Abstract
Background
Diagnosis of acute coronary syndrome (ACS) is important, due to the associated very high mortality. Failure to diagnose ACS is a problem both for the patients and the clinicians. Ischemia modified albumin (IMA) has already been licensed by the US Food and Drug Administration for the diagnosis of suspected myocardial ischemia.
Methods
Patients attending the emergency department (ED) within 6 h after having features of ACS were selected. IMA was done on admission. Blinded to the IMA results patients were fully evaluated and a diagnosis of non-ischemic chest pain (NICP), unstable angina (UA) or myocardial infarction (MI) was made. Later IMA results were correlated in each group.
Results
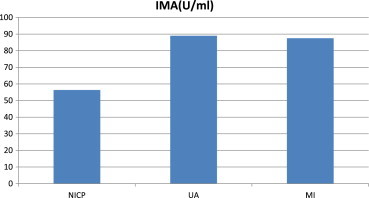
Mean IMA value was 56.38 ± 23.89 u/ml in NICP group whereas in UA group it was 89.00 ± 7.76 u/ml and MI group was 87.50 ± 9.62 u/ml. This showed a sensitivity of 92% and specificity of 87%. The positive predictive value of the test was 88% and negative predictive value was 94%. In 16 patients an early diagnosis could be made when compared with Trop-T. Of the 89 patients 11 patients died in hospital. The IMA value was compared between this group and the patients who survived. Patients who died had a mean IMA value of 88.5 with a standard deviation of 5.33 whereas in patients who survived the mean value was 78.26 which was not statistically significant.
Conclusion
In conclusion the benefit of the test would be to rule out ACS in patients who present early to ED with inconclusive diagnosis.
Keywords: Ischemia modified albumin, Acute coronary syndrome, Trop T
1. Introduction
The acute coronary syndrome (ACS) represents a spectrum of diseases which range from ‘Unstable Angina (UA)’ which is associated with a reversible myocardial cell injury, to an ST-segment elevation ‘Myocardial Infarction (MI)’ which is associated with irreversible myocardial necrosis.1 In today's world, about 17 million deaths occur due to cardiovascular disease. In India, the number of deaths which are caused by ischemic heart disease increased from 1.17 million in 1990 to 1.59 million in 2000 and to 2.03 million by 2010.2 The diagnostic approach and the clinical management of the patients who present with a suspected acute coronary syndrome or cardiac dysfunction are challenging3 (Fig. 1).
Fig. 1.
Mean IMA values.
The manifestations of myocardial ischemia are varied and multiple, like chest pain, epigastric discomfort, breathlessness, nausea and vomiting. However, these symptoms may be subtle and they may not be easily recognized. Because of their varied presentations and as they are associated with high mortality, an early identification of the patients with acute myocardial infarction is very critical.4
Assessment of the cardiac biomarker levels (Myoglobin, Creatine Kinase-MB and Troponins) is one of the most essential and effective ways for detecting myocardial damage. The current conventional cardiac markers, CK-MB, Troponin I (TnI) and T are sensitive and specific tests for the detection of myocardial necrosis, but they show a greater rise approximately 3–6 h after the onset of the myocardial cell injury and other diagnostic tools such as stress testing, and echocardiology are not routinely available.5
Recent research has found that ischemia modified albumin (IMA) is an ideal biomarker for ischemia. IMA is a form of human serum albumin in which the N-terminal amino acids have been modified by ischemia. This modification reduces the affinity of plasma albumin to bind to heavy metal ions such as cobalt.6 Bhagwan et al and others have shown increased IMA levels in patients with spontaneous coronary ischemia, with abnormal values which are detectable before the subsequent increases in the cardiac troponin.7 Initially, the test was named as the Albumin Cobalt Binding (ACB) assay, since it was based on human serum albumin for the metal ions (cobalt COII) in patients with ischemia.4
Failure to recognize ACS has unfavorable consequences not only for patients, but for physicians too. Missed acute cardiac ischemia continues to be one of the major causes of malpractice litigation against emergency physicians. Twenty percent of ED-related malpractice compensation is expended to patients with complications because of myocardial ischemia.8 The large number of patients presenting to EDs with symptoms suggestive of ACS, and the medical and legal consequences of an erroneous discharge from the ED, demand that clinicians pursue new diagnostic approaches to ACS.9
Very few studies have been reported on the serum IMA testing and on its application in the Indian context. This proposed study aims to evaluate the added diagnostic value of biomarker “Ischemia Modified Albumin (IMA)”, in patients with ACS beyond other diagnostic tests to reliably detect myocardial ischemia in the absence of necrosis, it's role in differentiating UA versus MI, it's role in providing clinical utility complementary to that of cardiac troponins, the established markers of necrosis in NSTEMI & STEMI, role of IMA in predicting in-hospital mortality.
2. Materials and methods
The study was conducted after getting the approval from the ethical committee of Narayana Medical College. Eighty nine subjects were chosen for the study. Both males and females in the age group of 20–75 years were included and an informed consent was obtained from all of them. In this, 24 subjects with symptoms of chest pain with normal, clinical, biochemical and ECG parameters served as the control group.
2.1. Patient selection
Data were collected from this 89 patients admitted to our emergency department with manifestations suggestive of acute myocardial ischemia, including those such as chest pain with or without radiation, chest heaviness, shortness of breath, lower jaw pain, left arm pain, epigastric pain, syncope, hypotension, palpitations, and other symptoms suggestive of an anginal equivalent. Cardiac biomarker of necrosis-Trop-T was measured and ECG taken in the ED within 30 min as part of the standard care. All patients received routine institutional care blinded to the IMA results.
2.2. Inclusion criteria
Patients admitted in the emergency room with a primary complaint of chest pain or angina equivalence evolving within 6 h and suspected as acute coronary syndrome.
2.3. Exclusion criteria
-
1)
Presence of renal diseases.
-
2)
Presence of cirrhosis.
-
3)
Presence of stroke, skeletal muscle injury, malignancy, trauma.
-
4)
Ongoing infectious diseases.
-
5)
Serum albumin <2 g/dl.
-
6)
Patient younger than 18 years old.
-
7)
Patients with complaints lasting more than 6 h, as IMA levels usually return to normal 8–12 h after onset of myocardial ischemia.
-
8)
Patients whose symptoms had ceased 2 h previously, because IMA levels fall rapidly once an ischemic event has ended.
-
9)
Asymptomatic patients, and those who unable to relate the time that their symptoms began or ended (if the pain was not persisting).
2.4. Clinical characteristics
Demographics, clinical information, and hospital course following enrollment were recorded for each patient. Data analyzed included clinical history and examination, all relevant electrocardiograms (ECGs), echocardiography, stress-testing data, cardiac catheterization data, hospital course documentation, and discharge summaries.
2.5. ECG classification
Positive ECGs were those with ST segment depression or elevation [greater than or equal to] 0.1 mV, or T wave inversion [greater than or equal to] 0.2 mV (in [greater than or equal to] two contiguous leads). ECGs showing no ST segment shifts or T wave changes (apart from lead III or VI) were considered negative. Equivocal or uninterruptable ECGs (that is, left bundle branch block, paced rhythm, extensive pathological Q waves, and/or persistent ST segment elevation after previous AMI) were considered to be negative in this study.
Based on available data, a diagnosis of myocardial ischemia was established or excluded for each patient. This decision was made in the light of objective and subjective data relevant to the nature of the patient's manifestations, including the results of history, physical examination, ECG, cardiac biomarkers other than IMA, hospital course (including results of diagnostic studies such as cardiac catheterization), and discharge summaries.
Patients were classified as non-ischemic chest pain (NICP) and acute coronary syndrome (ACS). ACS included unstable angina (UA), non-ST segment elevation myocardial infarction (NSTEMI), and ST segment elevation MI (STEMI). Practice guidelines for the redefinition of AMI (ESC/ACC) and the management of patients with UA (ACC/AHA), were used to diagnose ACS.10,11 STEMI was diagnosed if there was ST segment elevation [greater than or equal to] 0.1 mV in two or more contiguous leads, and NSTEMI was diagnosed if ECG was non-diagnostic and cTnT positive. All patients underwent serial cTnT testing at presentation and 6 h later as per institutional protocol for management of acute chest pain patients. UA was diagnosed in the presence of signs and symptoms of acute cardiac ischemia without evidence of myocardial necrosis. Positive indications for UA were a suggestive history and clinical examination; typical ischemic ECG changes at rest or during exercise; regional wall motion abnormality on echocardiography, significant stenosis (>70%) on coronary angiography. Patients were classified as non-ischemic chest pain (NICP) when (1) a reported non-cardiac mechanism was confirmed as the cause of chest pain; (2) all of the following criteria were met: atypical symptoms, negative cTnT results on serial sampling (over a 6–9 h interval), presence of normal ECGs, and negative stress test.
2.6. Angiography and final diagnosis
Coronary angiography was carried out on all ACS patients. All angiographic images were reviewed by an experienced cardiologist blinded to the patient's IMA results. A positive angiogram was defined as stenosis [greater than or equal to] 70% diameter reduction in any major epicardial vessel. Final diagnosis for this study was based on the history, clinical examination, serial cTnT results, ECG, exercise stress testing and coronary angiography, as available. Results of all investigations were reviewed blinded to IMA results.
2.7. Blood collection
5 ml of blood samples were collected by venous puncture with strict aseptic precaution as soon as the subjects got admitted as per the inclusion criteria. The samples were centrifuged and serum separated. One part of the sample was taken and analysis of cTnT, albumin and serum creatinine were done immediately. Remaining part of the sample was stored for analysis of ischemia modified albumin at 20 °C. cTnT was repeated after 6 h if the first sample was negative.
2.8. Albumin cobalt binding test
IMA was measured by the albumin cobalt binding test (ACB Test) on the Roche Cobas MIRA PLUS instrument. The mechanism whereby IMA represents a marker of ischemia is based upon the fact that human serum albumin (HSA) has the ability to bind certain transition metal ions, particularly cobalt and copper, at the N-terminus. Bar-Or has previously reported that exposure of albumin to ischemic tissue changes the structure of HSA N-terminus such that it can no longer bind cobalt.12,13
Blood was collected for the IMA test in serum separated tubes. Specimens were frozen at −20 °C or colder within 2 h. Frozen samples were gently vortexed after thawing. Specimens handled in this way showed no significant difference in assay results from the fresh specimens. In the ACB Test, 95 µ of a patient sample and 5 µ of cobalt chloride (CoII), are incubated for 5 min. During incubation, the Co(II) binds to the N-terminus of unaltered albumin in the sample; albumin for which the N-terminus is altered as a result of ischemic processes binds to the Co(II) to a far lesser extent. After incubation, 25 µ of dithiothreitol (DTT) is added to the mixture. DTT forms a coloured complex with Co(II) that is not bound at the N-terminus of albumin, and this complex is measured spectrophotometrically at 500 nm. Duplicate IMA values were obtained with the mean recorded as the result of the assay.
In our laboratory, the ACB test within-run duplicate CV% of patient samples averaged 1.9% (range 0.0%–6.5%). We applied the IMA upper limit of normal (95th percentile of 111 apparently healthy people) reported by the manufacturer. IMA values >80 u/ml were considered positive for cardiac ischemia.
2.9. Cardiac troponin T test
Blood was collected for the cTnT test in serum separated tubes and cTnT concentrations >0.05 ng/ml were considered positive. cTnT concentrations were measured by electrochemiluminescence assay.
2.10. Statistical analysis
The statistical analysis was executed by means of sigma graph pad prism software, USA Version-4. Continuous data is presented as mean, median, range and standard deviation. With-in group analysis was carried out by using paired “t” test and between group analyses by unpaired “t” test. Categorical data is presented as actual numbers and percentages. Categorical variables were analyzed with “Fischer's exact test”. Pearson correlations were used to determine the association between variables. 2 by 2 tables were used to assess the diagnostic value of IMA as positive and negative predictive values, sensitivity and specificity was calculated with 95% confidence interval.
2.11. Outcome measurements
The primary outcome analysis compared IMA values in patients diagnosed as ACS versus NICP. The use of IMA in early diagnosis of MI when compared with Trop T was also evaluated.
3. Results
Our study included a total of 89 patients (51 men, 38 women, median age 52.7 years). Sufficient data were available for all the patients for a final diagnosis to confirm or exclude CAD. The final diagnostic classification according to ESC/ACC criteria was 24 non-ischemic chest pain (NICP) and 65 coronary artery disease. Among the CAD patients, 26 had STEMI, 14 had NSTEMI, and 25 had UA.
Average duration of presentation was 3.27 h. Of the total of 89 patients 36 had history of hypertension and 43 had diabetes mellitus.36 patients were smokers.11 patients expired while in hospital (3 in UA group and 8 in MI group). Table 1 shows the patient characteristics in each individual group.
Table 1.
Patient clinical characteristics.
| Clinical Parameter | NICP |
UA |
MI |
|---|---|---|---|
| (n = 24) | (n = 25) | (n = 40) | |
| Age | 49.5 ± 1.3 | 54.7 ± 12.1 | 52.7 ± 1.4 |
| Gender | 12/12 | 13/12 | 13/27 |
| Time to hospitalization | 3.17 ± 2.40 | 2.86 ± 2.00 | 3.78 ± 1.83 |
| HTN | 11 | 11 | 14 |
| DM | 11 | 15 | 17 |
| Smoking | 10 | 7 | 19 |
| Mortality | 0 | 3 | 8 |
3.1. IMA in ischemic versus non-ischemic chest pain
Median IMA values were significantly higher in patients with ACS compared with NICP (p, 0.0001), in patients with UA compared with NICP (p, 0.0001), and in patients with NICP compared with MI (p = 0.001). But between UA and MI there was no significant difference (Table 2).
Table 2.
IMA values.
| NICP |
UA |
MI |
p value | |
|---|---|---|---|---|
| (n = 24) | (n = 25) | (n = 40) | ||
| IMA (Mean value) | 56.38 ± 23.89 | 89.00 ± 7.76 | 87.50 ± 9.62 | NICP vs UA, p = 0.001 NICP vs MI = 0.001 UA vs MI, p = 1.00 |
| Elevated IMA (number of patients) | 3 | 23 | 34 |
The normal IMA value was taken as 80 u/ml. In the NICP group mean IMA value was 56.38 ± 23.89 u/ml whereas in UA group it was 89.00 ± 7.76 u/ml and MI group was 87.50 ± 9.62 u/ml. IMA value was normal in 21 of 24 patients in NICP group whereas it was elevated in 23 of 25 patients in UA group and 34 of 40 patients in MI group.
This showed a sensitivity of 92% and specificity of 87%. The positive predictive value of the test was 88% and negative predictive value was 94%.
3.2. UA versus MI
Although non-significant the IMA value was elevated more in unstable angina than MI.
3.3. IMA in early diagnosis of MI
Comparing IMA with Trop-T showed that IMA was elevated in 34 of 40 patients at admission whereas Trop-T was positive in only 18 of the 40 patients. So in 16 patients an early diagnosis could be made when compared with Trop-T. IMA and Trop-T values are given in Table 3.
Table 3.
IMA versus cTnT.
| Biochemical parameters | NICP |
UA |
MI |
|---|---|---|---|
| (n = 24) | (n = 25) | (n = 40) | |
| Elevated IMA | 3 | 23 | 34 |
| Positive Troponin-T at admission | 0 | 0 | 18 |
| Positive Troponin-T after 6 h | 0 | 0 | 40 |
3.4. IMA and peri-hospital mortality
Of the 89 patients 11 patients died in hospital. The IMA value was compared between this group and the patients who survived. Patients who died had a mean IMA value of 88.5 with a standard deviation of 5.33 whereas in patients who survived the mean value was 78.26 which was not statistically significant. Comparison values are given in Table 4.
Table 4.
IMA values and in-hospital mortality.
| IMA values | Died | Alive |
|---|---|---|
| N | 11 | 78 |
| Minimum | 81 | 14.2 |
| Maximum | 99 | 112.6 |
| Mean | 88.55 | 78.26 |
| Std. Deviation | 5.336 | 21.17 |
| p value | 0.1139 |
4. Discussion
The use of biomarkers for the identification of suspected acute coronary syndromes depends on the presence of myonecrosis as a surrogate indicator for myocardial ischemia. However, many patients have myocardial ischemia in the absence of myonecrosis, and markers such as myoglobin, CK-MB, and the troponins, although mainstays for the diagnosis of acute cardiac myonecrosis, thus have limited role. Furthermore, the release of these markers is time-dependent; an initially negative result does not exclude the presence of MI. Therefore, a rapidly detectable, highly sensitive marker for myocardial ischemia would be desirable to identify patients with only ischemia and those early in the course of an acute coronary syndrome. For such a marker to be useful, it should be accompanied by a high negative predictive value. This is where the role of IMA comes in.
4.1. IMA in ischemic versus non-ischemic chest pain
In this study we found that the use of IMA at presentation could be used to both confirm and exclude a final diagnosis of coronary artery disease. It is apparent from the data shown above that for the emergency medicine physician, the role of this test will be to exclude those likely to have CAD at presentation. A negative test allows exclusion of CAD.
In the study by Bhagwan et al,7 the sensitivity and specificity for myocardial ischemia were 88% and 94%, respectively, and the positive and negative predictive values were 92% and 91%. The ACB test, however, was a poor discriminator between ischemic patients with and without MI. This compared well with our study.
The specificity values were very low in some of the studies. One study by Sinha et al14 evaluated IMA for diagnosis of cardiac ischemia in patients attending the ED with symptoms of ACS. In the whole patient group, sensitivity of IMA at presentation for an ischemic origin of chest pain was 82% (95% CI, 74–88%), specificity was 46% (34–57%), the negative predictive value was 59%, and the positive predictive value was 72% (prevalence, 63%). IMA, ECG, and cTnT combined identified 95% of patients whose chest pain was attributable to ischemic heart disease.
Roy et al15 studied 131 patients presenting to the ED with symptoms suggestive of ACS but with normal or non-diagnostic ECGs. All patients arrived to the ED within 3 h of the last episode of chest pain and had negative cTn results on admission to the ED. Cardiologists, unaware of IMA results, reviewed all the patients' notes and hospital test results (ECG exercise stress testing, dobutamine stress echo and coronary angiography) to establish a final diagnosis of ACS or non-ischemic chest pain. Ischemia modified albumin values were significantly higher in 64 patients with myocardial ischemia compared with 67 patients with non-ischaemic cardiac pain (98.3 ± 11 versus 85.5 ± 15, p < 0.0001). At the optimum cut-off point of 93.5 u/ml, IMA had a sensitivity of 75% for the diagnosis of myocardial ischaemia. The combination of IMA (measured at presentation to the ED) and serial cTnT (6–12 h) increased sensitivity to 82.8%.
Lee et al16 studied 413 patients who had visited the ED for symptoms suspicious of ACS. Sensitivity and specificity of IMA for identifying ACS were 93% and 35.6%, respectively, and the negative and positive predictive values were 91.8% and 39.6%, respectively. The combination of myoglobin, CK-MB, and troponin-T had a sensitivity of 80.2% and specificity 57% for the diagnosis of ACS. When IMA was included in the cardiac marker panel, sensitivity increased to 94.5% while specificity fell to 45.1%.
A multicenter study by Christenson et al,17 involving 224 patients who arrived at the ED within 3 h after onset of signs and symptoms suggestive of ACS, examined the ability of the ACB test to predict a positive or negative cTnI result within 6–24 h after presentation. All patients had a negative cTnI result at presentation. Patients were considered troponin positive if 1 or more cTnI values were above the upper reference limit within 6–24 h. At the optimum cutoff for the ACB test, sensitivity and specificity were 70% and 80%, respectively, with a negative predictive value of 96%. There were 6 false negatives and 131 true negatives. cTnI alone was used as the outcome measure, and electrocardiogram (ECG) status at presentation was not considered in the design of the study. The positive predictive value is only 33%.
A meta-analysis of more than 1800 patients concluded that in a large ED cohort with suspected myocardial ischaemia, the combination of ECG, troponin and IMA has 94.4% sensitivity and 97.1% negative predictive value for the final diagnosis.18
A study conducted in India by Chawla et al4 found that IMA demonstrated good discrimination between the ischemic and the non-ischemic patients with an Odds Ratio of 16.9 (6.29–46.87) than CK-MB which showed an Odds Ratio of 2.07 (1.18–6.08). Sensitivity and specificity of IMA for the detection of ACS was 78.0% and 82.7% compared to 58.0% and 60.0%, respectively for the CK-MB assay.
However not all studies had a positive outcome. The PRIMA study19 conducted in 399 patients in the emergency department of John Radcliff Hospital UK between 2005 and 2006 concluded that the sensitivity of IMA was insufficiently high, with a small number of false negatives undermining the safety of the test. Frequent false positives produce a low specificity that limits the practical value of the test. The disadvantage of this study was that it compared IMA with cTnT & UA was not a part of final diagnosis.
This was supported by other studies also. A study by Soren et al20 concluded that Ischemia modified albumin did not, at any time, provide superior sensitivity or specificity compared with other biomarkers and did not find the data supportive of IMA as a standard marker in the emergency department.
IMA, which appears to be an indicator of oxidative stress, may not be specific for cardiac ischemia. Data about IMA concentrations in non-cardiac ischemia are limited. Anecdotal evidence suggests that IMA increases in stroke, end-stage renal disease, liver disease, and some neoplasms.21 In a study evaluating the ACB test for forearm ischemia, increases in endogenous lactate inhibited the test.22 This result was recapitulated with exogenous lactate in vitro. Such a result raises caution concerning the significance of a negative IMA result in patients with poorly controlled diabetes, sepsis, and/or renal failure, all of which are situations where increased lactate may exist.
In a group of marathon runners, IMA did not increase immediately after a marathon run, indicating that skeletal muscle ischemia during exercise does not change IMA concentrations. However, there were significant increases 24–48 h after the run, which were attributed to exercise-induced latent gastrointestinal ischemia.23 This latent increase is an issue that may potentially complicate use of the test in clinical practice.
4.2. UA versus MI
When evaluating subsets of patients, we observed that IMA values were higher, albeit non-significantly, in patients with unstable angina compared to those with non-ST segment elevation myocardial infarction. A possible explanation for this may be that the opportune window to diagnose ischemia prior to the occurrence of myocardial damage in the patient subgroup that progressed to myocardial necrosis was missed. Previous studies have shown that IMA levels rise within minutes after ischemia and return to baseline within 6 h24 unfortunately, we did not carry out serial sampling measurements that may have given additional information about marker kinetics and may have increased assay sensitivity for ACS.
The results indicate that IMA could be a potential marker for early ruling out of ACS in chest pain patients because of its relatively high NPV, especially combined with cTn and ECG. Importantly IMA seems to add relevant diagnostic information to more readily available diagnostic parameters. However, problems with the stability of IMA and its lack of cardiospecificity have been reported.
A negative test allows exclusion of CAD but a positive IMA alone at presentation cannot differentiate between UA and MI. This will need a follow up Troponin measurement to confirm MI. There may be a number of reasons for this.
Firstly, IMA is a test for ischemia not infarction. Myocardial ischemia may occur without proceeding to infarction. Previous studies of the ability of IMA to predict a positive cTn have shown good performance with an area under the receiver operator characteristic (ROC) curve of 0.78 but did not show 100% concordance in patients admitted with chest pain.17
Secondly, the study used as diagnostic “gold standard” with elevated cTn according to ESC/ACC criteria, is for acute MI rather than myocardial ischemia. There is currently no method of reliably detecting myocardial ischemia. Previous studies of IMA have shown it is possible to distinguish reliably between the ACS and non-ACS populations (area under the ROC curve 0.95) but there is overlap when attempting to distinguish between AMI and unstable angina7 (area under the ROC curve 0.66).
4.3. IMA and peri-hospital mortality
Regarding the immediate prognosis our study could not find any significant correlation between IMA values and expired patients. A study conducted by Andrew Worster et al25 in 189 patients monitored patients who presented within 6 h of angina for 72 h for any serious cardiac outcome. When they correlated the IMA values there was no significant relation.
The long term outcome of IMA value was not considered in this study. The study which has monitored the long term correlation of IMA was the French nationwide OPERA study26 which found that the primary composite end point (death, resuscitated cardiac arrest, recurrent myocardial infarction or ischemia, heart failure, stroke) occurred in 75 (15.6%) patients in-hospital and in 144 (30.6%) at 1 year: 40% of patients in the highest IMA quartile (>104 IU/mL) reached the end point compared with 20% in the lowest (<83 IU/mL) by 1 year. They identified IMA as one of the 4 independent predictors of composite end point at 1 year the others being: plasma concentrations of brain natriuretic peptide (p = 0.001), heart failure (p = 0.005), and age (p = 0.003).
The results of our study support the evidence that IMA is an early marker of ischemia, increases before any detectable change in cardiac troponin occur, is elevated even in the absence of myocardial necrosis and is clinically useful in the ED setting.
4.4. Limitation
Our study was conducted in a single center, which may limit the generalizability of our results. Furthermore, our results may not be applicable to all patients suffering from acute chest pain, as we excluded patients with conditions known to increase IMA levels, and also limited our observations to patients with chest pain within 6 h prior to the ED admission; thus, the prognostic value of IMA in a more general population has yet to be determined. Finally, the relatively small number of patients included in the study dictates caution in the interpretation of the results.
IMA values are altered in the following settings:
(i) IMA may increase in patients with stroke, end-stage renal disease, liver disease, and some neoplasms. (ii) Increased endogenous lactate levels appear to reduce IMA concentrations, which raise concern about the true significance of a negative IMA result in patients with sepsis or renal failure where lactate may be present in the circulation. (iii) IMA levels raise after radiofrequency catheter ablation and direct current cardioversion, which could be due to the generation of reactive oxygen species following electrical and thermal myocardial injury.
A further limitation is that the ACB test (the test currently used to measure IMA) is a colorimetric assay, and therefore an indirect measurement of IMA production. New assay platforms (i.e. immunoassays), however, are expected to be available in the near future.
We did not do a serial assay of the IMA hence the kinetics of IMA in ischemia is not fully known. Further a long term follow up was also not done.
5. Conclusion
In our patients, IMA was useful to distinguish those with ACS from NICP subjects and in early diagnosis of MI in patients who present early. This biomarker may therefore constitute a useful adjunct to our current diagnostic armamentarium in the ED setting. However IMA could not differentiate between MI and UA. Neither was it useful in predicting in-hospital mortality.
Shortcomings are plenty. The influence of fluid shifts and albumin concentration changes need to be more fully understood. Additional information is needed for the clinical validation of this new assay including studies on reference distributions by gender and ethnicity; an optimum diagnostic cutoff value for ACS patients, comparing IMA concentrations in common disease states with or without accompanying cardiac disease; and common diseases that coexist with cardiac ischemia, such as congestive heart failure, diabetes mellitus, chronic renal failure, and hypertension. A better understanding of IMA kinetics over the early hours after the onset of an ACS is also essential.
In summary, many questions remain unanswered regarding IMA and the ACB test. The assay needs to be evaluated by incorporating it into decision-making algorithms under ED conditions. The highest expected benefit of the test would be to rule out ACS in patients with negative necrosis markers and a negative ECG. This was the language for which the ACB test was cleared by the FDA for clinical use.
Conflicts of interest
All authors have none to declare.
References
- 1.Eftihia S., Panagiota G., Dimitrios T., Kremastinos, Vasilious V. Ischemia modified Albumin: this marker of ischemia ready for prime time use? Hell J Cardiol. 2008;49:260–266. [PubMed] [PubMed] [Google Scholar]
- 2.Vamadevan S., Ajay Doraraj P. Prevalence. Indian J Med Res. 2010;132:561–566. doi: 10.4103/0971-5916.73396. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plebani M. Biochemical markers of cardiac damage: from efficiency to effectiveness. Clin Chim Acta. 2001;311:3–7. doi: 10.1016/s0009-8981(01)00551-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Chawla R., Navendu G., Rajneesh C., Goyal Shweta. Ischemia Modified Albumin: a novel marker for acute coronary syndrome. Indian J Clin Biochem. 2006;21:77–82. doi: 10.1007/BF02913070. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kritsanee M., Titiporn M., Saowanee L., Tomon T., Sarawut K. Combinational determination of ischemia modified albumin and protein carbonyl in the diagnosis of non ST-elevation Myocardial Infarction. Indian J Clin Biochem. 2011;26:389–395. doi: 10.1007/s12291-011-0118-2. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaoquing Ju, Juan N.I., Jianyou S.U., Min P.A.N., Jianhua Z. Ischemia modified albumin is increased in patients with unstable angina: a new potential diagnostic biomarker of this acute coronary syndrome? Science. 2008;39:668–670. [Google Scholar]
- 7.Bhagwan N., Ernest M., Rios P. Evolution of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem. 2003;49:581–585. doi: 10.1373/49.4.581. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Duseja R., Feldman J.A. Missed acute cardiac ischemia in the ED: limitations of diagnostic testing. Am J Emerg Med. 2004;22:219–225. doi: 10.1016/j.ajem.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Montagnana M., Lippi G., Guidi G.C. New perspectives in the diagnostic approach to acute coronary syndrome. Recenti Prog Med. 2005;96:171–177. [PubMed] [Google Scholar]
- 10.The Joint European Society of Cardiology and American College of Cardiology Committee Myocardial infarction redefined – a consensus document of the Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E., Antman E.M., Beasley J.W. ACC/AHA guidelines for the management of patients with unstable angina and non-ST segment elevation myocardial infarction. J Am Coll Cardiol. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Or D., Curtis G., Rao N. Characterization of the Co2+ and Ni2+ binding amino-acid residues of the N-terminus of human albumin. Eur J Biochem. 2001;268:42–47. doi: 10.1046/j.1432-1327.2001.01846.x. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Or D., Lau E., Winkler J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia – a preliminary report. J Emerg Med. 2000;19:311–315. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 14.Sinha M.K., Roy D., Gaze D.C., Collinson P.O., Kaski J.-C. Role of ischemia modified albumin a new biochemical marker of myocardial ischemia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004;21:29–34. doi: 10.1136/emj.2003.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy D., Quiles J., Aldama G. Ischemia modified albumin for the assessment of patients presenting to the emergency department with acute chest pain but normal or non-diagnostic 12-lead electrocardiograms and negative cardiac troponin T. Int J Cardiol. 2004;97:297–301. doi: 10.1016/j.ijcard.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y.W., Kim H.J., Cho Y.H., Shin H.B., Choi T.Y., Lee Y.K. Application of albumin-adjusted ischemia modified albumin index as an early screening marker for acute coronary syndrome. Clin Chim Acta. 2007;384:24–27. doi: 10.1016/j.cca.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Christenson R.H., Duh S.H., Sanhai W.R. Characteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: a multicentric study. Clin Chem. 2001;47:464–470. [PubMed] [Google Scholar]
- 18.Peacock F., Morris D.L., Anwaruddin S. Meta-analysis of ischemia-modified albumin to rule out acute coronary syndromes in the emergency department. Am Heart J. 2006;152:253–262. doi: 10.1016/j.ahj.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Keating L., Benger J.R., Beetham R. The PRIMA study: presentation ischaemia-modified albumin in the emergency department. Emerg Med J. 2006;23:764–768. doi: 10.1136/emj.2006.036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hjoiirtshøj Søren, Kristensen Risom, Ravkilde Jan. Diagnostic value of ischemia-modified albumin in patients with suspected acute coronary syndrome. Am J Emerg Med. 2010;28:170–176. doi: 10.1016/j.ajem.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Wu A.H.B. The ischemia-modified albumin biomarker for myocardial ischemia. MLO Med Lab Obs. 2003;6:36–40. [PubMed] [Google Scholar]
- 22.Zapico-Muniz E., Santalo-Bel M., Mercé-Muntanola J., Montiel J.A., Martinez-Rubio A., Ordonez-Llanos J. Ischemia-modified albumin during skeletal muscle ischemia. Clin Chem. 2004;50:1063–1065. doi: 10.1373/clinchem.2003.027789. [DOI] [PubMed] [Google Scholar]
- 23.Apple F.S., Quist H.E., Otto A.P., Mathews W.E., Murakami M.M. Release characteristics of cardiac biomarkers and ischemia modified albumin as measured by the albumin cobalt-binding test after a marathon race. Clin Chem. 2002;48:1097–1100. [PubMed] [Google Scholar]
- 24.Bar-Or D., Winkler J.V., Vanbenthuysen K., Harris L., Lau E., Hetzel F.W. Reduced albumin-cobalt binding with transient myocardial ischemia after elective percutaneous transluminal coronary angioplasty: a preliminary comparison to creatine kinase-MB, myoglobin, and troponin I. Am Heart J. 2001;141:985–991. doi: 10.1067/mhj.2001.114800. [DOI] [PubMed] [Google Scholar]
- 25.Worster Andrew, Devereaux P.J., Heels-Ansdell Diane, Opie John. Capability of ischemia-modified albumin to predict serious cardiac outcomes in the short term among patients with acute coronary syndrome. Can Med Assoc J. Jun 21, 2005;172:13. doi: 10.1503/cmaj.045194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Belle Eric, Dallongeville Jean, Vicaut Eric, Degrandsart Alexia, Baulac Cathrine, Montalescot Gilles. Ischemia-modified albumin levels predict long-term outcome in patients with acute myocardial infarction. The French Nationwide OPERA study. Am Heart J. 2010;159:570–576. doi: 10.1016/j.ahj.2009.12.026. [DOI] [PubMed] [Google Scholar]


