peptides of hypothalamic-hypophysial origin with morphinomimetic activity. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3942–3946. doi: 10.1073/pnas.73.11.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. N., Brown J. T. Paleozoic seeds with embryos. Science. 1973 Jan 12;179(4069):184–185. doi: 10.1126/science.179.4069.184. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Jacobowitz D. M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (mesencephalon, rhombencephalon). J Comp Neurol. 1974 Sep 1;157(1):29–42. doi: 10.1002/cne.901570104. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Goodman R., Snyder S. H. An endogenous morphine-like factor in mammalian brain. Life Sci. 1975 Jun 15;16(12):1765–1769. doi: 10.1016/0024-3205(75)90270-2. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Simantov R., Snyder S. H. Characterization of an endogenous morphine-like factor(enkephalin) in mammalian brain. Mol Pharmacol. 1976 May;12(3):504–513. [PubMed] [Google Scholar]
- Pert A., Yaksh T. Sites of morphine induced analgesia in the primate brain: relation to pain pathways. Brain Res. 1974 Nov 8;80(1):135–140. doi: 10.1016/0006-8993(74)90731-8. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Kuhar M. J., Snyder S. H. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Simantov R., Kuhar M. J., Pasternak G. W., Snyder S. H. The regional distribution of a morphine-like factors enkephalin in monkey brain. Brain Res. 1976 Apr 16;106(1):189–197. doi: 10.1016/0006-8993(76)90086-x. [DOI] [PubMed] [Google Scholar]
- Simantov R., Snowman A. M., Snyder S. H. A morphine-like factor 'enkephalin' in rat brain: subcellular localization. Brain Res. 1976 May 14;107(3):650–657. doi: 10.1016/0006-8993(76)90155-4. [DOI] [PubMed] [Google Scholar]
- Simantov R., Snyder S. H. Morphine-like peptides in mammalian brain: isolation, structure elucidation, and interactions with the opiate receptor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2515–2519. doi: 10.1073/pnas.73.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner T. J., Turner L. M. Cytoarchitecture of the rat spinal cord. J Physiol. 1972 Apr;222(2):123P–125P. [PubMed] [Google Scholar]
- Zieglgänsberger W., Fry J. P., Herz A., Moroder L., Wünsch E. Enkephalin-induced inhibition of cortical neurones and the lack of this effect in morphine tolerant/dependent rats. Brain Res. 1976 Oct 8;115(1):160–164. doi: 10.1016/0006-8993(76)90832-5. [DOI] [PubMed] [Google Scholar]