Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with characteristic neuropathological features that are accompanied by inflammatory processes and release of pro-inflammatory cytokines. There is evidence that microglial cells are a key mediator of damage in AD. The microglial compartment may arise to a greater part from activation and transmigration of circulating monocytes. The aim of the present pilot study was to explore, if different cell adhesion molecules (ICAM-1 and -3, PECAM-1, VCAM-1, P-, L- and E-selectins, E-cadherin), RAGE and CD14 are affected in monocytes of healthy subjects compared to patients suffering from AD or mild cognitive impairment (MCI). Monocytes were isolated by negative magnetic selection (MACS) from EDTA blood samples, and extracts were analyzed by Searchlight Multiplex ELISAs. When compared to healthy subjects, the ratio of monocytic ICAM-3/CD14 was significantly decreased in MCI and AD patients and the ratio of the monocytic P-selectin/CD14 was specifically decreased in AD patients. In conclusion, our data show that monocytic cell adhesion molecules are decreased in AD and MCI patients. Further larger longitudinal studies should then clarify whether any of these parameters may be useful as a diagnostic biomarker.
Keywords: CAMs, ICAM, VCAM, Selectins, CSF, Cerebrospinal fluid, Blood, Diagnosis, Alzheimer
1. Introduction
Progressive impairment in memory and cognition is a key clinical feature of Alzheimer’s disease (AD). The disorder is morphologically characterized by extracellular β-amyloid plaque deposition, intraneuronal tau pathology, neuronal cell death, vascular dysfunction and inflammatory processes. Mild cognitive impairment (MCI) represents a transition stage between normal aging and dementia. MCI can be defined as failing in one or more cognitive domains (such as memory) or a greater cognitive decline than that expected for the individual’s age or education, yet not interfering notably with activities of daily life (Petersen et al., 1999). Individuals which fulfill these criteria may have a 5- to 10-fold greater risk of developing clinical AD within 3-5 years.
Monocytes migrate through the blood-brain barrier (BBB) (Hickey, 1999; Floris et al., 2002; van der Goes et al., 2001) by interacting with specific cell adhesion molecules (CAM). Different CAMs are involved in this transmigration, such as, e.g., L-selectin (CD62L), P-selectin glycoprotein-ligand-1 (PSGL-1, CD162) and very late antigen-4 (VLA-4, CD49d) during tethering/rolling of monocytes along the BBB and platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) for early transmigration of monocytes (Muller, 2001, 2003; Muller and Randolph, 1999; Maslin et al., 2005). Thus, different CAMs on monocytes become activated during the inflammatory and neurodegenerative response, which could be useful as putative surrogate biomarkers in AD. CD14 is a 55-kDa glycosylphosphatidyl inositol (GPI)-anchored surface myeloid glycoprotein and is expressed on monocytes, macrophages and microglial cells and is responsible for the uptake of bacterial component lipopolysaccharide (LPS) via macropinocytosis (Poussin et al., 1998). The level of soluble form of monocyte differentiation antigen CD14 precursor has been shown to be elevated in CSF from AD patients compared with normal subjects as measured by proteomic analysis (Yin et al., 2009). It was also shown that soluble CD14 inhibited LPS induced nitric oxide production and cell death of microglia and astrocytes (Yin et al., 2009). Thus, blood monocytes may be a valuable model system correlating to the role of microglia cells in the pathogenesis of AD or MCI. Blood monocytes and in vitro differentiated macrophages express many genes related to the pathogenesis of AD (Wahle et al., 2006).
The aim of the present study was to explore the expression levels of different CAMs (ICAM-1 and -3, VCAM-1, P-, L- and E-selectins, E-cadherin) and receptor for advanced glycation endproducts (RAGE) on monocytes by Searchlight Multiplex ELISAs in comparison to the CD14 antigen. Differences among the CAMs and between RAGE and CAMs may point to specific pathogenetic mechanisms.
2. Materials and methods
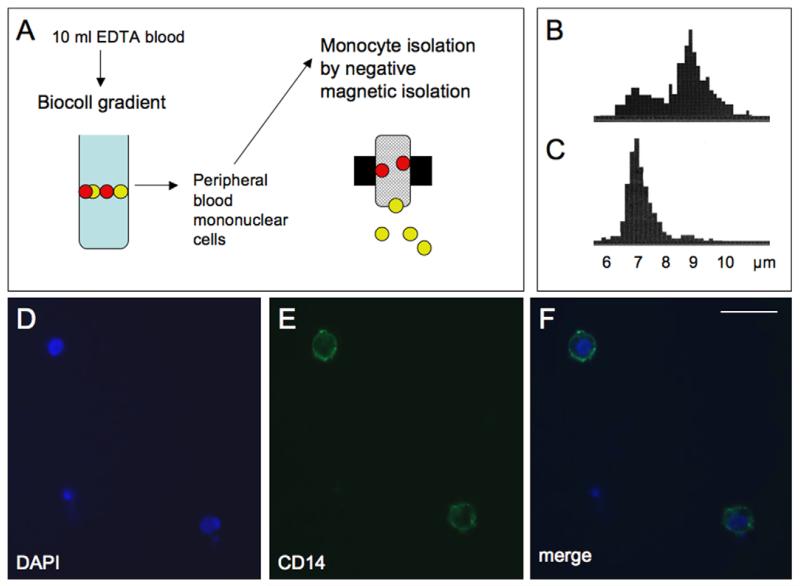
Healthy control subjects (n = 55) and patients with AD (n = 60) or MCI (n = 38) were assessed by the same diagnostic procedure. Psychiatrists clinically examined all subjects, performed a standardized neurological examination, reviewed medical records. Furthermore, neuropsychological assessment and magnetic resonance neuroimaging was performed as reported previously (Marksteiner et al., 2009). EDTA blood (10 ml) was collected during normal routine clinical treatments and processed within 3 h. Peripheral blood mononuclear cells (PBMC) were separated from whole blood (Fig. 1A) on a continuous Biocoll density gradient (1.077 g/ml) after centrifugation (400g, 30 min). PBMC were visible as a white stratum between plasma phase and Biocoll. The interphase with the PBMC was carefully removed, washed in 50 ml phosphate-buffered saline (PBS), and the pellet was dissolved in PBS + bovine serum albumin (BSA). Monoyctes were isolated by negative magnetic isolation as described by the manufacturer (Miltenyi Biotech, Germany). Briefly, PBMCs were incubated with a cocktail of different biotinylated antibodies (CD3, CD7, CD16, CD19, CD56, CD123, CD235a) for 10 min on ice. Then anti-biotin magnetic beads were added, incubated for further 15 min on ice, washed and the cells applied onto MACS MS columns (Miltenyi, Germany) on a strong magnet. The non-labeled monocytes were eluted and collected. Finally, the pellet was frozen at −80 °C until use. Then the pellet was dissolved in 100 μl PBS with a protease inhibitor cocktail (Sigma), sonicated on ice (30 s, 125 W/cm2, 140 μm amplitude, 100%), centrifuged 10 min 13,000g at 4 °C, and the supernatant used for the ELISA. Total protein was determined by Bradford assay using BSA. Some cells were spotted onto glass slides, air dried, and processed for immunohistochemical staining using a fluorescent Alexa Fluor 488 anti-human CD14 antibody (BioLegend) and counter-stained with DAPI to show nuclei.
Fig. 1.
Isolation and immunohistochemical characterization of human monocytes. Monocytes were isolated by negative magnetic isolation (MACS) from peripheral blood mononuclear cells (PBMS), which were collected from 10 ml EDTA blood after separation on a Biocoll gradient (A). PBMC counts displayed a double peak at approx. 7 and 9 μm (B), while after selective MACS isolation a single monocyte specific peak was visible at approx. 7 μm (C). Immunohistochemical staining of monocytes revealed intense CD14 immunoreactive cells (E), with DAPI positive nuclei (D), displaying that the majority of the isolated cells were monocytes (merge, F). Scale bar = 15 μm.
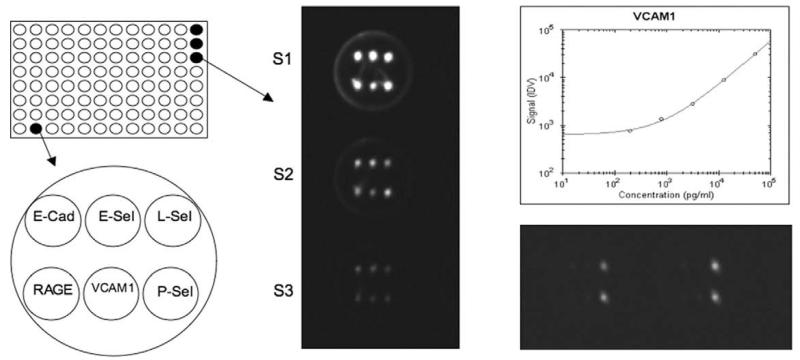
The detection of CAMs (E-cadherin, P-, E- and L-selectins, ICAM-1 and -3, VCAM-1, PECAM-1), RAGE and CD14 was performed using the Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Vienna). This method is a multiplexing sandwich-ELISA system based on chemiluminescent detection of analytes whose respective capture-antibodies are spotted in arrays within each well of a 96-well microplate (Fig. 2). Briefly, 50 μl calibrated protein standards or diluted extracts (1:21 for CD14 and 1:3 for all CAMs and RAGE) were added to coated wells, incubated for 60 min at room on a shaker, then washed 3 ×, and then the biotinylated antibodies (50 μl) were added that specifically bind to the captured proteins. After incubation for 30 min at room on a shaker, the wells were again washed (3 ×) and then incubated with streptavidin-horseradish peroxidase conjugate (50 μl), again washed (3×) and the Super-Signal ELISA Femto Chemiluminescent Substrate (50 μl) was added. The entire plate was imaged using a compatible CCD imaging system to capture the chemiluminescent signal from each spot within each well (Fig. 2). The concentration of each analyte in the array was quantified by comparing the spot intensities for each unknown sample to the corresponding standard curves calculated from the standard sample results by the Search-Light Array Analyst Software. Integrated density values were proportional to the concentrations of bound proteins. Individual analytes were identified by the position of each specific capture (Fig. 2) antibody within the well. Standard curves, raw data and final pg/ml concentrations for each analyte and each sample were reviewed in the array software and exported to Microsoft Excel Software for further statistical analysis. The detection limits for each markers are given in Table 1. To reduce interassay variations, all runs of the same multiplex assay were performed on the same day. Each plate contained an own standard curve. The controls and patient group samples were randomly distributed across all plates. For an easier and faster handling the sample dilutions were prepared the day before the experiment and stored frozen until use.
Fig. 2.
Analysis of cell adhesion molecules (CAMs) using Thermo Scientific SearchLight Protein Array Technology. A single well of a 96-well plate is spotted with different primary antibodies against different CAMs: E-cadherin (E-Cad), E-, L- and P-selectins (E-Sel, L-Sel and P-Sel), receptor for advanced glycosilation endproduct (RAGE) or vascular cell adhesion molecule-1 (VCAM-1). After processing of the wells the chemiluminescence is detected by a CCD camera: the spots in the wells S1-S3 correlate to standards of the respective CAMs, which are plotted as standard curves (e.g., for VCAM-1). The unknown biomarkers in the wells are calculated from the respective standard curves.
Table 1.
Cell adhesion molecules in monocytes of controls, patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD).
| DL | Intra-assay CV (%) | Controls | MCI | Alzheimer | |
|---|---|---|---|---|---|
| CD14 (pg/ml) | 6.2 | 6.3 | 13,673 ± 1284 (55) | 18,147 ± 1759 (38), p = 0.07 | 17,252 ± 1306 (60), p = 0.11 |
| ICAM-1/CD14 | 15.6 | 4.8 | 6.3 ± 0.4 (55) | 5.5 ± 0.4 (38), p = 0.27 | 6.1 ± 0.4 (60), p = 0.80 |
| ICAM-3/CD14 | 390.6 | 8.0 | 12.8 ± 1.3 (40) | 8.3 ± 0.7 (31), p= 0.03 | 9.0 ± 1.4 (48), p= 0.05 |
| VCAM-1/CD14 | 195.3 | 9.5 | 7.4 ± 10.9 (55) | 6.2 ± 1.2 (38), p= 0.55 | 5.2 ± 0.7 (60), p= 0.27 |
| PECAM-1/CD14 | 265.63 | 8.1 | 108 ± 13.2 (44) | 106.8 ± 14.3 (31), p= 1.0 | 97.0 ± 12.2 (48), p= 0.74 |
| P-selectin/CD14 | 977.6 | 6.7 | 1725 ± 156 (53) | 1498 ± 173 (38), p= 0.48 | 1282 ± 105 (60), p= 0.04 |
| L-selectin/CD14 | 93.8 | 7.6 | 47.1 ± 4.5 (55) | 44.6 ± 5.0 (38), p= 0.91 | 45.4 ± 4.8 (60), p= 0.95 |
| E-selectin | 46.9 | - | Not detectable | Not detectable | Not detectable |
| RAGE | 2.9 | - | Not detectable | Not detectable | Not detectable |
| E-cadherin | 39.1 | - | Not detectable | Not detectable | Not detectable |
Monocytic extracts were analyzed by Searchlight Multiplex ELISA and the following markers were tested: CD14, intracellular cell adhesion molecule-1 (ICAM-1) and -3 (ICAM-3), vascular cell adhesion molecule-1 (VCAM-1), platelet cell adhesion molecule-1 (PECAM-1), the P-, L- and E-selectins, E-cadherin and the receptor for advanced glycosilation endproducts (RAGE). The detection limits (DL) and CD14 values are given in pg/ml. Values are mean ± SEM, and the number of analyzed samples is given in parenthesis. The ratios CAMs/CD14 are multiplied by 1000. Statistical analysis was determined by two-way ANOVA (CD14) and one-way ANOVA with a subsequent Fisher PLSD post hoc test, where p < 0.05 is significant.
Statistical analysis was performed in two ways. First, CD14 values were normalized for healthy subjects, MCI and AD patients. Prior to normalization, statistical analysis revealed that there were no statistical differences in CD14 levels using two-way ANOVA. Second, marker values were log-transformed prior to analysis in order to approximate their distribution to normality. The ability of the individual markers to discriminate between diagnostic groups was tested by analysis of variance (ANOVA). Those markers for which a significant group effect had been detected in the ANOVA were followed up by post hoc pairwise comparisons of groups using Fisher’s least significant difference (LSD) method. No further adjustment for multiple testing is required as the number of diagnostic groups to be compared never exceeded three (due to appropriate combination of groups) in which case statistical significance in the global F-test and in the LSD testing (p ≤ 0.05) is sufficient to keep the family-wise error rate at 0.05.
3. Results
The age of the controls was 70.8 ± 0.8 years, of MCI patients 72.4 ± 1.2 years and of AD patients slightly higher (77.0 ± 1.0 years). Monocytes were isolated from whole blood by MACS selection with a very high selectivity and specificity, displaying approximately 7 μm in size (Fig. 1B and C) and an intense immunohistochemical staining for CD14 (Fig. 1D-F).
Searchlight analysis revealed that the CD14 marker slightly increased in MCI and AD patients compared to healthy controls (Table 1). E-selectin, RAGE and E-cadherin were not detectable in monocytic extracts. The ratios of ICAM-1/CD14, VCAM-1/CD14, PE-CAM-1/CD14 and L-selectin/CD14 were not different between MCI and AD patients compared to healthy subjects (Table 1). However, the ratio of ICAM-3/CD14 was significantly decreased in MCI and AD patients compared to controls (Table 1). Similarly, the ratio of P-selectin/CD14 was significantly decreased in AD patients but not in MCI patients (Table 1).
4. Discussion
In summary our study shows that monocytic ICAM-3 and P-selectin (related to the CD14 antigen) were significantly reduced in AD patients, while ICAM-3 was also reduced in MCI.
CD14 is a surface protein preferentially expressed on monocytes/macrophages. It binds lipopolysaccharide binding protein and recently has been shown to bind apoptotic cells. CD14 exists in two forms, it is either anchored into the membrane (mCD14) or it appears in a soluble form (sCD14). Recently, it has been reported that CD14 was enhanced in CSF of AD patients (Yin et al., 2009). In our study, we show that the staining intensity for CD14 immunoreactivity was slightly but not statistically significant enhanced in MCI and AD patients. In exploring the function of the different CAMs we have correlated the expression of the different CAMs to CD14 expression of the respective samples to correct the analysis for different amounts of monocytic extracts in the samples and to decrease the interassay variation.
Intercellular adhesion molecule-3 (ICAM-3, CD50) is constitutively and abundantly expressed by all leukocytes and is the most important ligand for LFA-1 in the initiation of the immune response. It functions not only as an adhesion molecule, but also as a potent signaling molecule. Our data show for the first time that ICAM-3 levels are decreased on monocytes of MCI and AD patients. Thus ICAM-3/CD14 may provide further insight into inflammatory processes of AD.
P-selectin (CD62P, GMP-140, PADGEM) is a CAM expressed on the surfaces of activated endothelial cells. P-selectin plays an essential role in the initial recruitment of leukocytes to the site of injury during inflammation. It has been reported that P-selectin plasma levels were decreased in AD (Kozubski et al., 2002). In our study, we show that P-selectin/CD14 was significantly decreased in AD patients, which goes in line with the plasma levels. Thus it seems likely that P-selectins are involved in the pathogenetic mechanisms of neurodegeneration.
Intercellular adhesion molecule-1 (ICAM-1, CD54) belongs to the IgG family and is expressed on activated endothelial cells, lymphocytes, monocytes or microglia. It is a membrane-bound cell adhesion molecule but it is also found in a soluble form. ICAM-1 can be induced by different cytokines, such as, e.g., interleukin-1. It has been shown that ICAM-plasma levels were increased in AD (Rentzos et al., 2005). Our data show that ICAM-1/CD14 was not changed in MCI and AD.
Vascular cell adhesion molecule-1 (VCAM-1) is a member of the IgG family and is expressed by endothelial cells and involved in adhesion of lymphocytes and monocytes. Thus, elevated VCAM-1 levels might reflect defects of the vascular system. Zuliani et al. (2008) found that VCAM-1 was elevated in plasma of AD patients and vascular dementia. Others showed a significant association between age and VCAM-1 independent of the cardiovascular risk (Richter et al., 2003). Our data did not reveal that VCAM-1 was affected in monocytes of AD and MCI patients.
Platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) is a 130-kDa protein which plays a significant role in the adhesion cascade. It is involved in leukocyte-endothelium interaction and in leukocyte transmigration during inflammation. In our hands PECAM-1 was not changed on monocytes of AD and MCI patients.
L-selectin (CD62L, LECAM-1 and LAM-1) is a cell adhesion molecule found on leukocytes. It belongs to the selectin family of CAMs. L-selectin is important for lymphocyte homing and adhesion to high endothelial cells of post capillary venules of peripheral lymph nodes. We did not find any changes of L-selectin in AD as well as MCI.
E-selectin (CD62E) is a member of the selectin family, which acts by helping circulating leukocytes to weakly attach to the endothelium. No changes were found for plasma levels of E-selectin in AD patients (Rentzos et al., 2005), while in vascular dementia increased levels have been reported (Zuliani et al., 2008). Thus E-selectin is mostly expressed on endothelial cells. In fact, we did not see expression of E-selectin on human monocytes.
E-cadherin (CDH1 and CD324) is a transmembrane protein and promotes the formation of adherens junctions and establishment of polarized cell monolayers. No expression of E-cadherin on human monocytes has been seen in our study.
The receptor for advanced glycation endproducts (RAGE) is a 35-kDa transmembrane receptor of the IgG family and is a multi-ligand cell surface receptor expressed by a wide variety of cells in the brain and on endothelial cells, pericytes and smooth muscle cells. It is involved in several chronic disorders including neurodegenerative diseases. RAGE plasma levels have been reported to be enhanced in AD (Miller et al., 2008), while decreased levels were seen in MCI patients (Ghidoni et al., 2008). We were not able to detect RAGE on human monocytes.
Our study has several limitations. (1) The present study is a cross-sectional study, therefore, we cannot draw any conclusions how the markers will change when disease progresses, or in the case of MCI remains stable or reverses. (2) The number of patients in this study is low and the differences are very small. Furthermore, we cannot exclude age-related effects, since Alzheimer patients were slightly older. (3) We extracted proteins by sonication of cells, thus we analyzed soluble cytoplasmic proteins. We do not know how membrane-bound proteins are changed. (4) The stability of the protein extracts was not tested. Although protease inhibitors were used and samples were frozen immediately, we cannot exclude that some proteins are degraded. (5) Monocytes were isolated within 3 h after blood collection. We cannot exclude that monocytes became activated during storage and transport of the samples. In summary, controlled standardized procedures to measure biomarkers in blood cells must be established to compare the data in different centers.
In conclusion we show that ICAM-3/CD14 and P-selectin/CD14 were decreased in AD monocytes, while ICAM-3/CD14 was also decreased in MCI. The data suggest that inflammatory processes are involved in AD, recruiting monocytes, which involve ICAM-3 and P-selectin. Further longitudinal larger studies should then clarify whether any of these parameters may be used as a diagnostic biomarker.
Acknowledgements
This study was supported by the Austrian Science Funds (L429-B05). We thank Ursula Kirzenberger-Winkler and Celine Ullrich for their help with monocyte isolation.
References
- Floris S, Ruuls SR, Wierinckx A, van der Pol SM, Dopp E, van der Meide PH, Dijkstra CD, De Vries HE. Interferon-beta directly influences monocyte infiltration into the central nervous system. Journal of Neuroimmunology. 2002;127:69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Benussi L, Glionna M, Franzoni M, Geroldi D, Emanuele E, Binetti G. Decreased plasma levels of soluble receptor for advanced glycation end products in mild cognitive impairment. Journal of Neural Transmission. 2008;115:1047–1050. doi: 10.1007/s00702-008-0069-9. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Seminars in Immunology. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- Kozubski W, Swiderek M, Kloszewska I, Gwozdzinski K, Watala C. Blood platelet membrane fluidity and the exposition of membrane protein receptors in Alzheimer disease (AD) patients - preliminary study. Alzheimer Disease and Associated Disorders. 2002;16(1):52–54. doi: 10.1097/00002093-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Kemmler G, Weiss EM, Knaus G, Ullrich C, Mechteriakov S, Oberbauer H, Auffinger S, Hinterhölzl J, Hinterhuber H, Humpel C. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslin CL, Kedzierska K, Webster NL, Muller WA, Crowe SM. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Current HIV Research. 2005;3:303–317. doi: 10.2174/157016205774370401. [DOI] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Research. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond molecules involved in the transmigration and fate of monocytes. Journal of Leukocyte Biology. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- Muller WA. New mechanisms and pathways for monocyte recruitment. Journal of Experimental Medicine. 2001;194:47–51. doi: 10.1084/jem.194.9.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends in Immunology. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Poussin C, Foti M, Carpentier JL, Pugin J. CD14-dependent endotoxin internalization via a macropinocytic pathway. The Journal of Biological Chemistry. 1998;273:20285–20291. doi: 10.1074/jbc.273.32.20285. [DOI] [PubMed] [Google Scholar]
- Rentzos M, Michalopoulou M, Nikolaou C, Cambouri C, Rombos A, Dimitrakopoulos A, Vassilopoulos D. The role of soluble intercellular adhesion molecules in neurodegenerative disorders. Journal of Neurological Science. 2005;228:129–135. doi: 10.1016/j.jns.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Richter V, Rassoul F, Purschwitz K, Hentschel B, Reuter W, Kuntze T. Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology. 2003;49(5):293–300. doi: 10.1159/000071710. [DOI] [PubMed] [Google Scholar]
- van der Goes A, Wouters D, vam der Pol SMA, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, De Vries HE. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. The FASEB Journal. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- Wahle T, Thal DR, Sastre M, et al. GGA1 is expressed in the human brain and affects the generation of amyloid β-peptide. The Journal of Neuroscience. 2006;26:12838–12846. doi: 10.1523/JNEUROSCI.1982-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin GN, Jeon H, Lee S, Lee HW, Cho J-Y, Suk K. Role of soluble CD14 in cerebrospinal fluid as a regulator of glial functions. Journal of Neuroscience Research. 2009 doi: 10.1002/jnr.22081. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Cavalieri M, Galvani M, Passaro MR, Munari MR, Bosi C, Zurlo A, Fellin R. Markers of endothelial dysfunction on older subjects with late onset Alzheimer’s disease or vascular dementia. Journal of Neurological Science. 2008;272:164–170. doi: 10.1016/j.jns.2008.05.020. [DOI] [PubMed] [Google Scholar]