Abstract
Aim: Attempts have been made to use CTC values for interpretation of treatment response and to guide change of chemotherapy by using a static cut-off of 5 CTC to stratify patients in favourable or unfavourable responders. We propose a new approach to interpret treatment effect using significant changes in CTC values (SCV-limits1) as grouping parameter for responders and non-responders to chemotherapy among metastatic breast cancer (mBC) patients. Method: CTC were analysed using the CellSearch System in blood from 47 mBC patients before the start of new chemotherapy and before the third cycle of therapy. The new and old approach to interpret changes in CTC values were compared in relation to progression free survival (PFS). Results: The new approach using significant CTC change (P = .032) and the old approach using static cut-off (P > .001) correlated significantly with PFS using a cohort of 47 patients. Conclusion: We propose a new approach to interpret significant changes between baseline and follow-up CTC values as a tool for assessing treatment effect in mBC. Our approach stratified patients in new risk groups that were stratified significantly with respect to PFS. More patients are needed to balance the size of the risk groups for better comparison to the existing approach based on a 5 CTC cut-off.
Introduction
CTC in peripheral blood have repeatedly been shown to have prognostic value in breast, colorectal, prostate, urothelial, and gastric cancer [1], [2], [3], [4], [5], [6]. The expectations are high when it comes to the use of CTC as a readily accessible liquid biopsy for monitoring treatment effect of therapy administration in the clinic [7], [8], [9], [10]. It is desirable to detect an early effect of the treatment so the patient can avoid the loss of valuable time in effective treatment and severe side effects from an inefficient treatment. Changes in CTC levels above and below cut-off values have been compared to clinical imaging of progressive disease [11]. The treatment effect using CTC is most often monitored as a baseline value compared to a follow-up value after a given number of weeks or cycles of therapy [6], [10], [12], [13], [14].
The long awaited randomized, prospective phase III clinical trial, SWOG S0500, had the primary objective to determine whether mBC patients with CTC ≥ 5/7.5 ml after the first follow-up could benefit from changing to an alternative chemotherapy regime rather than wait for clinical evidence of progressive disease before changing to an alternative chemotherapy. Preliminary results showed that the cut-off of 5 CTC could not guide clinicians in changing chemotherapy based on the hypothesis that a baseline value ≥ 5 CTC/7.5 ml blood and a follow-up value ≥ 5 CTC indicate a lack of treatment response [15], [16]. The cut-off value of 5 CTC was stated as prognostic for overall survival (OS) and progression free survival (PFS) by Cristofanilli et al. in 2004 using the FDA approved CellSearch System for CTC detection [1], [13]. Detecting these extremely rare CTC among billions of blood cells is technically challenging. The uncertainty of the CTC analysis value is accordingly high but even though the issues of CTC rarity have been addressed [5], [17], [18] the analytical uncertainty of the CTC result has not been taken into account when evaluating a difference between a patient baseline CTC value and follow-up CTC value after administration of therapy [15]. Given a baseline sample containing 5 CTC in 7.5 ml blood, a follow-up value after chemotherapy of 4 CTC could be accepted as a response to the treatment in the SWOG S0500 trial. This difference of one CTC between baseline and follow-up CTC values is hampered by the extreme rarity of the CTC in the blood and the associated lack of precision in the technology used to detect the CTC [5], [17], [18].
By taking the uncertainty of the CTC measurement into account, it is feasible to set up decision limits for a significant change between baseline and follow-up values of CTC. We suggest that such decision limits could give the clinicians a more accurate measure of treatment efficacy and thus spare the patients unnecessary toxicity and loss of time in ineffective treatment. The hypothesis addressed here was that a response to chemotherapy can be evaluated as a significant change between the number of CTC in the baseline and the follow-up sample. The primary aim of this study was to evaluate an alternative approach for clinicians to interpret chemotherapy treatment response based on a significant change in a baseline and a follow-up CTC value. The significant change was assessed by taking the significant change value (SCV) limits of the CellSearch System measurement into account [19].
Materials and Methods
This study was approved by The National Committee on Health Research Ethics in Denmark, registered as S20100102. After oral and printed information, all patients gave written consent for enrolment in the study at the Department of Oncology, Lillebaelt Hospital, Vejle, Denmark.
Patients
47 women entered the study cohort consecutively from November 2012 to December 2013 and in accordance with the inclusion criteria: Evaluable metastatic breast cancer, WHO performance status ≤ 2 and no concomitant inflammatory bowel disease. Patients were starting up in 1st–6th line of chemotherapy with or without HER2 targeted trastuzumab, bevacizumab or lapatinib therapy. Clinical evaluations were conducted in a standard manner and were blinded for CTC data. TNM score, histology, human epidermal growth factor receptor 2 (HER2), oestrogen receptor (ER) and progesterone receptor (PgR) status were evaluated on the primary disease. ER, PgR were considered positive by immunohistochemistry (IHC) when > 1% of tumour cells were stained positive. HER2 were evaluated using the Danish Breast Cancer Cooperative Group (DBCG) criteria (IHC = 0, 1, 2 or 3; if IHC = 2 FISH should be > 2.0 to confirm positive HER2 status). Progression free Survival (PFS) was measured from the time of the first baseline sample drawn until the time of the first confirmed suspicion of disease progression or death. Progression was evaluated according to the RECIST criteria version 1.1 such as alanine aminotransferase (ALAT) increase, computer tomography, magnetic resonance, and ultrasound scan evaluations of the tumour [20]. OS was measured from the time of the first baseline blood drawn to the time of death by all causes. Progression and survival data were gathered on 02-02-2014. The demographic data of the cohort are summarized in Table 1.
Table 1.
Patient Demographics
| Demographic Data | Patients |
||
|---|---|---|---|
| No. | % | ||
| Age | Mean (SD) [Range] |
59.8 (13.2) [30–81 y] |
|
| Metastatic site | Liver | 10 | 21 |
| Lung | 2 | 4 | |
| Bone | 4 | 9 | |
| Subcutaneous | 1 | 2 | |
| Lymph | 2 | 4 | |
| Two sites | 22 | 47 | |
| Multiple sites | 6 | 13 | |
| Performance status WHO baseline | 0 | 21 | 45 |
| 1 | 16 | 34 | |
| 2 | 4 | 9 | |
| Unknown | 6 | 13 | |
| Survival | Alive | 35 | 74 |
| Mors | 12 | 26 | |
| Progression | Progression | 26 | 55 |
| Censored | 21 | 45 | |
| Line of therapy | 1 | 21 | 45 |
| 2 | 12 | 26 | |
| 3 | 6 | 13 | |
| 4 + 5 + 6 | 8 | 17 | |
| Type of recidive therapy | Endocrine + Trastuzumab | 1 | 2 |
| Chemo | 20 | 43 | |
| Chemo + Endocrine | 15 | 32 | |
| Chemo + Trastuzumab | 7 | 15 | |
| Chemo + Endo + Trastu | 4 | 9 | |
| ER status (10) | ER + | 32 | 68 |
| ER- | 15 | 32 | |
| PgR status (10) | PgR + | 18 | 38 |
| PgR − | 21 | 45 | |
| Unknown | 8 | 17 | |
| HER2 status (10) | HER2 + | 11 | 23 |
| HER2- | 27 | 57 | |
| Unknown | 9 | 19 | |
| Histology (10) | Ductal invasive | 46 | 92 |
| Lobular invasive | 2 | 4 | |
| Others | 2 | 4 | |
| TNM score (10) | T1 | 8 | 17 |
| T2 | 18 | 38 | |
| T3 | 5 | 11 | |
| T4 | 9 | 19 | |
| Tx | 6 | 13 | |
| Unknown | 1 | 2 | |
| N0 | 10 | 21 | |
| N1 | 21 | 45 | |
| N2 | 7 | 15 | |
| N3 | 5 | 11 | |
| Nx | 1 | 2 | |
| Unknown | 3 | 6 | |
| M0 (at 10 diagnosis) | 33 | 70 | |
| M1 | 10 | 21 | |
| Mx | 2 | 4 | |
| Unknown | 2 | 4 | |
Pt: patients. 10: primary tumour; HER2: human epidermal growth factor receptor 2; ER: estrogen receptor; PgR: progesterone receptor; Chemo: chemotherapy.
Study design
CTC were evaluated in replicate blood samples collected in 9 ml CellSave tubes at baseline (C1, Table 2) before the start of the first cycle of a new line of chemotherapy and again in replicate blood samples before the 3 cycles of chemotherapy (C3, Table 2). The mean baseline to follow-up time was 6.3 weeks ranging [5.6-8.9].
Table 2.
Study Design
| Sample Cluster | Sample Tube | Analysis | Patient ID |
Total # Samples | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | ||||
| C1 | CellSave 1a | CTC CellSearch | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 47 |
| CellSave 1b | CTC CellSearch | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 47 | |
| C3 | CellSave 2a | CTC CellSearch | X | X | X | X | X | X | X | X | X | C | X | D | X | X | X | X | X | D | X | X | X | X | C | X | X | C | X | X | X | X | X | X | X | X | X | D | X | X | X | X | C | X | X | C | X | X | X | 39 |
| CellSave 2b | CTC CellSearch | X | X | X | X | X | X | X | X | X | C | X | D | X | X | X | X | X | D | X | X | X | X | C | X | X | C | X | X | X | X | X | X | X | X | X | D | X | X | X | X | C | X | X | C | X | X | X | 39 | |
D: Died before follow-up; C: changed treatment before follow-up; treatment effect experiment, CellSearch analysis: X. C1 = baseline and C3 = Follow-up: blood draw from same vein puncture before start of 1.-6.th line of therapy.
Evaluation
Treatment effect was evaluated according to the existing approach based on a cut-off of 5 CTC/7.5ml blood and the new approach based on significant change value (SCV) limits.
In the existing 5 CTC cut-off approach presented by Cristofanilli and Hayes et al. [12], [13] a change from unfavourable CTC ≥ 5 at baseline to favourable CTC < 5 at follow-up should improve survival and was expected to function as a marker for treatment response. The following grouping criteria were employed [12]: Group 1: Baseline C1 < 5 CTC/7.5 ml and follow-up C3 < 5 CTC/7.5 ml; Gr.2: C1 ≥ 5/C3 < 5; Gr.3: C1 < 5/C3 ≥ 5; Gr.4: C1 ≥ 5/C3 ≥ 5. Onwards this approach for evaluating treatment effect is referred to as “Gr1234” (Figure 3).
Figure 3.
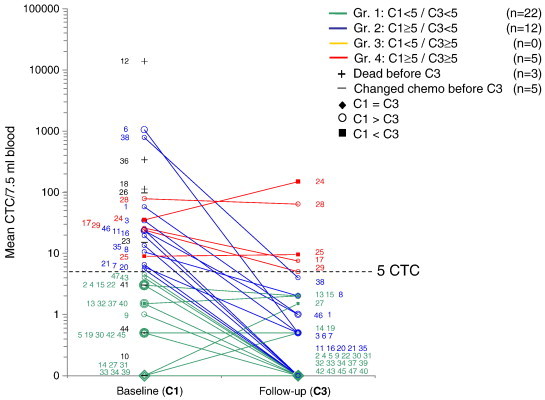
Logarithmic plot of replicate CTC mean values at baseline (C1) for n = 47 patients and at follow-up (C3) for n = 39 patients. Each line represents CTC development for a patient (ID in small numbers) and was grouped in accordance with the shown Gr1234 grouping approach.
The proposed alternative approach for evaluation of treatment effect of chemotherapy will be referred to as “GrABC”. The GrABC approach was based on a significant change between C1 baseline CTC value and the follow-up CTC value C3. The significant change was assessed using a clear and readable difference plot with significant change value (SCV) limits of the CellSearch System measurements plotted (Figure 1). The SCV-limits (also known as reference change values) in the difference plot (Equation 4) were based on the variation coefficients CV%(dd) of CTC measurements on replicate 7.5 ml blood samples collected from the same vein puncture of the individual patients at baseline [19]:
| (1) |
| (2) |
| (3) |
Figure 1.
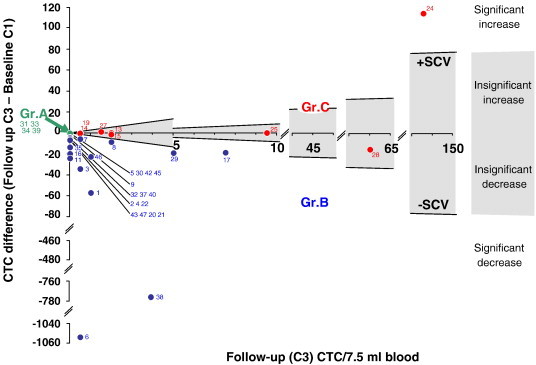
Difference plot for follow-up CTC as a function of follow-up (C3) minus baseline values (C1). Group A: (overlapping zero values shown stacked in the plot) Patients with mean baseline CTC(C1) = 0 + insignificant change; Gr.B: CTC(C1) > 0 + significant decrease; Gr.C: CTC(C3) > 0 + insignificant change or a significant increase. The grey area between the Significant Change Values (SCV) limits is where an insignificant change between C1 and C3 could be caused by analytical method variation instead of a change caused by treatment effect or disease progression. Patient ID is given as small pitch numbers.
Z = 1.96 for a 5% two-tailed gaussian distribution μ(dd) is the mean of the replicate CTC measurement of the individual patient’s baseline and σ(dd) was the standard deviation. SCV were calculated for the baseline values of CTC in the intervals [> 0–4], n = 18; [5–20], n = 8; [20–13.803], n = 14. Seven patients had mean baseline values equal to zero and were consequently not evaluable for CV%. The limit values were estimated as values in CTC units for follow-up value X
| (4) |
The SCV-limits in the difference plot determine if a difference between a CTC baseline and follow-up value is a significant increase, a significant decrease or an insignificant change. Based on these three categories, groups A, B and C can be established corresponding to groups 1, 2, 3 and 4 defined by the 5 CTC cut-off: Group A: Baseline CTC(C1) = 0 + insignificant change; Gr.B: Baseline CTC(C1) > 0 + significant decrease; Gr.C: CTC(C3) > 0 + insignificant change or significant increase (Figure 1).
CTC analyses
CellSearch CTC analyses on n = 47 patients at both C1 and C3 were done on replicate 7.5 ml whole blood samples collected from the same vein puncture in 9 ml CellSave tubes (Janssen) and analyzed within a mean of 43 hours, range [3–100 h]. Due to technical issues, three replicate samples were scanned after up to 100 hours instead of the recommended 96 hours. The replicate samples were reported as mean of the two samples in the unit CTC/7.5 ml blood except when using the individual replicate sampling to calculate CV%(dd) for SCV-limits (Equation 3).
The CTC analyses were carried out on the CellSearch System (formerly owned by Veridex, now Janssen). Assumed CTC were quantified using the FDA approved CellSearch Epithelial Cell Kit, ref 7900000, Janssen based on immunomagnetic enrichment of cells of ephithelial origin and positive for the epithelial cell adhesion molecule (EpCAM). Positive identification of suspected CTC was done by staining with phycoerythrin (PE) conjugated antibodies specific for cytokeratins 8, 18, and 19 and an associated DAPI stained nuclei. Residual leucocytes with positive DAPI were negatively discriminated by anti-CD45 antibodies conjugated with allophycocyanin (APC). All CTC evaluations after the Veridex criteria were performed by a single certified operator to avoid inter analyst variation [17].
Statistics
The main objective was to assess the ability of Gr1234 and GrABC to predict an effect of chemotherapy based on baseline and follow-up values of CTC in 7.5 ml of blood. Gr1234 used the cut-off of 5 CTC/7.5 ml as grouping parameter for treatment response and GrABC used SCV-limits to identify a significant change as grouping parameter. Kaplan-Meier PFS and OS curves for the groups were compared using log-rank test. All statistics were done using STATA11, StataCorp.
Results
The 47 patients enrolled had a mean age of 59.5 with a range of 30 to 81 years. A total of 12 deaths (26%) and 29 patients with disease progression (62%) were registered. Of the 29 patients with disease progression, 6 changed therapy or died before follow-up leaving n = 23 with disease progression evaluable for treatment effect. At baseline mean values of CTC replicate samples showed n = 34 had CTC ≥ 1/7.5 ml (72%) and n = 22 had CTC ≥ 5 (47%).
Prognostic value of 5 CTC cut-off at baseline
Stratifying the patients’ CTC values at baseline according to the classic prognostic cut-off of 5 CTC for worsened PFS and OS [1] showed no significant difference in OS (P = .975) for patients with CTC above and below 5 (Figure 2A). Similarly for PFS no difference (P = .887) between patients with CTC values above and below cut-off was found as illustrated by a median PFS(CTC < 5) = 183 days and median PFS(CTC ≥ 5) = 171 days (Figure 2B).
Figure 2.
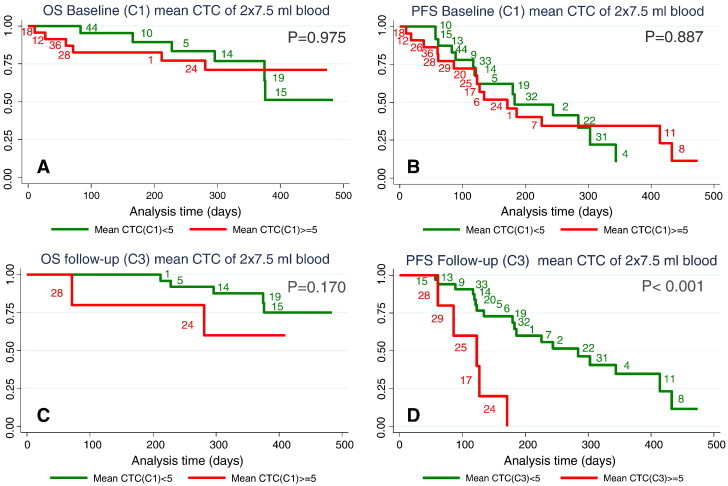
Kaplan-Meier plots for overall survival and progression free survival at baseline (A and B) and at follow-up (C and D). Red curves represent patients with mean CTC values of two 7.5 ml blood samples with CTC ≥ 5 and green curves represent patients with CTC < 5.
Prognostic value of 5 CTC cut-off at follow-up
Stratifying the patients’ CTC values at follow-up according to the classic prognostic cut-off of 5 CTC still resulted in non-significant difference for OS (Figure 2C, P = .170). In contrast there was a significant difference in PFS (P < .001) between patients with CTC values above and below the 5 CTC/7.5 ml cut-off (Figure 2D) after the administration of two cycles of chemotherapy.
Treatment effect
Median CTC values at baseline were 4 CTC/7.5 ml blood with 95% confidence interval [1.5-12.7], mean = 353 CTC/7.5 ml blood and range [0–13.803] (Figure 3). After two cycles of chemotherapy the median CTC value fell to 0 with 95%CI [0–0.5], mean = 6.4 CTC/7.5 ml and range [0–149] (Figure 3).
Two approaches for evaluation of the effect of chemotherapy were evaluated in this study. The Gr1234 approach used the cut-off of 5 CTC/7.5 ml as grouping parameter for treatment response. The new approach GrABC used SCV-limits to identify a significant change as grouping parameter by taking the analytical variation of the CellSearch method into account when assessing a difference between baseline and follow-up. For the present patient cohort, the GrABC grouping parameter was illustrated in the difference plot Figure 1. The classic Gr1234 grouping parameters [12], [13] are illustrated in Figure 3.
Figure 3 showed that Gr1234 had n = 22 patients (47%) in the low risk group 1 with CTC < 5 at C1 and CTC < 5 at C3. Group 2 had n = 12 (25%) at medium risk with CTC ≥ 5 at C1 and drop to CTC < 5 at C3. The high risk group 4 with CTC ≥ 5 at both C1 and C3 includes n = 5 (11%) of the patients. No patients fell into group 3 defined by low baseline CTC(C1) ≤ 5 and increased CTC(C3) ≥ 5 at follow-up and n = 8 (17%) patients died or changed treatment before follow-up.
The GrABC approach generated groups from the difference plot Figure 1. Accordingly the expected low risk group A counts n = 4 patients (9%) with CTC = 0 at C1 and no change at C3. Group B encompasses n = 27 (57%) at expected medium risk with CTC(C1) > 0 and a significant decrease in the CTC value at C3 compared to the CTC(C1) value. Group C had n = 8 (17%) patients with an expected high risk due to a value of CTC(C3) > 0 and no significant change or a significant increase in CTC value from C1 to C3 samples (Figure 1).
Treatment effect with respect to PFS
The Gr1234 groups and the GrABC groups were plotted in PFS Kaplan-Meyer plots (Figure 4). N = 39 patients had both baseline and follow-up CTC values for evaluation and n = 23 patients experienced disease progressions before the end of the study. OS Kaplan-Meyer is not shown as only n = 8 of 12 registered deaths had follow-up values. When stratified among 3 or 4 risk groups, 8 patients could not provide valid data.
Figure 4.
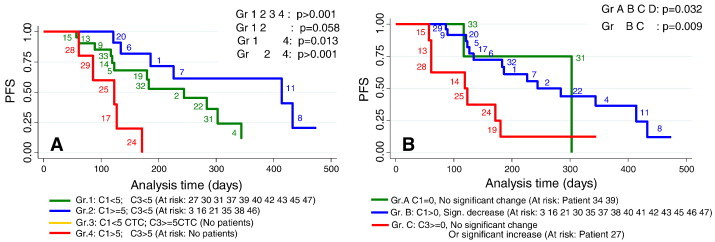
N = 23 patients with progressive disease who had both C1 and C3 values at the end of the study. Progression free survival curves for (A) risk group 1, 2, 3 and 4 assessed by the Gr1234 approach using 5 CTC cut-off as a grouping parameter for treatment effect; (B) risk groups A, B and C assessed by the GrABC approach using the significant change values limits as grouping parameter for treatment effect. The approaches are elaborated in the method section. Small pitch figures indicate patient ID number.
Risk groups 1, 2 and 4 generated by the Gr1234 approach had a significantly different (P > .001) progression free survival (Figure 4A) using the 5 CTC cut-off as a grouping parameter for treatment effect. It was of note that the expected low risk group 1 had shorter median PFS (244 days) than the expected medium risk group 2 (414 days). The low risk group 1 generated by Gr1234 (green curve Figure 4A) was identical to the low risk group 1 (green curve Figure 2B) except for patients 10 and 44 who changed therapy before follow-up. Also the high risk group for Gr1234 approach (Figure 4A, red curve) and the high risk group for CTC(C3) ≥ 5 at follow-up (Figure 2D, red curve) were identical. It follows that group 2 in Figure 4A generated by the Gr1234 approach was extracted from the high risk group CTC(C1) ≥ 5 (Figure 2B, red curve) by identifying patients 20, 6, 1, 7 11 and 8 who were responding to therapy and dropped at follow-up to CTC(C3) < 5 after two cycles of chemotherapy.
The GrABC approach generated three risk groups A, B and C also with a significant separation (P = .032) of the groups using significant change values limits as grouping parameter for treatment effect according to PFS (Figure 4B). There was an unequal distribution of patients in groups A, B, and C which affects the level of significance.
Higher risk groups by GrABC
Among the n = 23 patients with disease progression, the GrABC approach upgraded 10 patients to a higher risk group and downgraded the risk groups of 2 patients compared to the grouping obtained with the current Gr1234 approach. The two approaches agreed on the risk groups for 11 patients. The different grouping of the patients by the Gr1234 and GrABC approaches are visualised in Figure 4A versus B.
For the patients with disease progression, 8 patients were placed in the high risk group C by the GrABC approach compared to 5 patients placed in the high risk group 4 by the Gr1234 approach. GrABC agrees on three of these high risk patients (24, 25 and 28), but downgrades the risk group to medium risk B for two patients 17 and 29. In contrast four patients [13], [14], [15], [19] were upgraded to the high risk group C (Figure 4B) from the Gr1234 approach low risk group 1 (Figure 4A).
Likewise the GrABC approach upgraded 6 patients to the medium risk group B (Figure 4B) from the Gr1234 approach low risk group 1 (Figure 4A). Only 2 patients were left in the low risk group A. compared to Gr1234 that places 12 of the progressed patients in the low risk group 1. Figure 4A shows that low risk group 1 unexpectedly has a shorter median PFS (244 days) compared to the medium risk group 2 (414 days). The GrABC approach achieves a median PFS of 303 days for the low risk group A and 284 days for the medium risk group B by upgrading the risk group for all patients in the low risk group 1 (Figure 4A), except patients 31 and 33, to the medium risk group B (Figure 4B).
Discussion
In this study we proposed a new GrABC approach using the SCV-limits as grouping parameter for treatment effect. The main objective to compare GrABC with the existing approach Gr1234 and evaluate their grouping ability in terms of PFS both showed significant correlations. For GrABC P = .032 and for Gr1234 P > .001. Statistically stronger P values by the Gr1234 approach could be attributed to the even distribution of patients in the three groups compared to the uneven distribution of patients in the three groups by the GrABC approach (Figure 4A and B). Both Gr1234 and GrABC had the ability to group the patients in expected low risk groups (Groups A/1), expected responders to chemotherapy (Groups B/2) and expected non responders (Groups C, 3 and 4).
GrABC upgraded risk for CTC(C1) < 5 and CTC(C3) < 5
Using SCV-limits the GrABC approach had the ability to utilize the low values below 5 CTC. Patients 13, 14, 15 and 19 had low CTC values < 5 at all times making them a low risk group 1 in the Gr1234 approach (Figure 3). But the GrABC approach classified these patients as medium risk group 3 (or C) due to an insignificant change of the low baseline CTC value (Figure 1). This increased risk group by the GrABC approach was in accordance with the relatively short time to progression observed for patients 13, 14, 15 and 19 and death for patients 14, 15, 19 (Figures 2 and 4).
GrABC downgraded risk for CTC(C3) ≥ 5
Both approaches categorize the numeric CTC values into low risk groups, expected responders to chemotherapy and expected non responders. By loss of quantitative CTC values, valuable prognostic information is lost as reported by several authors who showed a non linear increase in risk of both progression and death with increasing number of CTC [14], [21], [22].This poses a potential problem for the GrABC approach since patients that progressed like patients 17 and 29 experienced a significant decrease from baseline to follow-up and ended up in the medium risk group B, but still had a follow-up CTC ≥ 5. By the Gr1234 approach patients 17 and 29 were categorized in the high risk group 4 (Table 3). According to GrABC and the hypothesis the CTC in patients 17 and 29 were responding to the chemotherapy even though their follow-up CTC(C3) ≥ 5 was associated with poor prognosis. Patients 17 and 29 had progressed but were still alive, and the hypothesis was supported by patient 38 who had also experienced a significant decrease from baseline to a follow-up CTC(C3) = 4 and was alive at the end of the study period without progression. More data are required to verify the hypothesis.
SCV intervals
The intervals [0–4], [5–20], [20–∞] used for construction of the SCV-limits in the difference plot (Figure 1) were adopted from Botteri et al. who showed increase in risk of both progression and death with increasing numbers of CTC [22]. These intervals were implemented for the SCV-limits to limit the impact of the biological variation on CTC in the mBC patient cohort. The biological variation between subjects ranged between 0–13.808 CTC/7.5 ml in the present study. Ranges from 0–23.618 CTC/7.5 ml have been reported [5], [12]. A range of this magnitude would introduce a total variation (Equation 2) so wide that SCV-limits (Equation 4) would be useless to detect small differences between baseline and follow-up. Also the analytical variation differed when counting replicate samples containing 5 CTC/7.5 ml or 50 CTC/7,5 ml blood [5]. A tendency towards high CTC values for the mastitis BC subtype was observed. Furthermore the different levels of CTC can be associated with different BC subtype [23]. Therefore it seemed eligible to construct SCV-limits in intervals due to both analytical and clinical arguments.
Total CTC in 15 ml blood samples
The study could have been based on a total of 15 ml blood samples due to the replicate samples for assessing analytical variation of the CellSearch System. Increased blood volume could have increased the sensitivity of the analysis according to Coumans et al. [21]. Unexpectedly the use of total CTC in 15 ml blood gave a statistically reduced separation (P = 0.025, plot not shown) of groups 1, 2 and 4 similar to Figure 4A for the Gr1234 approach. The use of total CTC in 15 ml did not change the distribution of patients in the groups for the GrABC approach, since it is based on differences (Figure 1). The mean values of the 2×7.5 ml were therefore chosen and it also facilitates comparison with other studies.
Prognostic value of CTC ≥ 5 at baseline with respect to OS and PFS
In a large study, Pierga et al. found that 65% had CTC ≥ 1 and 44% had CTC ≥ 5/7.5 ml in mBC patients measured with the CellSearch System [6]. Our rates of 72% and 47% are in accordance with these findings, given that Pierga et al. only enrolled patients in 1st line of therapy. The prognostic value of CTC ≥ 5 CTC/7.5 ml at baseline has repeatedly been confirmed [1], [6], [14], [24]. This study could not validate the prognostic value of CTC(C1) ≥ 5 for OS or PFS. This could be due to a lack of power linked to progression data from only n = 23, shorter median follow-up time or, as indicated by the crossing of the Kaplan-Meier curves (Figure 2B), an interaction possibly due to a patient cohort in worse condition than the other study cohorts.
The difference in the patients’ condition is illustrated in Figure 4A where the green risk group 1 had unexpectedly longer median PFS compared to the blue medium risk group 2. All six patients in group 2 were in 1st line treatment which could lessen their risk of progression compared to group 1 where only 5 of 13 (38%) patients were in 1st line therapy while 62% were in an advanced line of therapy. More patients with different lines of therapy in the cohort could balance this issue.
Prognostic value of CTC ≥ 5 at follow-up with respect to OS and PFS
At follow-up a significant difference in PFS (P < .001) between the groups above and below 5 CTC/7.5 ml was shown (Figure 2D) in concordance with Cristofanilli et al. [13]. This was expected as the two cycles of chemotherapy have split the red high risk PFS curve at baseline with CTC ≥ 5 (Figure 2B) into the expected medium risk group 2 responders with CTC(C3) < 5 (blue curve, Figure 4A) and expected high risk group 4 non-responders with CTC(C3) ≥ 5) (red curve, Figure 4A) by means of the Gr1234 approach. The Figure 4A low risk group 1 (green) and medium risk group 2 (blue) of the Gr1234 approach were again merged into the green PFS curve in Figure 2D representing low risk CTC(C3) < 5 leaving the red high risk PFS curves of Figures 2D and 4A identical. Given that the red curve (high risk group CTC ≥ 5) in Figure 2B was comprised of risk groups 2 and 4 (Figure 4A), the curves in Figure 2B would possibly be more separated if group 2 had the expected shorter median PFS than group 1 (Figure 4A).
At follow-up the OS for patients above and below 5 CTC cut-off had only two patients in the high risk group with CTC ≥ 5 (Figure 2C) and is not statistically valid.
The challenge of the 5 CTC cut-off
Coumans et al. made a large retrospective study on interpreting changes in circulating tumour cell count on a cohort of cancer patients all with baseline ≥ 5 CTC/7.5 ml [21]. They challenged the static cut-off of 5 CTC by using the Poisson distributed nature of CTC in blood samples to set up probability percentage based criteria for a CTC reduction. Unfortunately they sat the limit at 99.9% due to statistical reason and found this approach less adequate compared to the static cut-off of 5 CTC. Test values illustrated that the 99.9% probability approach is incomparable to the SCV-limits used in the present study (data not shown). An advantage of the GrABC approach is that the Poisson distributed nature of CTC is part of the analytical variation of the true replicate samples from the same vein puncture.
Our proposed GrABC approach gives the clinician well defined SCV-limits to identify changes in repeated measurements. SCV-limits are already used for clinical purposes in algorithms for management of anticoagulant treatment operating in a narrow therapeutic interval [19]. The SCV-limits presented here hold true for a broad metastatic setting of BC patients with mixed lines of treatment and a single operator for CTC evaluation using a CellSearch System with similar analytical variation. Applying the SCV-limits retrospectively on a larger patient cohort could provide solid data to determine the value of the GrABC approach for simple and more factual interpretation of changes in CTC for treatment effect.
Conclusion
We propose a new approach to evaluate treatment effect using SCV-limits to interpret significant changes in mBC patients’ baseline and follow-up CTC values. The SCV guided approach for grouping patients in favourable or unfavourable risk groups correlated with PFS (P = .032). The existing approach based on a 5 CTC cut-off for grouping high and low risk patients correlated with PFS (P > .001), but inverted the expected PFS time for the low and medium risk groups in this cohort. More patients are needed to balance the size of the groups for better comparison and hence to demonstrate the possible benefit of using SCV-limits to interpret significant changes in CTC as a predictive factor for treatment effect in mBC.
Funding
The authors wish to thank “Region Syddanmarks forskningspulje” and “FornyelsesFonden” for economic support.
Disclosure
All authors declare no conflicts of interest.
Acknowledgment
The authors would like to thank Camilla Davidsen and Sara Egsgaard, Department of Clinical Biochemistry, for outstanding laboratory work; Trine Gregersen and Annette Rehmeier and the physicians at the Department of Oncology for providing immaculate patient enrolment, samples and data.
Footnotes
Significant change value (SCV) limits signify a significant difference between two serial measurements when taking the variability of the measuring method into account.
References
- 1.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L.W. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S.J., Punt C.J., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 3.de Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 4.Naoe M., Ogawa Y., Morita J., Omori K., Takeshita K., Shichijyo T., Okumura T., Igarashi A., Yanaihara A., Iwamoto S. Detection of circulating urothelial cancer cells in the blood using the Cell Search System. Cancer. 2007;109:1439–1445. doi: 10.1002/cncr.22543. [DOI] [PubMed] [Google Scholar]
- 5.Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G., Uhr J.W., Terstappen L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 6.Pierga J.Y., Hajage D., Bachelot T., Delaloge S., Brain E., Campone M., Dieras V., Rolland E., Mignot L., Mathiot C. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2012;23:618–624. doi: 10.1093/annonc/mdr263. [DOI] [PubMed] [Google Scholar]
- 7.Bidard F.C., Mathiot C., Degeorges A., Etienne-Grimaldi M.C., Delva R., Pivot X., Veyret C., Bergougnoux L., de C.P., Milano G. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol. 2010;21:1765–1771. doi: 10.1093/annonc/mdq052. [DOI] [PubMed] [Google Scholar]
- 8.Liu M.C., Shields P.G., Warren R.D., Cohen P., Wilkinson M., Ottaviano Y.L., Rao S.B., Eng-Wong J., Seillier-Moiseiwitsch F., Noone A.M. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27:5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danila D.C., Pantel K., Fleisher M., Scher H.I. Circulating tumors cells as biomarkers: progress toward biomarker qualification. Cancer J. 2011;17:438–450. doi: 10.1097/PPO.0b013e31823e69ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidard F.C., Fehm T., Ignatiadis M., Smerage J.B., ix-Panabieres C., Janni W., Messina C., Paoletti C., Muller V., Hayes D.F. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32:179–188. doi: 10.1007/s10555-012-9398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd G.T., Cristofanilli M., Ellis M.J., Stopeck A., Borden E., Miller M.C., Matera J., Repollet M., Doyle G.V., Terstappen L.W. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 12.Hayes D.F., Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Miller M.C., Matera J., Allard W.J., Doyle G.V., Terstappen L.W. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M., Hayes D.F., Budd G.T., Ellis M.J., Stopeck A., Reuben J.M., Doyle G.V., Matera J., Allard W.J., Miller M.C. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura S., Yagata H., Ohno S., Yamaguchi H., Iwata H., Tsunoda N., Ito Y., Tokudome N., Toi M., Kuroi K. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer. 2010;17:199–204. doi: 10.1007/s12282-009-0139-3. [DOI] [PubMed] [Google Scholar]
- 15.Smerage J. SABCS Press Release; 2014. Changing chemotherapy Not beneficial for metastatic breast cancer patients with elevated circulating tumor cells. [Google Scholar]
- 16.Southwest Oncology Group S0500 treatment decision making based on blood levels of tumor cells in women with metastatic breast cancer receiving chemotherapy. 2014. http://clinicaltrials.gov/show/NCT00382018
- 17.Tibbe A.G., Miller M.C., Terstappen L.W. Statistical considerations for enumeration of circulating tumor cells. Cytometry A. 2007;71:154–162. doi: 10.1002/cyto.a.20369. [DOI] [PubMed] [Google Scholar]
- 18.Allan A.L., Keeney M. Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J Oncol. 2010;2010:1–10. doi: 10.1155/2010/426218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassen J.F., Kjeldsen J., Antonsen S., Hyltoft P.P., Brandslund I. Interpretation of serial measurements of international normalized ratio for prothrombin times in monitoring oral anticoagulant. Clin Chem. 1995;41:1171–1176. [PubMed] [Google Scholar]
- 20.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Coumans F.A., Ligthart S.T., Terstappen L.W. Interpretation of changes in circulating tumor cell counts. Transl Oncol. 2012;5:486–491. doi: 10.1593/tlo.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botteri E., Sandri M.T., Bagnardi V., Munzone E., Zorzino L., Rotmensz N., Casadio C., Cassatella M.C., Esposito A., Curigliano G. Modeling the relationship between circulating tumour cells number and prognosis of metastatic breast cancer. Breast Cancer Res Treat. 2010;122:211–217. doi: 10.1007/s10549-009-0668-7. [DOI] [PubMed] [Google Scholar]
- 23.Punnoose E.A., Atwal S.K., Spoerke J.M., Savage H., Pandita A., Yeh R.F., Pirzkall A., Fine B.M., Amler L.C., Chen D.S. Molecular biomarker analyses using circulating tumor cells. PLoS O. 2010;5:1–12. doi: 10.1371/journal.pone.0012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bidard F.C., Hajage D., Bachelot T., Delaloge S., Brain E., Campone M., Cottu P., Beuzeboc P., Rolland E., Mathiot C. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 2012;14:1–10. doi: 10.1186/bcr3114. [DOI] [PMC free article] [PubMed] [Google Scholar]


