Abstract
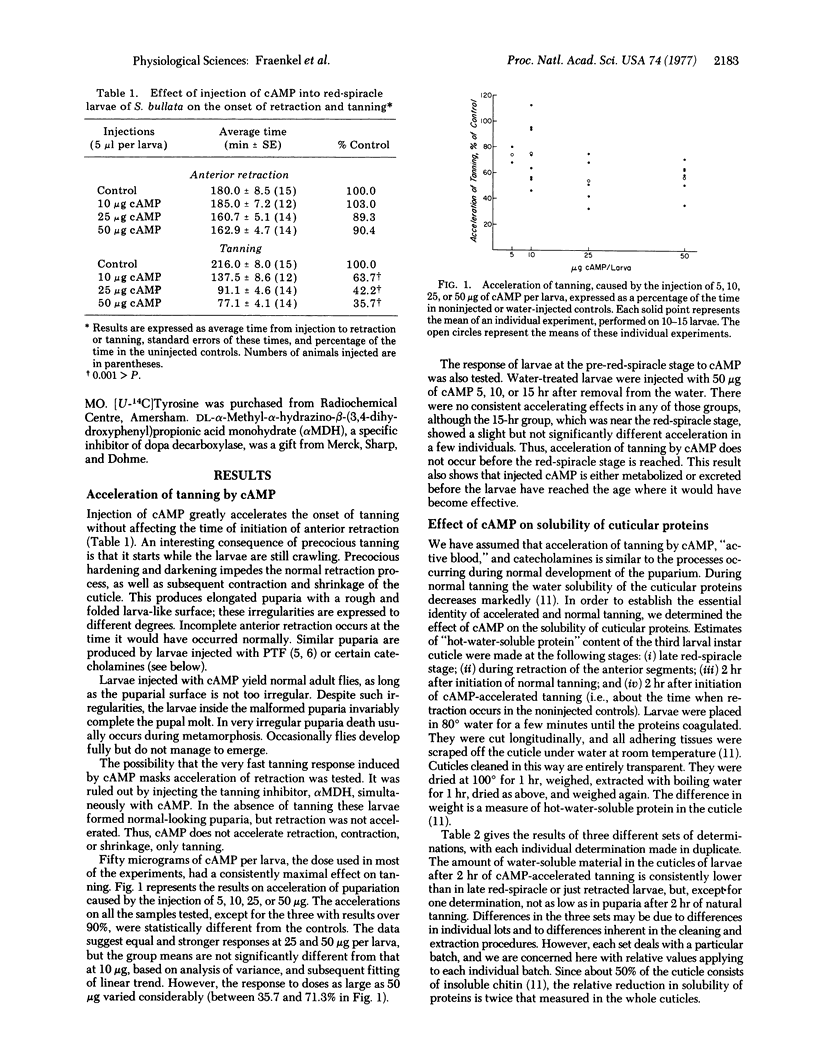
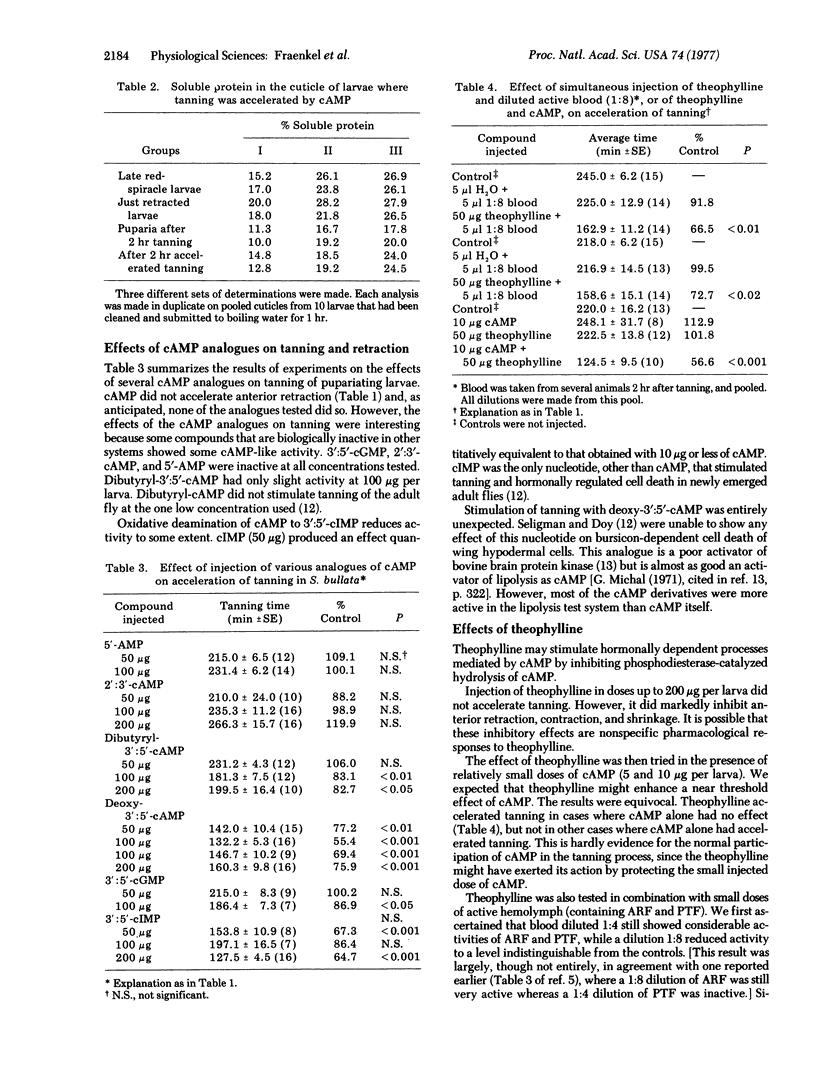
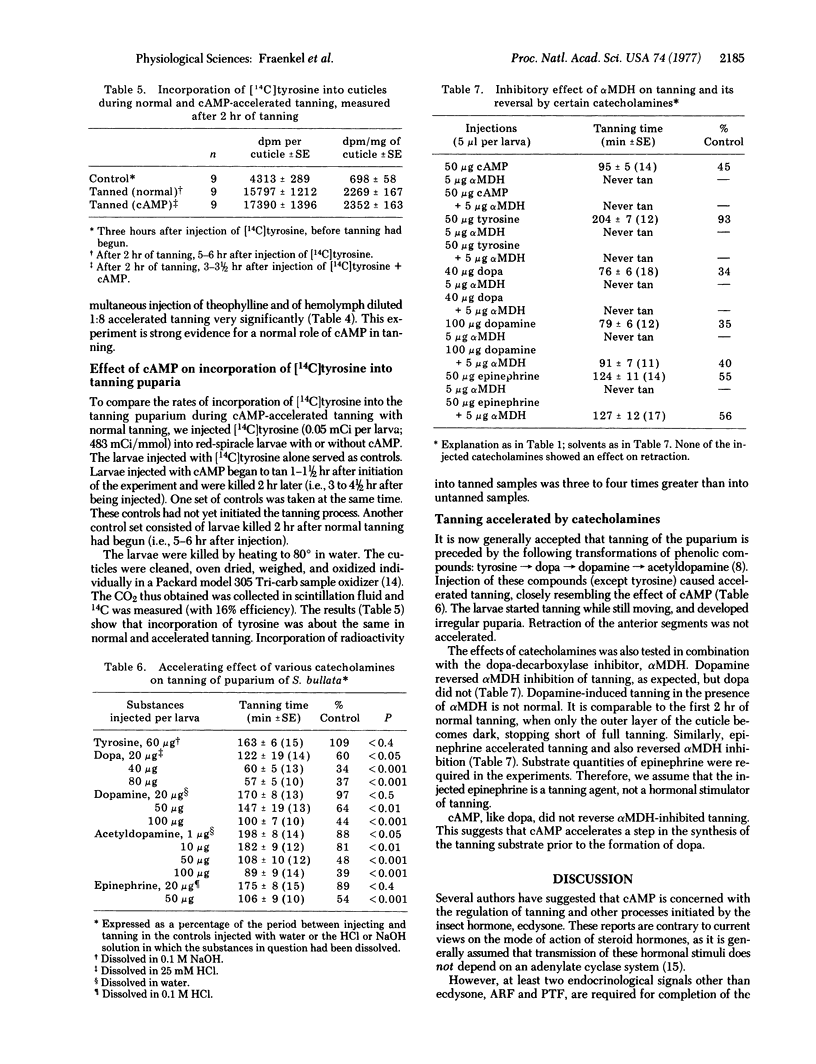
Injection of 3':5'-cyclic AMP (cAMP) into larvae of the fly Sarcophaga bullata 3-4 hr before the beginning of puparium formation (red-spiracle stage) greatly accelerates the onset of tanning without affecting initiation of puparium formation (anterior retraction). Accelerated tanning resembles real tanning in two important respects: the solubility of cuticular proteins becomes reduced and [U-14C]tyrosine is incorporated into the cuticle. Of a number of cAMP analogues tested, 3':5'- cyclic GMP, 2':3'-cyclic AMP, and 5'-AMP were inactive, dibutyryl-3':5'-cAMP had only slight activity, and cyclic IMP and deoxy-3':5'-cAMP showed some activity. Theophylline enhanced the effect of small doses of cAMP or of blood, diluted 1:8, active in the puparium tanning factor. Injection of dopa, dopamine, acetyldopamine, or epinephrine, but not of tyrosine, had an accelerating effect similar to that of cAMP. The tanning-inhibiting effect of DL-alpha-methyl-alpha-hydrazino-beta-(3,4-dihydroxyphenyl)propionic acid monohydrate is reversed by dopamine or epinephrine, but not by tyrosine, dopa, or cAMP. Evidence is presented to indicate that the responses to cAMP are not artifacts but reflect actual biochemical events during tanning.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuchs M. S., Schlaeger D. A. The stimulation of dopa decarboxylase activity by ecdysone and its enhancement by cyclic AMP in adult mosquitoes. Biochem Biophys Res Commun. 1973 Sep 18;54(2):784–789. doi: 10.1016/0006-291x(73)91492-7. [DOI] [PubMed] [Google Scholar]
- Simon L. N., Shuman D. A., Robins R. K. The chemistry and biological properties of nucleotides related to nucleoside 3',5'-cyclic phosphates. Adv Cyclic Nucleotide Res. 1973;3:225–353. [PubMed] [Google Scholar]
- Sivasubramanian P., Friedman S., Fraenkel G. Nature and role of proteinaceous hormonal factors acting during puparium formation in flies. Biol Bull. 1974 Aug;147(1):163–185. doi: 10.2307/1540576. [DOI] [PubMed] [Google Scholar]
- Vedeckis W. V., Gilbert L. I. Production of cyclic AMP and adenosine by the brain and prothoracic glands of Manduca sexta. J Insect Physiol. 1973 Dec;19(12):2445–2457. doi: 10.1016/0022-1910(73)90248-5. [DOI] [PubMed] [Google Scholar]
- Zdarek J., Fraenkel G. Correlated effects of ecdysone and neurosecretion in puparium formation (pupariation) of flies. Proc Natl Acad Sci U S A. 1969 Oct;64(2):565–572. doi: 10.1073/pnas.64.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reggi M. L., Cailla H. L. Cyclic AMP levels in Drosophila during postembryonic development and in the adults. J Insect Physiol. 1975 Oct;21(10):1671–1674. doi: 10.1016/0022-1910(75)90177-8. [DOI] [PubMed] [Google Scholar]


