Abstract
Background and objective
The promotion of medication adherence is considered as an integral component of pharmaceutical care practice and patient healthcare. An approach which focuses on the choice and dose of antiepileptic drug will have limited success without medication adherence. This study sought to assess medication adherence for improvement among adolescents who are suffering from epilepsy.
Methods
A total of 116 patients affected with idiopathic epilepsy and fulfilled the inclusion criteria were recruited in the current study. Adherence to the treatment was evaluated during patients’ hospitalization in the Department of Neurology at Riyadh National Hospital, Riyadh, Saudi Arabia, between December 2011 and January 2014. The medication adherence has been assessed during semi-structured interviews with each patient and/or his parents using a multiple choice graded questionnaire.
Results
From the selected group of patients, only 94 patients (81.0%) fulfilled the inclusion criteria within the study period. Thirty-six of respondents (38.3%) were non adherent to antiepileptic treatment. No statistical differences were found between males and females regarding their ages, age at diagnosis of epilepsy, mother age, epilepsy duration, family numbers, number of poor-adherents or seizure frequency. The most important factors that were significantly affecting patients’ adherence to the prescribed medications were age of mother, family number, number of administered drugs, the stability of parents’ marriage, family support, and seizure frequency as well as the regularity of the relationship between patients and their healthcare providers. Forgetfulness was the most common cause of non-adherence among this group of patients followed by inability to obtain medication and fear from side effects of drugs. Our results revealed also that the number of patients who felt to be stigmatized is significantly more in non-adherent group as compared to patients with a strong sense of normality (P < 0.05). A positive relationship between adherence and the necessity and benefit scales at which patients have a stronger belief in the necessity of medication for controlling illness was associated with good adherence.
Conclusion
The assessment of medication adherence among epileptic patients should be a routine part of the management process to improve the health care and quality of lives of those patients.
Keywords: Epilepsy, Compliance, Medication adherence, Pharmaceutical care
1. Introduction
Epilepsy is a neurological condition, which affects the nervous system. Epilepsy is also known as a seizure disorder (England et al., 2012). It is usually diagnosed after a person has had at least two seizures that were not caused by some known medical condition like alcohol withdrawal, extremely low blood sugar, heart problems or some other medical condition (Berg et al., 2013). Sometimes, according to the International League Against Epilepsy, epilepsy can be diagnosed after one seizure, if a person has a condition that places him/her at a high risk of having another (Kaiboriboon et al., 2013). Epilepsy is the second common disease among chronic nervous diseases next to stroke (Ray et al., 2002) which affects approximately over 50 million patients worldwide (de Boer et al., 2008), with a prevalence rate ranging from 16 to 51 per 100,000 population in the developed countries and 35–111 per 100,000 population in the developing countries (Banerjee et al., 2009).
Epilepsy may promote limitations and restrain activities, interfering with the occupational ability, professional goals and social integration of patients (Gomes et al., 1998). It increases morbidity and symptomatic epilepsy reduces life expectancy by 18 years at maximum (Gaitatzis et al., 2004). It continues to be a highly stigmatized and disabling chronic condition (Paschal et al., 2014) requiring a lifelong process of adherence to the prescriber’s instructions and drug regimens (Michaud et al., 1991; Adams et al., 1997). Medication adherence or the older term, drug compliance, is defined as the extent to which patients follow the instructions they are given for prescribed treatments and persistence as the duration of time from initiation to discontinuation of therapy (Shams and Barakat, 2010). Medication non-adherence includes delaying prescription fills, failing to fill prescriptions, cutting dosages, and reducing the frequency of administration.
When treating an individual with epilepsy, there are several factors that cannot be modified such as the age of onset, the etiology of the seizures and the location of the epileptogenic zone. There are also some factors that may be amenable to an intervention to improve outcomes. An obvious consideration for the clinician is the choice of medication to prescribe. Despite medication, it has been found that seizures persist in 20–35% of cases (Devinsky, 1999). It is necessary therefore to identify other “modifiable factors” which could lead to improved seizure control if targeted effectively (Jones et al., 2006). Current estimates of non-adherence in epilepsy are similar to those in other chronic illnesses and range from 30% to 50% and non adherence may be the most important cause of poorly controlled epilepsy (Gomes et al., 1998).
The promotion of medication adherence is considered nowadays as an important component of pharmaceutical care practice (Shams and Barakat, 2010). Medication adherence should be discussed regularly with the patient, and in particular when a treatment seems to fail (Jones et al., 2006). There are several types of non adherence. Therapeutic or medication non adherence which includes failure to have the prescription dispensed or renewed, omission of doses, errors of dosage, incorrect administration, errors in the time and frequency of administration, and premature discontinuation of the drug regimen. A second type of non-adherence is dietary/exercise non-adherence in which the patient fails to follow the diet and exercise recommendations. A third type is the appointment non adherence at which the patient fails to show up at clinics for the scheduled check up (Hughes and Manns, 2000; Shams and Barakat, 2010).
Several methods are used to measure therapeutic adherence. Indirect methods, like self reports and interviews with patient, are the simplest and most common methods for measuring medication adherence (Girerd et al., 2001; Shams and Barakat, 2010). Although medication adherence and factors associated with it have been extensively studied, very little is known about the factors associated with good medication adherence among epileptic patients (Kyngas, 2001).
Non-adherence leads to considerable morbidity, mortality, and avoidable health care costs. In epilepsy, non-adherence leads to lack of control over seizures, recurrence, increased absenteeism from work and, possibly, injury to oneself or to others (Asawavichienjinda et al., 2003; Enriquez-Caceres and Soto-Santillana, 2006).
For the previous reasons, we conducted this research study to identify the different factors which could affect the medication adherence among adolescent epileptic patients, to investigate factors that may reduce morbidity caused by recurrent seizures, and to know how we can improve the medication adherence among those patients for optimum therapy outcome and enhancement of their quality of lives.
2. Materials and methods
2.1. Patients’ characteristics
From December 2011 to January 2014, total 116 patients affected with idiopathic epilepsy were recruited in the current research study. These patients were randomly selected from Department of Neurology at Riyadh National Hospital, Riyadh, Saudi Arabia. Approval for this study was granted by the scientific committee at Riyadh National Hospital and with the Helsinki Declaration of 1975, as revised in 1983. Informed consent was taken from the patients to participate in this study. Patients were informed that personal information will never be disclosed to a third party.
Inclusion criteria for all participants were:
-
•
aged between 13 and 18 years,
-
•
diagnosed with epilepsy for at least one year,
-
•
administered at least one antiepileptic drug,
-
•
with normal neurological and cognitive development,
-
•
without other severe co-morbidities,
-
•
consented to participate in the current study.
Age selection was based on the expectation that by the age of thirteen, self-care responsibilities had been assumed by the adolescents.
2.2. Assessment of medication adherence
A database of the selected patients who underwent one or more semi-structured interviews was created in the hospital outpatient clinics after signing an informed consent. The data were collected by means of interview with the patients and/or their parents, by using a questionnaire of known reliability and validity to assess treatment adherence (Morisky et al., 1986). Beliefs about illness (illness perception questionnaire, IPQ) and treatment (beliefs about medicines questionnaire, BMQ) were also measured. Patients’ histories were taken and they were asked about the age of onset of epilepsy to calculate the disease duration, recent seizure frequency, details of prescribed antiepileptic drugs and feelings of stigma.
2.3. The Morisky Medication Adherence Scale
The Morisky Medication Adherence Scale (MMAS) consists of four items with a scoring scheme of “Yes” = 0 and “No” = 1. The items are summed to give a range of scores from 0 to 4. The four questions included in this scale are: (1) Do you ever forget to take your medicine? (2) Do you ever have problems remembering to take your medication? (3) When you feel better, do you sometimes stop taking your (name of health condition) medicine?) When you felt better, did you sometimes stop taking your medicines? (4) Sometimes, if you felt worse, did you stop taking your medicines? In our study, patients were considered poor-adherent if they scored 1 or more.
2.4. Beliefs about medicines questionnaire, (BMQ)
This questionnaire was developed in the UK and published by Horne and Weinman (1999) and comprises two parts (general and specific sections). Subjects are asked the extent to which they agree or disagree with the statement on a five-point Likert scale, where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree and 5 = strongly agree, to investigate the participant’s opinion for each item. The questionnaire is divided into two sections, measuring beliefs about medicines in general and beliefs about a specified medication. In this evaluation, items in the specific section were worded to relate to ‘antiepileptic drugs’, where personal beliefs about the necessity of the medication for maintaining or improving health (5 items, e.g. “my health at present depends upon my antiepileptic drugs”) are balanced against concerns about the potential adverse effects of taking it (5 items, e.g. “having to take my antiepileptic drugs worries me”). Total scores for the necessity and concerns scales ranged from 5 to 25. Higher scores indicate stronger beliefs (Horne et al., 1999). A necessity–concerns differential is calculated as the difference between the necessity and the concerns scales, with a possible range of −20 to +20. This differential can be thought of as the cost–benefit analysis for each patient, for whom costs (concerns) are weighed against their perceived benefits (necessity beliefs) (Horne et al., 1999). A positive differential score indicates stronger necessity beliefs than concerns, and a negative score indicates the contrary i.e., stronger concerns. The general section consists of the overuse subscale (e.g. “doctors use too many medicines”), the Harm-Benefit Subscale (e.g. “medicines do more harm than good”). Only completed questionnaires were included in the analyses. The scores for each item in a subscale are summed to give a total score which ranges from 4 to 20 for the Harm and Benefit scale and ranged from 3 to 15 for the Overuse Scale. The total score for each sub-scale was then divided by the number of items in the scale. Higher scores indicate stronger beliefs in the concepts represented by the scale.
2.5. Data management and statistical analysis
Computer software GraphPad InStat version 6.00, GraphPad Software, San Diego, California, USA was used to analyze the data obtained from the questionnaire. For descriptive statistics, the frequency and percentage were calculated for qualitative variables while the mean values ± standard deviation (SD), and range were used for quantitative variables. For comparison between two groups Student’s t-test was used. For correlation, Pearson correlation test was used. Chi-square test and contingency coefficient test were used to analyze the significant correlations between adherence and the tested factors. For certain two variables, when P value is less than 0.05, there is a statistically significant relationship between the two variables.
3. Results
3.1. Patient’s characteristics
From a total of 116 patients who were clinically examined during the study period in the Neurology Department at Riyadh National Hospital, Riyadh, Saudi Arabia, only 94 patients (81.0%) fulfilled the inclusion criteria within the study period. The characteristics of the selected group of patients are summarized in Table 1.
Table 1.
Characteristics of participants (N = 94).
| Total | Male | Female | P value t-test | ||
|---|---|---|---|---|---|
| Number of patients (%) | 94 (100%) | 44 (46.8%) | 50 (53.2%) | ||
| Mean age (±SD) | 14.96 (1.74) | 14.68 (1.59) | 15.20 (1.87) | 0.32 | |
| Mean age at diagnosis of epilepsy in years (±SD) | 8.72 (2.53) | 8.59 (2.04) | 8.84 (2.94) | 0.74 | |
| Mean age of the mother (±SD) | 38.32 (5.51) | 38.91 (5.91) | 37.80 (5.19) | 0.50 | |
| Mean duration of epilepsy in years (±SD) | 6.23(2.20) | 6.09 (2.20) | 6.36 (2.23) | 0.32 | |
| Mean family number (±SD) | 7.38 (1.62) | 7.64 (1.76) | 7.16 (1.49) | 0.64 | |
| Number (%) of non-adherents (⩾1 on Morisky) | 36 (100.0) | 20 (55.5%) | 16 (44.5%) | Chi-square = 1.79 (P = 0.18) | |
| Number (%) of adherents (=0 on Morisky) | 58 (100.0) | 24 (41.4) | 34 (58.6) | ||
| Seizure frequency [Number of cases (%)] | <once/month | 48 (51.0) | 22 (23.4) | 26 (27.6) | Chi-square = 1.356 (P = 0.715) |
| >one/Month but <one/week | 20 (21.4) | 10 (10.7) | 10 (10.7) | ||
| >one/week but <one/day | 16 (17.0) | 6 (6.3) | 10 (10.7) | ||
| >one/day | 10 (10.6) | 6 (6.3) | 4 (4.3) | ||
∗Statistically significant (t-test) or Chi-square at P < 0.05.
From all patients, only 38.3% (N = 36) of them were non adherent to antiepileptic treatment. No statistical differences were found between males and females regarding their ages, age at diagnosis of epilepsy (age of the disease onset), mother age, epilepsy duration, family numbers, number of poor-adherents or seizure frequency (Table 1).
Epilepsy in 83% of the patients (N = 78) was generalized, in 14.9% of them (N = 14) was partial while in 2.1% (N = 2) was unclassified. The most common types of epilepsy were generalized tonic–clonic seizures (65%, N = 61), and to a less extent myoclonic seizures (6.4%, N = 6), and tonic seizures (4.2%, N = 4).
3.2. Disease management
Monotherapy was used to control the disease condition in 76.6% of the patients (N = 72). Valproic acid was the most common prescribed drug to control epilepsy among those patients (59.6%, N = 56) followed by carbamazepine (12.8%, N = 12), and levetiracetam (4.3%, N = 4). A combination of two drugs was used in 17% of them (N = 16) while 6.3% of patients (N = 6) required more than two drugs to control their conditions.
3.3. Characteristics of adherent and non-adherent epileptic patients to medication
Characteristics of patients’ adherence toward antiepileptic drugs were significantly affected by many factors like age of mother, family number, number of administered drugs, the stability of parents’ marriage, family support, and seizure frequency as well as the regularity of the relationship between patients and their healthcare providers (medical support) (Table 2). Medication adherence in patients with generalized epilepsy did not differ significantly from the patients with focal epilepsy (P = 0.860). The commonest causes of non-adherence in the patients sample are summarized in Fig. 1. Cultural believes that the main cause of the convulsion may be due to possession by external powers or Evil Spirit constitutes 8.4% of non adherent patients. Our results revealed that patients felt to be stigmatized are significantly more in non-adherent group as compared to patients with a strong sense of normality (P < 0.05).
Table 2.
Characteristics of adherent and non-adherent epileptic patients.
| Adherent | Non-adherent | Test | P value | ||
|---|---|---|---|---|---|
| Total number (N = 94, 100%) | 58 (61.7) | 36 (38.3) | |||
| Number of males (N = 44, 100%) | 24 (54.5) | 20 (45.5) | Chi-square = 1.793 | 0.18 | |
| Number of females (N = 50, 100%) | 34 (68.0) | 16 (32.0) | |||
| Mean age (±SD) | 14.93(1.77) | 15.00(1.75) | t = −0.130 | 0.89 | |
| Mean age (year) at diagnosis of epilepsy (±SD) | 2.49(0.46) | 2.66(0.63) | t = −0.585 | 0.56 | |
| Mean duration of epilepsy in years (±SD) | 2.32(0.43) | 2.03(0.48) | t = 0.571 | 0.57 | |
| Mean age of Mother (±SD) | 36.76(5.64) | 40.83(4.33) | t = −2.619 | <0.05⁎ | |
| Mean Family Number (±SD) | 6.66(1.42) | 8.56(1.20) | t = −4.721 | <0.05⁎ | |
| Family history of similar illness (%) | 10 (38.5%) | 16 (61.5%) | |||
| Patients with monotherapy [N (%)] | 40 (71.4) | 16 (28.6) | Chi-square = 5.546 | <0.05⁎ | |
| Patients with polypharmacy [N (%)] | 18 (47.4) | 20 (52.6) | |||
| Stable parent marriage [N (%)] | 44 (68.8) | 20 (31.2) | Chi-square = 4.215 | <0.05⁎ | |
| Unstable parent marriage [N (%)] | 14 (46.6) | 16 (53.4) | |||
| Patients with good family support | 39 | 12 | Chi-square = 10.290 | <0.01⁎ | |
| Patients with poor family support | 19 | 24 | |||
| Seizure Frequency [number of cases (%)] | <once/month | 42 (44.7) | 6 (6.3) | Chi-square = 33.06 | <0.0001⁎⁎ |
| >one/Month but < one/week | 10 (10.6) | 10 (10.6) | |||
| >one/week but < one/day | 2 (2.1) | 14 (14.9) | |||
| >one/day | 4 (4.2) | 6 (6.3) | |||
| Patients with regular medical support | 37 (75.5) | 12 (24.5) | Chi-square = 8.259 | <0.05⁎ | |
| Patients without regular medical support | 21 (46.6) | 24 (53.5) | |||
Statistically significant (t-test) or Chi-square at P < 0.05,
Statistically significant (t-test) or Chi-square at P < 0.01.
Figure 1.
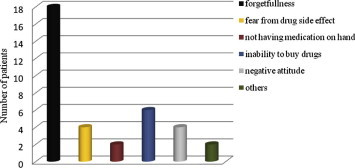
Causes of non-adherence to antiepileptic medications.
3.4. Beliefs about medicines questionnaire (BMQ)
Specific necessity and specific concerns scales, and the necessity–concerns differential, are presented in Table 3. No gender differences were identified. In calculations of the necessity–concerns differential, 78 respondents reported positive scores, meaning that their belief about the necessity of taking medication to control their illness was stronger than their concerns regarding medication. For sixteen of the respondents, the necessity concerns differential score was negative.
Table 3.
A comparison in the mean of beliefs about medicines questionnaire scores (BMQ) in both sexes.
| Total | Male | Female | P value | |
|---|---|---|---|---|
| Necessity median | 18 (13–25) | 18.5 (15–25) | 18(13–23) | 0.140 |
| Concern median | 11 (5–22) | 12 (5–22.) | 10 (7–22) | 0.327 |
| Necessity–concerns differential score | 8 (−7–17) | 7.5 (−4–17.) | 9 (−7–16) | 0.960 |
| Overuse median | 9 (3–15) | 9 (3–14) | 10 (5–15) | 0.214 |
| Harm median | 14 (4–19) | 12.5 (4–19) | 15 (10–19) | 0.055 |
| Benefit median | 12 (5–20) | 14.5 (8–20) | 12 (5–18) | 0.097 |
Associations between the BMQ scale and adherence to medication are found in Table 4. A statistically significant relationship between specific necessity, specific concerns, the necessity–concerns differential, and benefit–harm scales with the adherence to administered medications was observed. Stronger concerns about adverse consequences of taking the prescribed medication and fears from the possible medication harm in non-adherent group were observed. A positive relationship between adherence and the necessity and benefit scales at which patients with stronger belief in the necessity of medication for controlling illness was associated with good adherence (Table 4).
Table 4.
Comparison between adherent and non-adherent groups of patients regarding BMQ.
| Adherent group | Non-adherent group | Statistical test | ||
|---|---|---|---|---|
| Necessity median | 20 (16–25) | 15.5 (13–21) | Z = −4.450 | P < 0.01 |
| Concern median | 9 (5–15) | 15.5 (13–22) | Z = −5.570 | P < 0.01 |
| Necessity–concerns differential score | 11 (2–17) | 1 (−7–8) | Z = -5.552 | P < 0.01 |
| Overuse median | 8 (3–12) | 12 (9–15) | Z = −5.281 | P < 0.01 |
| Harm median | 12 (3–16) | 16 (11–19) | Z = −4.290 | P < 0.01 |
| Benefit median | 16 (8–20) | 10 (5–15) | Z = −4.202 | P < 0.01 |
4. Discussion
Medication adherence among adolescents who are suffering from epilepsy disorder has been studied less extensively, although patients’ adherence to medication poses difficult issues for all clinicians. Poor patients’ adherence is considered as one of the major causes of non-responsiveness to antiepileptic drug therapy (Kyngas, 2000). More than 95% adherence may be necessary to adequately suppress the epileptic seizures. This means missing one or more doses of a regimen per week may be enough to cause treatment failure and trigger seizures (Johnbull et al., 2011). For this reason we classify our patients either adherent or non-adherent to their medications (All or None Rule). All literature reported that factors which influence patients ‘adherence to their medications are multiple and complex’. For these reasons, the primary purpose of this current research study was to assess factors influencing medication adherence among adolescent epileptic Arabic patients who have different cultures, believes and daily habits to provide recommendations for improvement in their healthcare and quality of lives.
In this current study, 61.7% of the patients (N = 58) were adherent to their medications according to their self or parental reports (Table 1) but this percentage has to be improved. In other research study which was carried out by Kyngas (2000), only one-fifth (22%) of the adolescents with epilepsy felt that they are adherent to medication. On the other hand, the rate of patient adherence measured by Liu et al. (2013) was 51.9% while it was only 41% in another study carried out by Jones et al. (2006). Asadi-Pooya (2005) found also that the compliance rate in his sample of patients was satisfactory in almost three-fourths (75%) of the patients with epilepsy. These variation in rate of compliance between different studies may reflect the differences in patient attitude toward the prescribed drug due to different cultures, beliefs, education, physician approach to the patient with epilepsy or the degree of medical and parental support.
The effect of gender as an important contributing factor affecting the medication adherence was studied. No statistical differences were found between both genders regarding their ages, age of the disease onset, mother age, epilepsy duration, family numbers, and seizure frequency or even in the adherence to their medications (Table 1). These results are in agreement with those carried out by Johnbull et al. (2011) who also found that the gender does not affect adherence rate. Liu et al. (2013) also concluded in other research study that there were no demographic differences based on gender between adherent and non- adherent patients which support our findings.
Other factors that may have a role on the rate of patient adherence to antiepileptic drugs were also studied. The age of disease onset and duration of the disease did not have a significant correlation with the rate of adherence among our patients. In contrast, Kyngas (2000) found that the duration of the disease is significantly related to patient adherence.
Maternal age was significantly higher in non-adherent patients compared to adherent ones (P < 0.05). Family size was also found to play a significant negative role on patient adherence to their medications (P < 0.05). Patients with positive family history of epilepsy were more non-adherent than patients with negative family history (61.5% versus 38.5%).
Complex treatment is believed to threaten patient’s adherence (Shams and Barakat, 2010). This study revealed that patients receiving monotherapy are significantly more adherent than patient treated with polypharmacy (P < 0.05). This is in agreement with the findings of another researcher who found that the medication adherence was affected by the complexity of the treatment at which taking many pills at different times can lead patients to miss doses (Johnbull et al. (2011)). On the other hand, Sweileh et al. (2011) stated that there was no significant difference in the rate of adherence between patients on monotherapy and those on polypharmacy. The latter study was carried out at Al-Makhfya Governmental Outpatient Center in Nablus, Palestine and all ages are included in the research study but not only for adolescent which may explain the result differences.
The frequency of seizure did not differ significantly between men and women (Table 1), but differed significantly between adherent and non-adherent groups (P < 0.0001). There was an increase in the frequency of seizure among non-adherent patients and this may be attributed to their incompliance with the prescribed medications (Table 2). In addition, patients living in family with stable marriage were significantly more adherent (P < 0.05) than patients with broken family such as divorce, widow or separated parents and consequently are living with only one parent.
The parent, other family member and friend support is considered as the corner stone to the medication adherence among adolescents with epilepsy (Chigier, 1992; Desai et al., 1998). Parental strategies found to be effective include planning of self-care with adolescents, giving regular positive feedback, and providing other rewards to promote medication adherence. Family members and friends are important people to whom adolescents want to talk (Hauser et al., 1993; Woodgate, 1998). Our results showed that patients having good family support were significantly more adherent (P < 0.01) compared to patients complaining of lack of family support (Table 2). The same conclusion was reached by Kyngas (2000) who stated that family support explained the good compliance in his studied group of patients.
A healthy relationship is based on patients’ trust in prescribers and empathy from the physicians, pharmacists and nurses. Studies have found that adherence to medication is good when healthcare providers are emotionally supportive, giving reassurance or respect, and treating patients as an equal partner (Chigier, 1992; Hauser et al., 1993; Kyngas, 2000; Lawson et al., 2005). These studies stress the importance of enabling patients to see their doctors and clinical pharmacists regularly and to talk about epilepsy and explaining how to live with it. Our results showed that patients with satisfactory regular medical relationship, supervision and support were significantly more adherent to their medication than patients feeling not enough support from their health providers (P < 0.05). Liu et al. (2013) also found that the cause of non-adherence in 9.5% of his patients’ group was bad patient–prescriber relationship.
The reasons for non-adherence were found to include discomfort resulting from treatment, expense of treatment, decisions based on personal judgments about the effectiveness of the proposed treatment, maladaptive coping styles (e.g., denial of illness), or mental disorders (Blackwell, 2000; Kyngas, 2000). Forgetfulness was the most common cause of non-adherence among this group of patients followed by inability to obtain medication and then fear from side effects of drugs (drowsiness, gastro-intestinal upset) or negative attitude toward medication as explained in Fig. 1.These results are supported by the findings in other research studies. For example, Liu et al. (2013), found that the primary reason for non-adherence was forgetfulness in 69.6% of his studied group of patients while Johnbull et al. (2011) observed that the cause of non-adherence was forgetfulness in more than 40% of his cases. In addition, Paschal et al. (2014) concluded that “forgetfulness” was the primary reason for non-adherence in his studied group of patients.
The role possibly played by the stigma of epilepsy in patients’ adherence to medication is unclear (Buck et al., 1999; Austin et al., 2004). Dell defined stigma as a distinctive feature in an individual and the devaluation society places on that difference. Stigma felt by people with epilepsy was more profound in some countries than in others. Stigmatization is most effective if the stigmatized person holds the same belief as the society, as it often occurs in people with epilepsy (Dell, 2014). In adolescents with epilepsy, stigma is a complex concept to investigate because it involves personal attitudes and beliefs, elements of secrecy and disclosure management, and influences from the social environment (DiIorio et al., 2003). It was obvious from our results that patients felt to be stigmatized were significantly more non-adherent as compared to patients with a strong sense of normality (P-value < 0.05). Buck et al. (1997), DiIorio et al. (2003) and Johnbull et al. (2011) found also that participants reporting higher levels of perceived stigma also reported lower levels of adherence.
Patients’ beliefs about their illness and the effectiveness of medication are predictive of their adherence and the control of seizure (Buck et al., 1997; Miner et al., 2013). Good motivation with a positive attitude toward disease and treatment, no fear of complications and no fear of seizures explain good adherence (Kyngas, 2000). The effect of patient beliefs on medication adherence was studied in this current research study. It was evident that there was an increase in adherence with stronger beliefs in necessity of treatment and with low concern beliefs as shown in Table 3. Patients with positive necessity–concerns differential scores were more adherent compared to patients with negative scores. In addition, patients who believed that antiepileptic drugs benefit more than harm were significantly more adherent (Tables 3 and 4). This is congruent with the results obtained by Jones et al. (2006) who found that epileptic patients, who had a greater belief in the need for medication, were significantly more adherent than those with uncontrolled epilepsy.
5. Conclusion
Our study population of patients with epilepsy has demonstrated that adherence to medications has to be improved. Living with epilepsy is challenging because of its complex bio-psychosocial characteristics. Diagnosis of epilepsy and pharmacological treatment is not enough for epilepsy management. Assessment of medication adherence among epileptic patients should be a routine part of the management process. Healthcare providers have to find out all factors connected to each adolescent’s adherence and try to modify them in an individualized form and not as a package suitable for all. Parental strategies found to be effective include planning of self-care with adolescents, giving regular positive feedback, and providing other rewards to promote medication adherence.
Adolescents need frequent support, encouragement, and positive feedback. Supportive care is much more important than controlling relationships between adolescents, their parents, and health care staff to improve their adherence to medications. In addition, healthcare providers should continuously evaluate the role of the family and patient feeling and beliefs. Further studies are needed to evaluate the effect of medication adherence and psychological care on seizure control.
Conflict of interest
We declare that there is no conflict of interest on this research study. The research study did not receive funds or support from any source.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams S., Pill R., Jones A. Medication, chronic illness and identity: the perspective of people with asthma. Soc. Sci. Med. 1997;45:189–201. doi: 10.1016/s0277-9536(96)00333-4. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya A.A. Drug compliance of children and adolescents with epilepsy. Seizure. 2005;14:393–395. doi: 10.1016/j.seizure.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Asawavichienjinda T., Sitthi-Amorn C., Tanyanont W. Compliance with treatment of adult epileptics in a rural district of Thailand. J. Med. Assoc. Thai. 2003;86:46–51. [PubMed] [Google Scholar]
- Austin J.K., MacLeod J., Dunn D.W., Shen J., Perkins S.M. Measuring stigma in children with epilepsy and their parents: instrument development and testing. Epilepsy Behav. 2004;5:472–482. doi: 10.1016/j.yebeh.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Banerjee P.N., Filippi D., Allen-Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. 2009;85(1):31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A.T., Jallon P., Preux P.M. The epidemiology of seizure disorders in infancy and childhood: definitions and classifications. Handb. Clin. Neurol. 2013;3:391–398. doi: 10.1016/B978-0-444-52891-9.00043-9. [DOI] [PubMed] [Google Scholar]
- Blackwell B. Lippincott Williams & Wilkins; Philadelphia: 2000. Treatment Compliance. Textbook of Psychiatry. pp. 1893–1898. [Google Scholar]
- Buck D., Jacoby A., Baker G.A., Chadwick D.W. Factors influencing compliance with antiepileptic drug regimes. Seizure. 1997;6:87–93. doi: 10.1016/s1059-1311(97)80060-x. [DOI] [PubMed] [Google Scholar]
- Buck D., Jacoby A., Baker G.A., Ley H., Steen N. Cross-cultural differences in health-related quality of life of people with epilepsy: findings from a European study. Qual. Life Res. 1999;8:675–685. doi: 10.1023/a:1008916326411. [DOI] [PubMed] [Google Scholar]
- Chigier E. Compliance in adolescents with epilepsy or diabetes. J. Adolesc. Health. 1992;13:375–379. doi: 10.1016/1054-139x(92)90032-7. [DOI] [PubMed] [Google Scholar]
- de Boer H.M., Mula M., Sander J.W. The global burden and stigma of epilepsy. Epilepsy Behav. 2008;12(4):540–546. doi: 10.1016/j.yebeh.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Dell J.L. Oxford University Press; New York: 2014. Stigma and response. Social dimensions of epilepsy. pp. 185–210. [Google Scholar]
- Desai P., Padma M.V., Jain S., Maheshwari M.C. Knowledge, attitudes and practice of epilepsy: experience at a comprehensive rural health services project. Seizure. 1998;7:133–138. [PubMed] [Google Scholar]
- Devinsky O. Patients with refractory seizures. N. Engl. J. Med. 1999;340:1565–1570. doi: 10.1056/NEJM199905203402008. [DOI] [PubMed] [Google Scholar]
- DiIorio C., Osborne S.P., Letz R., Henry T., Schomer D.L., Yeager K. The association of stigma with self-management and perceptions of health care among adults with epilepsy. Epilepsy Behav. 2003;4:259–267. doi: 10.1016/s1525-5050(03)00103-3. [DOI] [PubMed] [Google Scholar]
- England M.J., Liverman C.T., Schultz A.M., Strawbridge L.M. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 2012;25:266–276. doi: 10.1016/j.yebeh.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Caceres M., Soto-Santillana M. Non-compliance with pharmacological treatment in patients with epilepsy. Rev. Neurol. 2006;42:647–654. [PubMed] [Google Scholar]
- Gaitatzis A., Trimble M.R., Sander J.W. The psychiatric comorbidity of epilepsy. Acta Neurol. Scand. 2004;110:207–220. doi: 10.1111/j.1600-0404.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- Girerd X., Hanon O., Anagnostopoulos K., Ciupek C., Mourad J.J., Consoli S. Assessment of antihypertensive compliance using a self-administered questionnaire: development and use in a hypertension clinic. Presse Medicale. 2001;30:1044–1048. [PubMed] [Google Scholar]
- Gomes M.M., Filho H.S., Noe R.A. Anti-epileptic drug intake adherence. The value of the blood drug level measurement and the clinical approach. Arq Neuro-Psiquiatr. 1998;56:708–713. doi: 10.1590/s0004-282x1998000500002. [DOI] [PubMed] [Google Scholar]
- Hauser S.T., DiPlacido J., Jacobson A.M., Willett J., Cole C. Family coping with an adolescent’s chronic illness: an approach and three studies. J. Adolesc. 1993;16:305–329. doi: 10.1006/jado.1993.1027. [DOI] [PubMed] [Google Scholar]
- Horne R., Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999;47:555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Horne R., Weinman J., Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health. 1999;14:1–24. [Google Scholar]
- Hughes D., Manns B. Patient compliance with drug therapy for diabetic nephropathy. CMAJ. 2000;162:1553–1554. [PMC free article] [PubMed] [Google Scholar]
- Johnbull O., Farounb B., Adeleye A., Uche A. Evaluation of factors influencing medication adherence in patients with epilepsy in rural communities of Kaduna State, Nigeria. Neurosc. Med. 2011;2(4):299–305. [Google Scholar]
- Jones R.M., Butler J.A., Thomas V.A., Peveler R.C., Prevett M. Adherence to treatment in patients with epilepsy: associations with seizure control and illness beliefs. Seizure. 2006;15:504–508. doi: 10.1016/j.seizure.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kaiboriboon K., Bakaki P.M., Lhatoo S.D., Koroukian S. Incidence and prevalence of treated epilepsy among poor health and low-income Americans. Neurology. 2013;80(21):1942–1949. doi: 10.1212/WNL.0b013e318293e1b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyngas H. Compliance with health regimens of adolescents with epilepsy. Seizure. 2000;9:598–604. doi: 10.1053/seiz.2000.0470. [DOI] [PubMed] [Google Scholar]
- Kyngas H. Predictors of good compliance in adolescents with epilepsy. Seizure. 2001;10:549–553. doi: 10.1053/seiz.2001.0557. [DOI] [PubMed] [Google Scholar]
- Lawson V.L., Lyne P.A., Harvey J.N. Understanding why people with type 1 diabetes do not attend for specialist advice. A qualitative analysis of the views of people with insulin-dependent diabetes who do not attend diabetes clinic. J. Health Psychol. 2005;10:409–423. doi: 10.1177/1359105305051426. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Z., Ding H., Yang X. Adherence to treatment and influencing factors in a sample of Chinese epilepsy patients. Epileptic Disord. 2013;15:289–294. doi: 10.1684/epd.2013.0588. [DOI] [PubMed] [Google Scholar]
- Michaud P.A., Frappier J.Y., Pless I.B. Compliance in adolescents with chronic disease. Arch. Fr. Pediatr. 1991;48:329–336. [PubMed] [Google Scholar]
- Miner P.J., Alexander J., Ewing H., Gerace L. Caregivers’ beliefs associated with medication adherence among children and adolescents with epilepsy. J. Neurosci. Nurs. 2013;45(4):211–218. doi: 10.1097/JNN.0b013e3182986127. [DOI] [PubMed] [Google Scholar]
- Morisky D.E., Green L.W., Levine D.M. Concurrent and predictive validity of self-reported measure of medication adherence. Med. Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Paschal A.M., Rush S.E., Sadler T. Factors associated with medication adherence in patients with epilepsy and recommendations for improvement. Epilepsy Behav. 2014;31:346–350. doi: 10.1016/j.yebeh.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Ray B.K., Bhattacharya S., Kundu T.N., Saha S.P., Das S.K. Epidemiology of epilepsy-Indian perspective. J. Indian Med. Assoc. 2002;100(5):322–326. [PubMed] [Google Scholar]
- Shams M.E., Barakat E.A. Measuring the rate of therapeutic adherence among outpatients with T2DM in Egypt. Saudi Pharm. J. 2010;18(4):225–232. doi: 10.1016/j.jsps.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweileh W.M., Ihbesheh M.S., Jarar I.S., Taha A.S., Sawalha A.F., Zyoud S.H., Jamous R.M., Morisky D.E. Self-reported medication adherence and treatment satisfaction in patients with epilepsy. Epilepsy Behav. 2011;21:301–305. doi: 10.1016/j.yebeh.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Woodgate R.L. Adolescents’ perspectives of chronic illness: “it’s hard”. J. Pediatr. Nurs. 1998;13:210–223. doi: 10.1016/S0882-5963(98)80048-1. [DOI] [PubMed] [Google Scholar]



