Abstract
Mantle cell lymphoma (MCL) is a hematological malignancy with unfavorable prognosis. Novel therapeutic approaches for treating the disease are aimed at the mechanisms regulating growth signals, cellular proliferation, and survival pathways of the malignant clones. Bortezomib (Brt), a proteasome inhibitor with pleiotropic activities was shown to be active in MCL and is currently implemented in therapeutic combinations for this disease. Telomerase activity is essential for survival of malignant cells and as such is considered a valid therapeutic target. This study evaluated the effects of bortezomib on telomerase activity and its regulation in MCL cells in vitro and ex vivo.
Our study shows that bortezomib exerts a cytotoxic effect in a dose dependent manner in two MCL cell lines, with differential sensitivity. While the IC50 for HBL-2 cells ranged between 2.5 ng/ml to 1.5 ng/ml during 24-72 h respectively, the IC50 for the NCEB cells was twice. Bortezomib differentially inhibited telomerase activity (TA): in HBL-2 cells there was a decline of 20%-55% during 24-72 h respectively. However in NCEB cells the decline was much smaller, and did not exceed 25%. Inhibition of telomerase activity is shown to be operated by two separate mechanisms: reduction of the hTERT mRNA expression (controlled by the binding of transcription factors) and reduction in phosphorylation of the catalytic subunit of hTERT by its kinases, AKT and PKCα.
A decrease in telomerase activity was demonstrated also in mononuclear cells, isolated from three MCL patients following incubation of the cells in the presence of bortezomib for 24-72 h. In one patient the decrease in TA ranged between 17%-37% respectively, in the second patient between 63%-76% and in the third patient between 70-100% for 24-72 h respectively.
The current study indicates that a combination of bortezomib and rapamycin, (an m-Tor pathway inhibitor used in MCL treatment) induced synergistic inhibition of telomerase activity. In HBL-2 cells, the combined treatment of bortezomib and rapamycin decreased TA by 80% compared to the expected value (40%) and for NCEB cells a similar trend was observed. In contrast, there was neither additive nor synergistic effect of this combination on cell proliferation.
In the light of the crucial role of telomerase in cancer cells, it was important to characterize the possible relations between telomerase and bortezomib and to distinguish the biochemical mechanisms of its regulation and its interactions with other signal transduction inhibitors such as rapamycin. The results of this work encourage the in vivo examination of the therapeutic potential of the combination of bortezomib and rapamycin in Mantle Cell Lymphoma patients.
Introduction
MCL is a subtype of B-cell lymphoma accounting for 5–10% of all non-Hodgkin’s lymphomas [12]. MCL patients have one of the worst prognoses among lymphomas with a median survival of approximately 3 years [12].
Recent studies have identified new drugs active in MCL, among them proteasome inhibitors and mammalian target of rapamycin (mTOR) inhibitors [13]. The proteasome inhibitor bortezomib (Brt) has been recently approved for treatment of this disease.
The ubiquitin-proteasome pathway plays a critical role in many cellular functions such as cell cycle control and modulation of the transcription factor NFκB [1]. Proteasome inhibitors lead to tumor growth arrest, induce cell death, and inhibit tumor metastasis and angiogenesis [6]. Although many mechanisms of Brt action on MCL cells are known, the fact that it is a proteasome inhibitor suggests that other cellular targets may be affected by its inhibition as well. This inhibition is achieved by the binding of Brt to the catalytic site of the 26S proteasome with high affinity and specificity.
The mTOR kinase, another key player in the pathogenesis of MCL, regulates mRNA translation which enhances the translation of cyclin-D1. The activity of mTOR can be inhibited by rapamycin analogs [13] and which are effective in MCL treatment.
The importance of telomerase in the biology and prognosis of many types of cancers including MCL is well established [17]. Telomerase is a unique reverse transcriptase expressed almost exclusively in > 90% of cancer cells. It compensates for telomeric loss in each DNA replication (Blackburn and Collins, 2010) thus conferring endless replicative potential to the cancer cell. Due to its essentiality and specificity to the malignant cell it may serve as a valid anticancer drug target and indeed active compounds that target telomerase are already in advanced phases of clinical trials (Shay and Wright, 2005). The importance of telomerase in MM has been demonstrated convincingly both in vitro and clinically. Telomerase activity has been found in MCL cells of 90% of the newly diagnosed and relapsed patients, while only in 13% of patients in remission [17]. It has also a prognostic value as elevated activity of the enzyme is correlated with poor prognosis [18]. These may be related to the fact that a recurrent breakpoint region in MCL involves the hTERT (human telomerase) locus on chromosome 5p [15]. Numerous cytotoxic drugs target telomerase (Mor-Tzuntz et al, 2010; Uziel et al, 2005; Dong et al, 2009). We also showed that bortezomib down regulates telomerase activity in myeloma cells and this inhibition may have clinical implications [16]. In the light of common signaling pathways connecting telomerase regulation and bortezomib mechanisms of action such as the NFκB axis we surmised that the drug may affect the activity of telomerase in MCL cells. In the current paper we present data regarding the effect of the drug on TA in MCL cells and analyze the regulatory pathways leading to this inhibitory effect. We also evaluated the possible synergistic effect of Brt and rapamycin on telomerase activity. Additionally, we assessed the effect of Brt on TA in other cancer and non-cancer cell lines. While most of the work was performed in vitro we were able to show also that the same mechanisms are relevant in vivo, by analyzing cells isolated from bone marrow aspirates of MCL patients before and after Brt treatment and ex vivo exposure to the drug. In addition, we found that telomerase response to bortezomib may be correlated with clinical response in patients with MCL.
Materials and methods
Cell culture
The experimental system was based on two MCL cell lines, Ewing sarcoma cell line and non malignant keratinocyte line. MCL cell lines HBL-2 and NCEB were obtained from the Germany Type Culture Collection and from Prof. Priel's laboratory at the Ben-Gurion University, Beer- Sheva, Israel. SK-N-MC cell line (Ewing sarcoma) was kindly provided by Dr. Gad Lavie (Sheba Medical Center, Ramat-Gan, Israel). Cells were cultured in RPMI-1640 with 10% FBS (HBL-2, SK-N-MC), or 20% (NCEB), containing 2 mM L-Glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. All ingredients were purchased from Biological Industries Beit Haemek, Israel. Keratinocytes (kindly donated by Dr. Amiram Ravid, FMRC, Israel) were cultured in MEM-EAGLE, supplemented with 10% fetal calf serum, containing 1% L-Glutamine, 20 μg/l D-calcium pantothenate, 100units/ml penicillin, and 100 μg/ml streptomycin. Cells from MCL patients (after obtaining informed consent) were isolated from blood samples by the Ficoll-Hypaque density gradient.
Cell viability and proliferation assays
Proliferation and viability were assessed by the WST-1 assay (Roche, Basel Switzerland) and by Trypan Blue exclusion. WST-1 measures the activity of mitochondrial dehydrogenases that cleave the WST-1 to a formazan dye and is proportional to cell number. This is followed by measuring the absorbance at 450 nm in a microplate reader. Cells were cultured in 96-well plates for 24, 48, and 72 h with or without Brt. After incubation, 10% of the WST-1 reagent was added and further incubated at 37 °C for 2 h [2]. The effect of rapamycin on cell proliferation was evaluated by the WST-1 method. Control cells were grown in the presence of DMSO (used as a solvent for rapamycin) at 0 - 500 nM. SK-N-MC and keratinocytes proliferation was determined by the sulforhodamine B (SRB) assay as previously described [19]. Basically, this assay measures the amounts of cellular proteins which correlate to the number of viable cells.
Telomerase activity assay
Telomerase activity was assessed by the TRAP assay (TRAPeze kit, Millipore) according to the manufacturer’s instructions in samples which were exposed to the IC50 dose of bortezomib. Cells were lysed with ice cold CHAPS lysis buffer, 50-100 ng of protein extracts were subjected to PCR in the presence of TS primer. The PCR products were separated on 12.5% PAGE and were stained with Nucleic Acid Gel Stain (Lonza, Basel Switzerland). Quantification was performed by the Quantity One software in the Versa-Doc device. TA was calculated according to the following formula: TPG = [(X − B)/C]:[(r − B)/Cr⁎100], where TPG is the total product generated, X signifies each sample signal, B is the gel background, C represents the 36 bp internal PCR control, r is the TSR8 quantification control.
Real-time PCR for hTERT expression
The expression of the hTERT gene was detected by Real Time PCR. Total RNA was extracted from cells using EZ-RNA Isolation Kit reagent (Biological Industries Beit Haemek, Israel). The extracted RNA was reverse transcribed according to the manufacturer's instructions of the High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, CA. USA). The real-time quantitative RT-PCR is based on the TaqMan methodology, ABI PRISM 7000 Sequence Detection System (Applied Biosystems, CA. USA). hTERT gene expression was calculated relatively to the expression of the control gene HPRT-1.
Chromatin Immunoprecipitation (ChIP) assay
The involvement of NFκB, c-Myc and SP1 in the inhibitory effect of bortezomib in MCL cells was assessed by the Chromatin Immunoprecipitation (ChIP) assay [5]. ChIP was performed with the EZ ChIP kit according to the manufacturer’s instructions (Upstate, Temecula, CA) and as previously described [16]. The level of the DNA binding of SP1, c-Myc and NFκB was evaluated relatively to the total DNA input of each sample that was not immunoprecipitated with any of the antibodies. The antibodies against SP1 and c-Myc were purchased from Millipore MA, USA. The antibodies against NFκB were purchased from Abcam MA, USA. The relevant primers with the following sequences are as follows:
For the region of c-Myc and SP-1 binding site:
Forward primer: AGTGGATTCGCGGGCACAGA;
Reverse primer: TTCCCACGTGCGCAGCAGGA
For the region of NFκB binding site:
Forward primer: GCCTCCTAGCTCTGCAGT
Reverse primer: ACCCGAGGACGCATTGCT
Due to the high GC content in the hTERT promoter region, PCR reactions included several additional temperature steps (in the polymerization stage) to ensure proper products formation. PCR conditions were as follows:
94°C – 3 min, followed by 36 cycles of: 94°C- 20 sec, 58°C- 30 sec, 72°C- 20 sec, 76°C- 20 sec, 80°C- 20 sec 84°C- 20 sec, and finally 72°C- 5 min. The products were separated on a 1% agarose gels and analyzed by the VersaDoc software on the Gel Doc documentation system (BioRad, Israel).
Immunoprecipitation and Western blot analysis
To determine the phosphorylated hTERT level, an immunoprecipitation assay was used. 500-1000 μg of protein was precipitated with 10 μg/mg anti-phosphoserine antibody (StressMarq Biosciences Inc.) or with 20 μg/mg anti-total hTERT antibody (Santa Cruz, CA, USA). The protein-antibody complexes were mixed with 20 μg of protein G agarose beads for 16 h agitation in 4°C. The immunoprecipitated lysates were washed with 1XCHAPS lysis buffer (obtained from the TRAPEZE kit) and boiled for 3 min. for standard Western blot analysis, as described below. The primary anti-hTERT antibody for the immunobloting (1:500-1:1000) was purchased from Epitomics, CA, USA.
The phosphorylation states of telomerase and its kinases, phospho-AKT (p-AKT) and phospho PKCα (p-PCKα), were evaluated by Western blotting. Cells were grown in absence of serum for 24 h, Brt and serum were added for 30 min and lysates were prepared by using the CHAPS lysis buffer (from the TRAPEZE kit) containing phosphatase and protease inhibitors. Identical protein amounts (100 μg) were separated by 10% SDS-PAGE, transferred to nitrocellulose membrane and then detected by the following antibodies: anti-pAKT Ser473/pPKCα Ser657 and anti-total AKT/PKCα. (anti-AKT: 1:1000, Cell signaling SC, USA; anti-PKCα: 1:500, Santa Cruz Biotechnology, CA, USA). Signals were visualized after exposing the membranes to 2nd fluorescent antibodies and quantified by the Odyssey analysis software. The protein phosphatase level of total PP2A in cellular lysates was determined after Brt treatment for 24 h by Western blot analysis. Antibodies against the three subunits of PP2A: a, b and c were used (Cell Signaling, Boston, MA, USA).
Statistical Analysis
A two-tailed One-sample Student t test with unequal variance and ANOVA one way were used to calculate the P values in SPSS for Windows version 11.5 software (SPSS, Inc., Chicago, IL). In all assays, P values < 0.05 and 0.001 were considered statistically significant and highly significant, respectively.
Results
The effect of Brt on proliferation of MCL cell lines
The proliferation of HBL-2 and NCEB cells was monitored after the administration of bortezomib for 24, 48 and 72 h. Bortezomib inhibited cell proliferation in a dose dependent manner in both cell lines (Figure 1 A, B). NCEB cells were less sensitive to inhibition with IC50 value of 5 ng/ml while the IC50 for HBL-2 cells was 2.5 ng/ml after 24 h. After exposure of 48 and 72 h the IC50 of HBL-2 decreased to 2 ng/ml and 1.5 ng/ml respectively. The IC50 of NCEB did not decrease over time and remained 5 ng/ml (Figure 1B).
Figure 1.
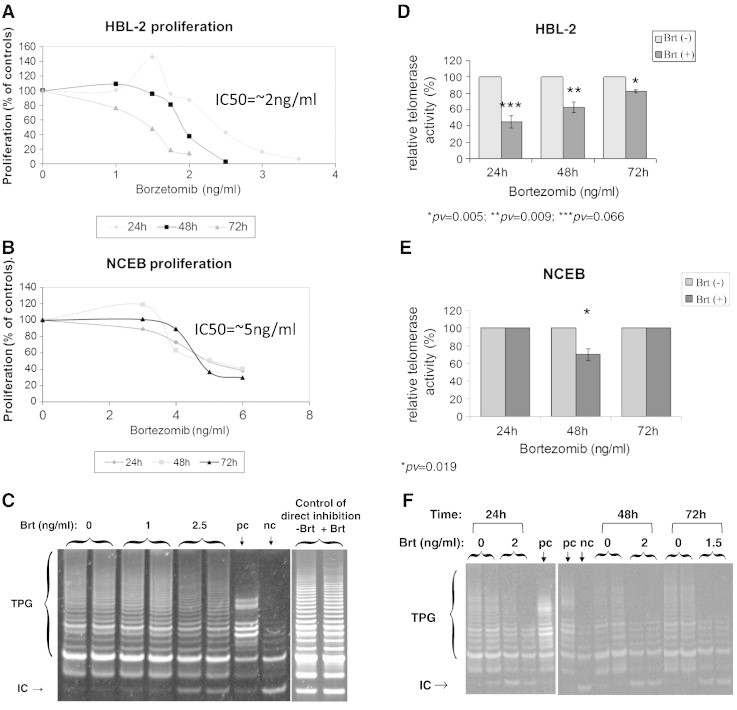
Proliferation and telomerase activity in response to Brt treatment. MCl cells were grown in the presence of a range of Brt dosages and their proliferation was assessed by the WST-1 assay. A. HBL-2 c; B. NCEB cells. Cells were exposed to the IC50 concentration of Brt and their telomerase activity was measured by the TRAP assay. C. A representative example of the TRAP assay; D. The activity of telomerase in HBL-2 cells; E. The activity of telomerase in NCEB cells. Mononuclear cells from bone marrow aspirates were subjected to Brt at 2 or 1.5ng/ml for 24-72h and telomerase activity was measured thereafter. F. Representative TRAP assay.
TA following treatment of MCL cell lines by Brt
MCL cells were exposed for 24 h, 48 h and 72 h to the respective IC50 concentrations of Brt. TA was assessed by the TRAP assay. Bortezomib decreased TA in HBL-2 cells by 55%, 40% and 20% at 24, 48 and 72 h, respectively (Figure 1D, E). In NCEB cells however, TA was not affected by bortezomib (Figure 1 F). In addition, mononuclear cells were isolated from three MCL patients, exposed ex vivo to 2 ng/ml of Brt for 24 h, 48 h and 1.5 ng/ml for 72 h (Figure 1G). The effect of bortezomib varied widely and ranged from 0% to more than 80% decrease in TA (Table 1). To examine whether the down regulation of TA was a nonspecific result of reduced proliferation the cell lysates of treated and non- treated cells were analyzed for DNA polymerization activity of DNA polymerase α (done in the laboratory of Dr. Mary Bakhanashvily, Sheba Medical Center, Israel). There was no decrease in DNA polymerization capacity of the enzyme indicating a specific effect of the drug on TA (not shown). To clarify whether telomerase inhibition was mediated by a direct interaction of the drug with the enzyme, relevant doses of Brt were added to cell lysates for 30 min prior to the TRAP assay. Brt had no effect on TA in these settings (Figure 1C).
Table 1.
Telomerase Activity in Mononuclear Cells Isolated from MCL Patients After Exposure to Brt
| 24h | 48h | 72h | |
|---|---|---|---|
| Patient 1 | 37 | 17 | 20 |
| Patient 2 | 76 | 67 | 63 |
| Patient 3 | 100 | 81 | 70 |
The effect of Brt on proliferation and TA of SK-N-MC and keratinocytes
SK-N-MC cells were used to test the effect of Brt on non-MCL cells. Cells were exposed to a wide range of Brt concentrations for 24 h and 48 h. Following 24 h treatment of the cells with Brt a decrease of about 45% was observed at a dose of 20 ng/ml. Higher doses did not changed this effect significantly (Figure 2A). Exposure of SK-N-MC cells to Brt for 48 h resulted in a dose dependent decrease in cell number. At 20 ng/ml all cells died (Figure 2B). The effect of Brt on keratinocytes, non-cancer human epithelial cells [14] was determined as well. Keratinocytes were also found to be sensitive to the cytotoxic effect of Brt for 48 h with an IC50 of 4-8 ng/ml for 24 and 48 h, respectively (Figure 2 C).
Figure 2.
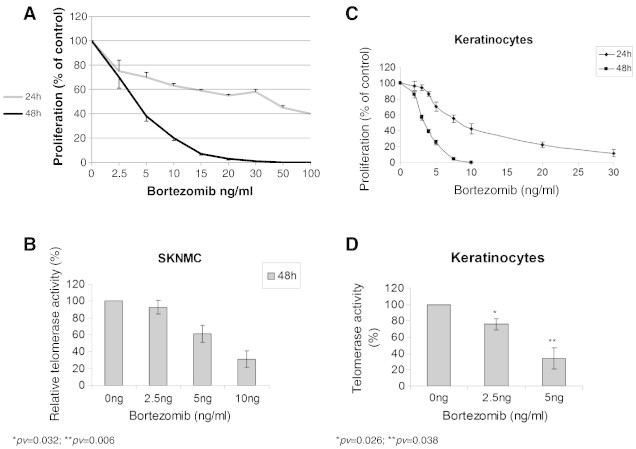
The activity of telomerase and cell proliferations in response to Brt treatments to non MCL cells. A. Proliferation of SK-N-MC cells. Cells were exposed to Brt in escalating concentrations and their proliferation was measure by the SRB assay. B. Telomerase activity of these cells after exposure of the IC50 dosage of Brt for 48h; C. Proliferation of keratinocytes. Cells were exposed to Brt in escalating concentrations and their proliferation was measure by the SRB assay. D. Telomerase activity of these cells after exposure of the IC50 dosage of Brt for 48h.
TA of SK-N-MC and keratinocytes following exposure to several concentrations of Brt for 48 h was determined. The activity of telomerase in SK-N-MC and in keratinocytes was reduced in a dose-dependent manner (Figure 2A, B).
The effect of Brt on telomerase regulation
The regulation of telomerase is exerted on both transcriptional and posttranslational levels.
Inhibition of hTERT expression by Brt
Cells were grown in the presence of Brt (2.5 ng/ml for HBL-2 and 5 ng/ml for NCEB, for 16 h and 24 h). Expression of hTERT was evaluated by real time PCR. Treatment with Brt reduced the hTERT mRNA levels in HBL-2 cells. However, the reduction in hTERT mRNA expression level of NCEB cells was observed only at 16 h and not at 24 h. The estimation of the hTERT gene expression was calculated relatively to the expression of the control housekeeping gene, HPRT-1. The decrease of hTERT mRNA in HBL-2 after exposure to bortezomib for 16 h and 24 h was 45% and 25%, respectively (Figure 3A). Decreases of 30% and 5% in hTERT mRNA was observed in NCEB cells after the administration of Brt for 16 h and 24 h, respectively (Figure 3B).
Figure 3.
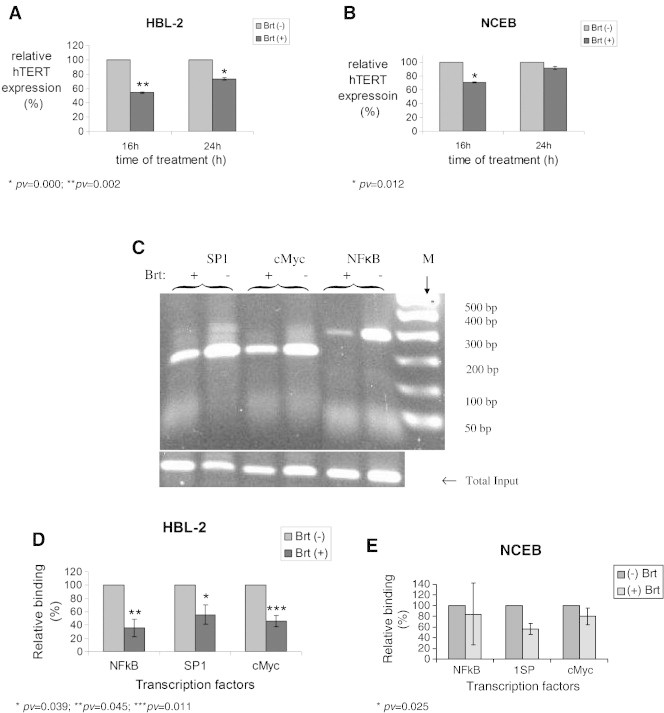
The transcriptional regulation of telomerase activity in response to Brt exposure. The expression of hTERT after Brt treatment. Cells were exposed to the IC50 of Brt for 16h or 24h and the expression of hTERT was measured in the cells. A. HBL-2 cells; B. NCEB cells. ChIP assay demonstrating the binding of SP1, C- Myc and NFkB to the hTERT promoter. C. An example of the ChIP assay, D. Chip assay using HBL-2 cells; E. ChIP assay using NCEB cells.
Transcription factors binding to the hTERT promoter
The involvement of NFkB, c-Myc and SP-1 in the transcriptional regulation of hTERT was assessed by the ChIP assay, which measures the level of the transcription factors binding to its promoter. The level of the DNA binding of SP1, c-Myc and NFkB was evaluated relatively to the total DNA input of each sample, which was not immunoprecipitated with any of the antibodies. Brt reduced the binding of all transcription factors to the promoter of hTERT in HBL-2 cells (Figure 3 C, D). In NCEB cells, only the binding of SP1 was decreased after bortezomib treatment (Figure 3E).
post-translational modification of telomerase by Brt
The degree of hTERT phosphorylation (which enables its activity) was determined by immunoprecipitation. 500-1000 μg of protein were precipitated with anti-phosphoserine antibody or with anti-total hTERT antibody. Brt caused ~ 60% and 40% reduction in the levels of the phosphorylation of hTERT protein in HBL-2 and NCEB, respectively (Figure 4A). Telomerase is phosphorylated by the two kinases: AKT and PKCα. The possible effects of Brt on the phosphorylated form of AKT and PKCα were monitored by Western blot analysis using specific p-AKT/p-PKCα and AKT/PKCα antibodies. Brt caused 20% and 35% reduction in the level of the phosphorylated form of AKT in HBL-2 and NCEB, respectively (Figure 4B). The phosphorylation levels of PKCα decreased approximately by 50% in both MCL cell lines (Figure 4C).
Figure 4.
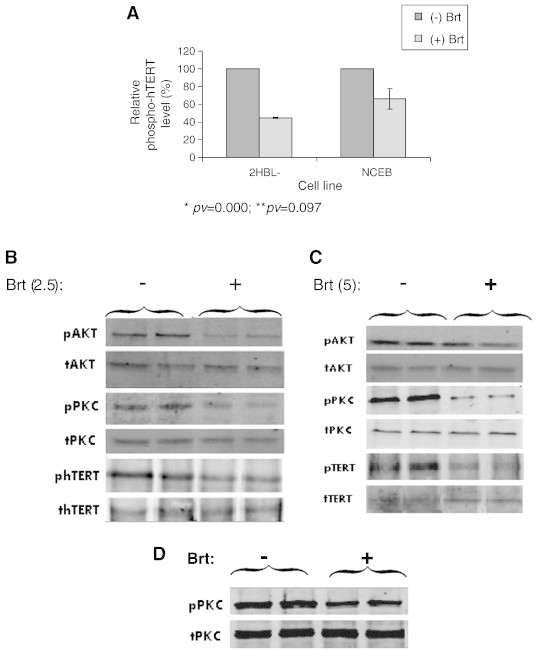
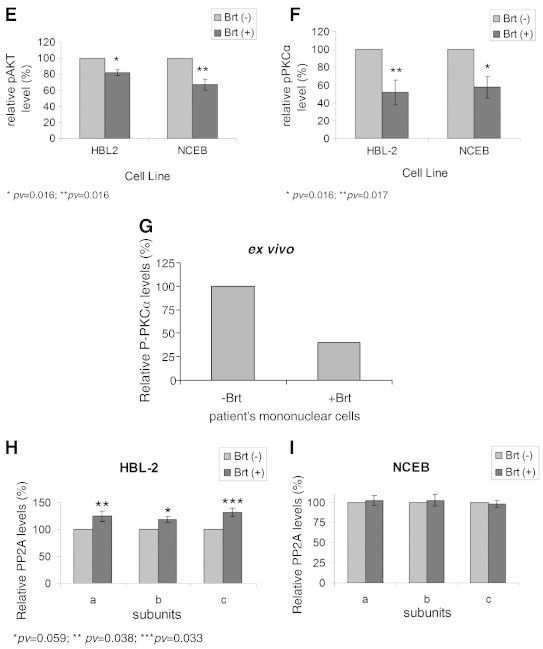
The post-translational regulation of telomerase activity in response to Brt exposure. Cells were exposed to the IC50 of Brt and the phosphorylation status of hTERT, PKCα and AKT were measured by IP and Western blot. A, Phospho-hTERT expression in MCL cells. B, C. D. Examples of Western blot results for HBL-2, NCEB and mononuclear cells isolated from a MCL patient respectively; E, F, G. Quantitation of the results (obtained after three repetitions and measurements of three MCL patients), respectively. H, I. The cellular levels of PP2A in HBL-2 and NCEB cells, respectively.
Mononuclear cells obtained from one patient were exposed to 2 ng/ml bortezomib ex vivo, and the phosphorylation level of PKCα was determined. Phospho-PKCα level decreased by 60% following Brt treatment compared to the untreated patient cells (Figure 4D). To understand whether this inhibition was due to a lower kinase activity or a higher phosphatase activity we followed the expression of PP2A in our system. Brt caused a slight increase in total protein phosphatase level of all PP2A subunits in HBL-2 cells and not in NCEB cells (Figure 4H, I).
The effect of rapamycin treatment on proliferation of MCL cell lines
The proliferation of HBL-2 and NCEB cells was evaluated after the exposure of the cells to rapamycin for 48 h. The cells were grown in the presence of the indicated rapamycin concentrations and cell proliferation was determined by the WST-1 assay. Rapamycin had a cytostatic effect on MCL cells. HBL-2 cells reached a maximal inhibitory effect of 40% at 30nM rapamycin after 48 h of exposure (Figure 5A). NCEB cells reached a plateau curve at 2nM of rapamycin (Figure 5A).
Figure 5.
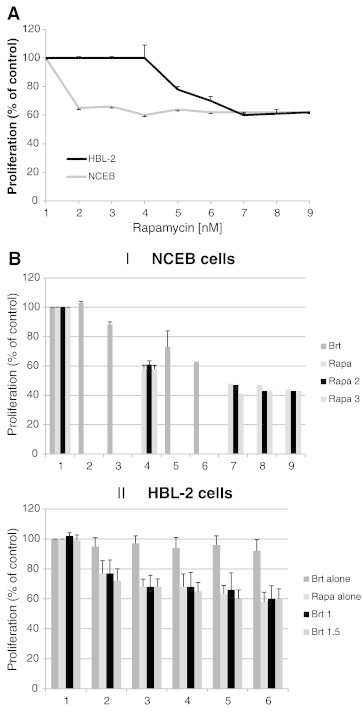
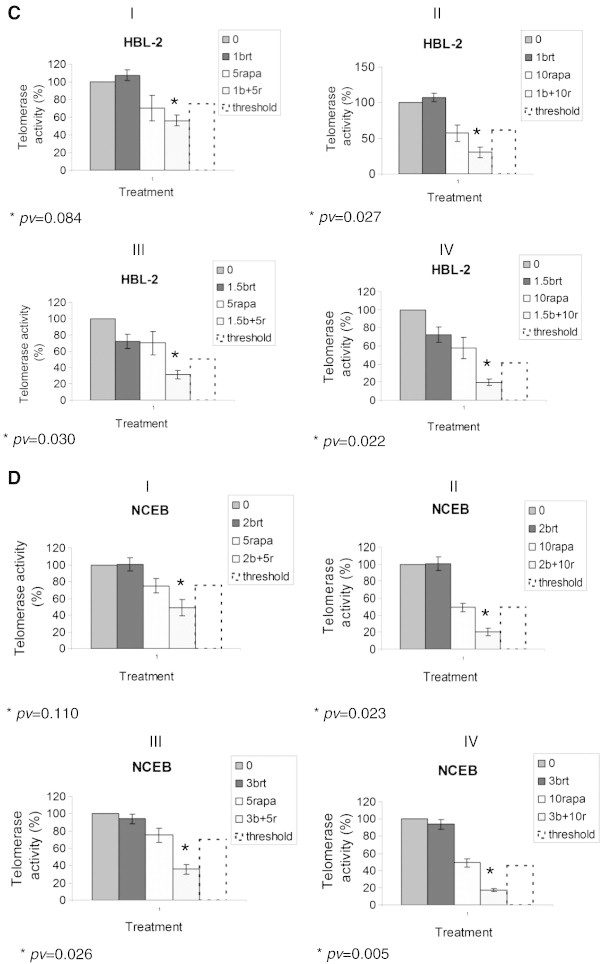
The effects of Rapamycin on the proliferation and the activity of telomerase in MCL cells. A. MCL cells were exposed to Rapamycin for 48 h and their proliferation in response to the drug was examined by the SRB assay (detailed in the materials and methods); B. The effect of the combined treatments of Bortezomib and Rapamycin on cell proliferation;. C, D. Telomerase activity in response to the combination of Brt and Rapamycin in HBL-2 and NCEB cells respectively. Drug concentrations are listed in the graph's legend.
The combined effect of bortezomib and rapamycin on cell proliferation and telomerase activity
MCL cell lines were exposed to several concentrations of rapamycin with or without Brt for 48 h. The proliferative effect of the combined treatments of Brt and rapamycin did not enhance the cells proliferation, as the extent of proliferation inhibition of the combined treatments was similar to that of each drug alone (Figure 5 B). However, when TA of cells treated both with Brt and rapamycin was compared to its activity in cells treated with each drug alone, the effect was totally different. Both HBL-2 and NCEB exhibited a synergistic effect on TA (Figure 5 C, D). The synergistic effect was calculated according to the following formula:
- S
Synergism;
- X(B)
% of TA after bortezomib exposure;
- X(R)
% of TA after rapamycin exposure;
- X(B+R)
TA after bortezomib and rapamycin.
For HBL-2 cells, the expected combined effect of 1 ng/ml Brt and 5 nM rapamycin should reduce TA by 20% but it actually reached 40% reduction. At 1 ng/ml Brt and 10 nM rapamycin, the expected combined effect should reduce TA by 40% but it actually reached 70% reduction. Likewise, at 1.5 ng/ml Brt and 5 nM rapamycin, the expected combined effect should reduce TA by 45% but it actually reached 70% reduction. At 1.5 ng/ml Brt and 10 nM rapamycin, the expected combined effect should reduce TA by 55% but it actually reached 80% reduction. Similarly, in NCEB cells the synergism was as follows: The expected reduction in TA when combining 2 ng/ml Brt with 5 nM rapamycin was 50% but it was 80% in fact. Combining 2 ng/ml Brt with 10 nM rapamycin resulted in 80% reduction in TA instead of 55%; Combining 3 ng/ml Brt with 5 nM rapamycin resulted in 65% reduction in TA instead of 25%; and 3 ng/ml Brt with 10 nM rapamycin resulted in 82% reduction in TA instead of 50%.
Discussion
Our results (summarized in Table 2A, Table 2B) demonstrate the ability of Brt to inhibit TA in MCL cells by decreasing the hTERT mRNA expression and the phosphorylation of the catalytic subunit hTERT by its kinases. HBL-2 and NCEB cells exhibited differential sensitivity to the anti proliferative effect induced by Brt. HBL-2 cells were found to exhibit a higher sensitivity to the drug than NCEB cells. In addition, SK-N-MC cells (Ewing Sarcoma) representing cancer cells, and keratinocytes (epithelial skin cells) also showed a high sensitivity to Brt. when The effect of the drug on SK-N-MC after 48 h of exposure was cytotoxic, while at 24 h it was probably cytostatic (Figure 4). The decrease in TA in these cells indicates that the effect of Brt is more general and not related only to MCL cells. The effect of Brt on TA differed between the two cell lines of MCL. Telomerase activity is highly variable among cancer cells and is specific for each one. The HBL-2 cells showed a substantial decrease in TA after 24 h of exposure to Brt. This decrease in TA persisted after 48 and 72 hours also. On the other hand, NCEB cells were more resistant to Brt and the decrease in TA occurred 48 h post exposure. It is inhibited wherever it is expressed, in all kinds of cells. The increase in TA during 24-72 h exposure of HBL-2 cells to the drug may stem from the fact that the drug was not stable in the growth media and degraded during cell exposure. Of note, since Brt could inhibit cellular proteasome activity much prior to that, even after 15 min, the changes are probably caused by that inhibition as expected.
Table 2A.
A Summary of the Changes Induced by Bortezomib in MCL Cells
| Cells | Effect | Change Extent |
|---|---|---|
| NCEB cells | proliferation | IC50 = 5-2ng/ml |
| HBL-2 cells | proliferation | IC50 = 2.5-1.5ng/ml |
| SK-N-MC cells | proliferation | IC50 = 20ng/ml |
| keratinocytes | proliferation | IC50 = 4-7.5ng/ml |
| NCEB cells | TA | No effect |
| HBL-2 cells | TA | ↓45-80% |
| mononuclear cells isolated from MCL patients | TA | ↓0-80% |
| SK-N-MC cells | TA | ↓20-70% |
| keratinocytes | TA | ↓25-70% |
| NCEB cells | hTERT expression | ↓30% and 5% |
| HBL-2 cells | hTERT expression | ↓45% and 25% |
| NCEB cells | SP-1 Transcription factor binding to the hTERT promoter | ↓40% |
| HBL-2 cells | SP-1 Transcription factor binding to the hTERT promoter | ↓50% |
| HBL-2 cells | NFκB Transcription factor binding to the hTERT promoter | ↓65% |
| HBL-2 cells | c-Myc Transcription factor binding to the hTERT promoter | ↓58% |
| NCEB cells | Phosphorylation of telomerase | ↓40% |
| HBL-2 cells | Phosphorylation of telomerase | ↓60% |
| NCEB cells | Phosphorylation of AKT | ↓20% |
| HBL-2 cells | Phosphorylation of AKT | ↓35% |
| NCEB cells | Phosphorylation of PKCα | ↓50% |
| HBL-2 cells | Phosphorylation of PKCα | ↓50% |
| mononuclear cells isolated from MCL patients | Phosphorylation of PKCα | ↓60% |
| NCEB cells | Expression of PP2A | No change |
| NCEB cells | Expression of PP2A | A slight increase |
Table 2B.
A summary of the changes induced by rapamycin in MCL cells
| Cells | Effect | Change extent |
|---|---|---|
| NCEB cells | proliferation | IC40 = 2nM |
| HBL-2 cells | proliferation | IC40 = 30nM |
| NCEB cells | proliferation | No synergistic or additive effect of Brt and rapamycin |
| HBL-2 cells | proliferation | No synergistic or additive effect of Brt and rapamycin |
| NCEB cells | TA | Synergistic effect of Brt and rapamycin expressed as↓40-80% |
| HBL-2 cells | TA | Synergistic effect of Brt and rapamycin expressed as ↓20-80% |
TA is regulated at multiple levels, including transcription, mRNA splicing, maturation and modifications of hTR and hTERT, transport and sub cellular localization of each component, assembly of the holoenzyme to an active ribonucleoprotein, accessibility and proper function on its telomeric substrates [4]. Previous studies showed that TERT promoter activity is usually regulated by a variety of transcription factors like: AP-1, NFκB, c-Myc, SP1 and the estrogen receptor [8], [11]. Our results show that Brt down-regulates TA by two separate mechanisms, operating at different time points. hTERT mRNA expression in HBL-2 was reduced by 40% following Brt treatment. This reduction was controlled by a decreased binding of transcription factors NFκB, c-Myc and SP1 to the hTERT promoter. In contrast, NCEB cells showed a slight decrease in hTERT expression, which was modulated by the transcription factors SP1 only. The observation that the transcription factors NFκB and c-Myc did not show decreased binding to hTERT prompter may identify them as mediators, among others, of the resistance of the NCEB cells to Brt, with respect to TA and the expression of its gene.
Post-translational changes of telomerase were evaluated by the determination of the phosphorylated hTERT and of its kinases levels, which phosphorylate telomerase to an active form. We show that Brt inhibits the phosphorylation of the hTERT protein. Western blot analysis revealed a slight decrease in phospho-AKT in the two MCL cell lines. Other studies have shown different effects of Brt on p-AKT, depending on the cell line. In the work of Kuen-Feng Chen et al., Brt down-regulated p-AKT in a dose- and time-dependent manner in all sensitive hepatocellular carcinoma cells, whereas no alterations of p-AKT were found in PLC5 cells. In contrast, the effect of Brt on p-PKCα was stronger and similar in these two cell lines. Accordingly, the reduction of p-hTERT may be mediated mainly by a decrease in the level of p-PKCα. Our data are consistent with other reports showing that AKT and PKCα directly control phosphorylation and activity of hTERT protein [3], [7]. Another telomerase regulator is PP2A, which deactivates telomerase by dephosphorylation (He [10]). The treatment by Brt caused a slight increase in total protein phosphatase level of all PP2A subunits in HBL-2 cells, whereas no alterations of PP2A were found in NCEB cells. Together, these results suggest that Brt reduces TA via transcriptional – reduction in hTERT mRNA level and post-translational modifications – a decrease in the phosphorylated form of the enzyme.
The ex vivo exposures of mononuclear cells isolated from three MCL patients to Bortezomib showed variable effects on TA activity. These differences may reflect the resistance or sensitivity of MCL patients to chemotherapy with Brt and may therefore be clinically relevant.
The m-TOR inhibitor rapamycin has been reported to be active against MCL cells [13]. In the work by C. Zhou et al., 2003 it was demonstrated that rapamycin potently reduces TA by decreasing the hTERT mRNA level in endometrial cancer cells. Previous studies showed that rapamycin synergies with Brt to enhance its cytotoxicity in MCL lines, in vitro [9]. In this work, we show for the first time that Brt and rapamycin synergistically reduce TA in MCL cell lines (Figure 5). This is important in light of the fact that TA in NCEB cells, which appeared to be relatively resistant to Brt was reduced by the drug combination. Although there was no anti proliferative advantage when the two drugs - Brt and rapamycin were used together, the fact the two drugs induced a synergistic decrease in TA activity may bear an anti- cancer advantage, at least for the long run (e.g. shortening of telomers) in MCL. However, the inspected sensitivity of the keratinocytes to Brt should be taken into consideration if and when the results of our study will be translated into clinical usage.
MCL is a Non-Hodgkin’s lymphoma characterized by an aggressive course, a poor prognosis and a low chemosensitivity to common drugs [12]. This work suggests that Brt may be a potentially useful component of targeted therapy against MCL and that loss of TA in vitro and ex vivo may be a good surrogate biomarker for predicting the anti-tumor activity of the sensitivity. In addition, evidence of synergistic influence of proteasome and m-TOR inhibitors on TA provides the framework for clinical studies in which the combination of both agents will be administered to patients with MCL.
In the light of the crucial role of telomerase in cancer cells, it was important to characterize the possible relation between TA and Brt and to distinguish the biochemical mechanism of its regulation. The phenotype of a given cell after exposure to the proteasome inhibitor results from the effects of many signal transduction processes that are either inhibited or catalyzed by that treatment. Therefore, we cannot exclude the possibility that additional routes may be involved in the regulation of telomerase inhibition and therefore other intra-cellular mechanism should be examined in these settings. We are also aware to the fact that some of the changes reported here are only moderate.
Another potentially significant point is the possibility of getting an anti-cancer synergistic effect in MCL cells from a drug combination such as Brt and GRN163L (Imetelstat), a very effective telomerase inhibitor which is clinically evaluated in numerous cancer types. Hopefully the results of the clinical studies will be translated to improved treatment for MCL patients.
References
- 1.Adams J, Palombella VJ, Elliott PJ. Proteasome inhibition: A new strategy in cancer treatment. Invest New Drugs. 2000;18:109–121. doi: 10.1023/a:1006321828515. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MV, Tan AS, McCoy KD, Wang R. The biochemical and cellular basis of Cell Proliferation assays that use tetrazolium salts. Biochemical. 1996;4:15–19. [Google Scholar]
- 3.Chang JT, Lu YC, Chen YJ, Tseng CP, Chen YL, Fang CW, Cheng AJ. 96th American Association of Cancer Research Meeting, Anaheim, CA, USA. 2005. hTERT phosphorylation by PKC is essential for telomerase holoprotein integrity and enzyme activity in head neck cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cong YS. Human Telomerase and Its Regulation. Microbiol Mol Biol Rev. 2002;3:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das PM, Ramachandran K, vanWert J, Singal R. Chromatin immunoprecipitation assay. Biotechniques. 2004;37:961–969. doi: 10.2144/04376RV01. [DOI] [PubMed] [Google Scholar]
- 6.Delic J, Masdehors P, Omura S, Cosset JM, Dumont J, Binet JL, Magdelénat H. The proteasome inhibitor lactacystin induces apoptosis and sensitizes chemo- and radioresistant human chronic lymphocytic leukemia lymphocytes to TNF-a-initiated apoptosis. Br J Cancer. 1998;77:1103–1107. doi: 10.1038/bjc.1998.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 8.Kyo S. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT) Nucleic Acids Res. 2000;28:669–677. doi: 10.1093/nar/28.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail LK, Timm M, Novak A, Stenson M, Ansell SM, Witzig TE. Rapamycin Enhances the Cytotoxicity of Bortezomib and Rituximab on Mantle Cell Lymphoma (MCL) Cell Lines. Blood. 2005;106:2411–2419. [Google Scholar]
- 10.Li H, Zhao LL, Funder JW, Liu JP. Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem. 1997;272:16729–16732. doi: 10.1074/jbc.272.27.16729. [DOI] [PubMed] [Google Scholar]
- 11.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Nathwani BN, Berger F, Piris MA, Isaacson PI, Harris NL. Mantle cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press; Lyon, France: 2001. pp. 168–170. (World Health Organization classification of tumours). [Google Scholar]
- 13.Witzig TE. Current Treatment Approaches for Mantle-Cell Lymphoma. J Clin Oncol. 2005;23(26):6409–6414. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 14.Yasumoto S, Kunimura C, Kikuchi K, Tahara H, Ohji H, Yamamoto H, Ide T, Utakoji T. Telomerase activity in normal human epithelial cells. Oncogene. 1996;13(2):433–439. [PubMed] [Google Scholar]
- 15.Schilling G, Penas EM, Janjetovic S, Oliveira-Ferrer L, Braig M, Behrmann P, Bokemeyer C, Dierlamm J. Molecular characterization of chromosomal band 5p15.33: a recurrent breakpoint region in mantle cell lymphoma involving the TERT-CLPTM1L locus. Leuk Res. 2013;37:280–286. doi: 10.1016/j.leukres.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Weiss C, Uziel O, Wolach O, Nordenberg J, Beery E, Bulvick S, Kanfer G, Cohen O, Ram R, Bakhanashvili M. Differential downregulation of telomerase activity by bortezomib in multiple myeloma cells-multiple regulatory pathways in vitro and ex vivo. Br J Cancer. 2012;107:1844–1852. doi: 10.1038/bjc.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trentin L, Ballon G, Ometto L, Perin A, Basso U, Chieco-Bianchi L, Semenzato G, De Rossi A. Telomerase activity in chronic lymphoproliferative disorders of B-cell lineage. Br J Haematol. 1999;106:662–668. doi: 10.1046/j.1365-2141.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiu KC, Fine M, Ikle D, Slovak ML, Arber DA. Telomerase activity and proliferation index in aggressive mature B-cell lymphoma: comparison to germinal center phenotypic markers. Hum Pathol. 2003;34:1259–1264. doi: 10.1016/j.humpath.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Nordenberg J, Perlmutter I, Lavie G, Beery E, Uziel O, Morgenstern C, Fenig E, Weizman A. Anti-proliferative activity of haloperidol in B16 mouse and human SK-MEL-28 melanoma cell lines. Int J Oncol. 2005;27:1097–1103. [PubMed] [Google Scholar]


