Abstract
Cholinergic neurons are intensively studied, because they degenerate in Alzheimer’s disease. Although neurotracer techniques are widely used to study axonal transport, guidance, regeneration or sprouting it is not clear if cholinergic neurons can be stained by tracer techniques and studied in brain slices. The aim of the present study was to evaluate the characteristics of the neurotracer Miniruby in organotypic brain slices of the basal nucleus of Meynert (nBM), focusing on cholinergic neurons. Miniruby is a biotinylated dextran amine and is taken up very fast by a variety of cells. When 2-week old nerve growth factor-incubated brain slices of the nBM were treated with Mini-ruby crystals for 1 h, only a few (2–3%) cholinergic neurons were clearly labeled as shown by co-localization with choline acetyltransferase. The staining was found in neuN-positive neurons and microtubule associated protein-2 (MAP-2)-positive nerve fibers. A very rapid dynamic change was observed in these labeled varicosities within seconds. However, Mini-ruby was taken up also by many glutamine synthethase-positive astrocytes. At the site of Mini-ruby application an intense CD11b-positive microglial staining was evident. In conclusion, neurons and astrocytes in organotypic brain slices can be labeled very fast with the fluorescent dye Mini-ruby which undergoes dynamic processes.
Keywords: Cholinergic neuron, Mini-ruby, Brain slice, Neurotracing
Introduction
In Alzheimer’s disease a decline of cholinergic neurons directly correlates with cognitive deficits and memory loss. Thus, the characterization of cholinergic neurons is of high importance, especially to study survival and nerve fiber innervations. The organotypic brain slice model has been demonstrated as an excellent in vitro model to study the survival of different cell types, the cytoarchitecture of the tissue, and the connections between cells [1-4]. Individual cells within the brain slices remain in close contact and do not lose density-dependent regulatory mechanisms, three-dimensional architecture as well as tissue-specific transport and diffusion probabilities. The organotypic brain slice model has been used to study survival of cholinergic neurons of the septum, as well as their nerve fiber innervations to the hippocampus [2]. In our research group we have extensively studied cholinergic neurons of the basal nucleus of Meynert (nBM) in organotypic brain slices when cultured with nerve growth factor [5-7].
Neurotracing techniques [8] are widely used to study axonal transport [9], guidance [10] regeneration [11] or sprouting [11-13]. However, no reports are available on the neurotracing of nBM cholinergic neurons. The present study uses a fluorescently tagged biotinylated dextran amine, Mini-ruby, which is widely used to trace neuronal projections in vitro and in vivo [9, 17]. Mini-ruby is a 10,000 MW conjugate of tetramethylrhodamine and biotin with dextran. Such dextran conjugates can function efficiently as anterograde or retrograde tracers. The purpose of the present study was to explore if cholinergic neurons of the nBM can be selectively labeled by Mini-ruby. This tracer seems to be very useful because it is taken up very fast. We expect that at least partly cholinergic neurons take up the neurotracer, which would give us a potent tool to further characterize these neurons in brain slices.
Experimental Procedure
Organotypic Brain Slice Cultures
All experiments conformed to Austrian guidelines on the ethical use of animals and all efforts were made to minimize the number of animals used and their suffering. Organotypic brain slice cultures were established as described by us in detail [5, 6]. Briefly, the nBM of postnatal day 7–9 (P7–9) Sprague–Dawley rats was dissected under aseptic conditions, 400 μm slices were cut with a tissue chopper (McIlwain, USA), and the slices were placed on a 30 mm diameter Millicell-CM 0.4 μm membrane insert (Millipore, Austria), where they become attached to the membrane after 2 weeks of incubation. It is important to note, that the ipsilateral as well as contralateral brain areas were dissected at the same time, cut on the tissue chopper and all slices pooled in medium. Slices were cultured in 6-well plates (Greiner) at 37°C and 5% CO2 with 1.2 ml/well of the following culture medium: 50% MEM/HEPES (Gibco), 25% heat inactivated horse serum (Gibco/Lifetech, Austria), 25% Hanks’ solution (Gibco), 2 mM NaHCO3 (Merck, Austria), 6.5 mg/ml glucose (Merck, Germany), 2 mM glutamine (Merck, Germany), and 10 ng/ml nerve growth factor (NGF, Sigma) at pH 7.2. Medium was changed twice a week. After 2 weeks in culture, a few crystals of Mini-ruby (Invitrogen, D3312) were placed directly onto the surface of the slice using the tip of a small needle. The slices were incubated for 1-24 h and were then observed under the fluorescence Leica DMIRB inverse microscope using a Y3 filter (excitation 535/50 nm, emission 610/75 nm). Afterwards, slices were fixed for 3 h at 4°C in 4% paraformaldehyde/10 mM phosphate buffered saline (PBS) and then stored at 4°C in PBS. In addition and for comparison, we also tested the NeuroTrace tissue labeling paste DiO (Invitrogen, N22881).
Immunohistochemistry
Immunohistochemistry was performed similarly as previously described [5]. All incubations were performed free floating for 2 days including 0.1% Triton, such that the antibodies can penetrate from both sides of the slices, which allows good penetration of the antibody into the brain slices. Slices were washed 30 min with 0.3% Triton/PBS (T-PBS) at room temperature. After thorough rinsing, the slices were blocked with 20% horse serum/0.2% BSA/T-PBS and then incubated for 2 days at 4°C with primary antibodies against choline acetyltransferase (ChAT, 1:750, Millipore), neuN (Millipore, 1:1,000), glutamine synthethase (GS, 1:2,000, Abcam) and against microtubule associated protein-2 (MAP-2, 1:200, Millipore). Then the slices were washed again with PBS and incubated with secondary anti-goat (ChAT), anti-rabbit (GS) and anti-mouse (neuN, MAP-2) Alexa-488 antibodies (1:400, Invitrogen) for 1 h at room temperature. To label nuclei, slices were incubated with 4,6-diamidino-2-phenylindole (DAPI, 1:10,000, Sigma) for 30 min. Immunolabeling was visualized with a Leica DMIRB fluorescence inverse microscope equipped to an Apple computer with Openlab 4 software (Improvision). The following filters have been used to visualize DAPI (Filter A4, excitation 360/40, emission 470/40), Alexa 488 (Filter L5, excitation 480/40, emission 527/30) and Mini-Ruby (Filter Y3, excitation 535/50, emission 610/75).
Results
Mini-Ruby Application in Organotypic Brain Slices of the nBM
When Mini-ruby was applied to the brain slices, several fluorescent areas were visible all over the brain slice already after 1 h (Fig. 1a, b). Because the Mini-ruby crystals were applied very carefully, a homogenous application could not be obtained (Fig. 1b). The Mini-ruby dye seemed to be taken up mainly by cells at the surface of the slices.
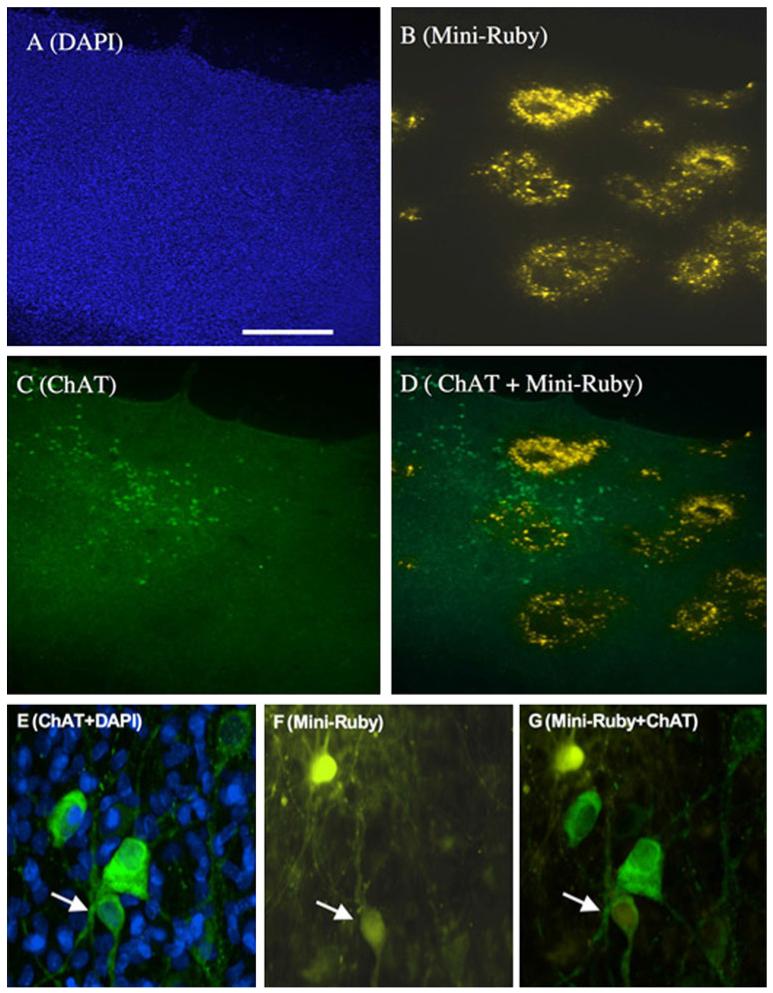
Fig. 1.
Overview of a brain slice shows DAPI-positive nuclei (a, blue, excitation 360/40, emission 470/40), Mini-ruby-positive areas (b, yellow, excitation 535/50, emission 610/75) and ChAT-positive cholinergic neurons (c, green, excitation 480/40, emission 527/30). A merged picture (d) shows the distribution of cholinergic neurons and Mini-ruby positive areas in a representative slice. Co-staining of ChAT-positive neurons (e) with Mini-ruby (f) displayed a clear neurotracing of a few cholinergic neurons as shown in a merged picture (g). Scale bar in a = 250 μm (a–d), 50 μm (e–g)
Mini-Ruby in Cholinergic Neurons
Cholinergic neurons were visualized by immunohistochemistry for ChAT (Fig. 1c–g). In the nBM brain slices 97 ± 5 ChAT-positive neurons/slice (n = 12) were counted, but only 3 ± 0.5 neurons were Mini-ruby positive (Fig. 1d–g).
Mini-Ruby in Neurons and Astrocytes
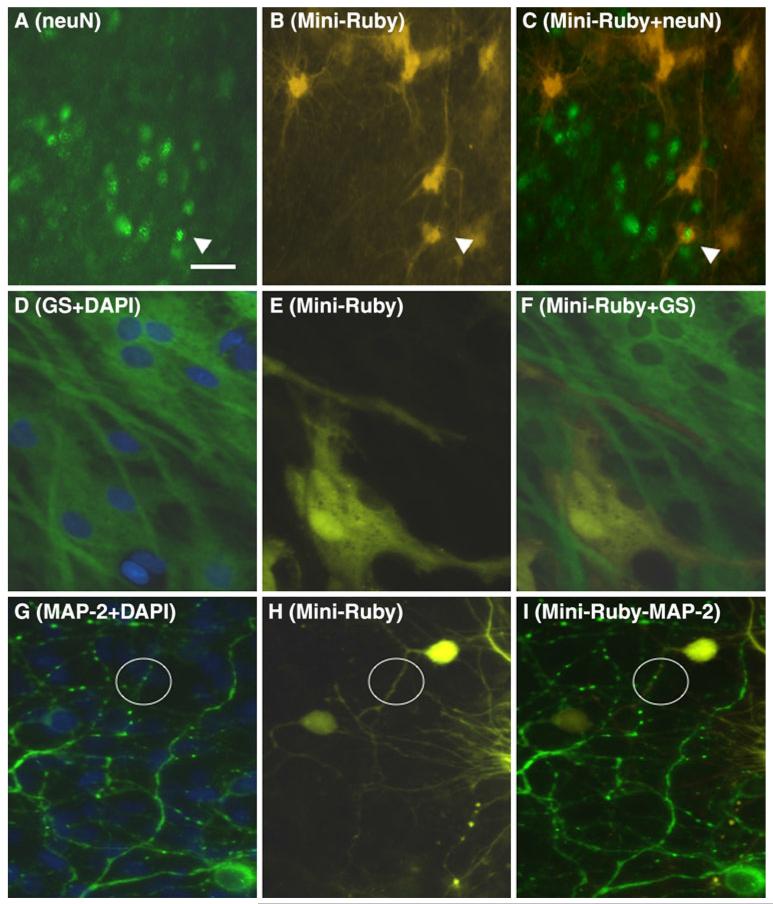
Neurons in brain slices were stained by neuN (Fig. 2a). Co-staining showed that neuN positive neurons were labeled by Mini-ruby, however, a large population of Mini-ruby positive cells was neuN negative (Fig. 2b–c). Immunohistochemistry for glutamine synthetase stained astrocytes (Fig. 2d) and for MAP-2 stained neuronal processes (Fig. 2g). Mini-ruby clearly labeled mainly GS-positive astrocytes (Fig. 2e–f). Double labeling of brain slices with Mini-ruby and MAP-2 indicated some Mini-ruby-positive neuronal perikarya (Fig. 2h), but mainly positively stained nerve fibers and varicosities were seen (Fig. 2i). In order to compare Mini-ruby with another neurotracer, the tissue labeling paste DiO was applied onto slices. However, this tracer was not easy to apply onto the slices and did not yield clearly labeled cells in brain slices.
Fig. 2.
Neuronal and glial uptake of Mini-ruby in organotypic brain slices of basal nucleus of Meynert. Brain slices were stained for neurons by neuN (a), for astrocytes by glutamine synthetase (GS) (d) or neuronal microtubuli-associated protein-2 (MAP-2) (g). One hour after Mini-ruby application several strongly or weakly stained small or large cells (b, e, h) were found, but also an intense nerve fiber network was visible (h). Co-staining clearly identified that a neuron (c, arrow) was labeled with Mini-ruby, however, several astrocytes were identified to be positive (f). Neuronal processes (varicosities) were clearly labeled with Mini-ruby (circle, i). Some cells were stained with nuclear DAPI. DAPI was visualized with filter A4 (excitation 360/40, emission 470/40), neuN, GS or MAP-2 by Alexa 488 with filter L5 (excitation 480/40, emission 527/30) and Mini-Ruby with filter Y3 (excitation 535/50, emission 610/75). Scale bar in a = 50 μm
Effects of Mini-Ruby Application on Microglia in Organotypic Brain Slices
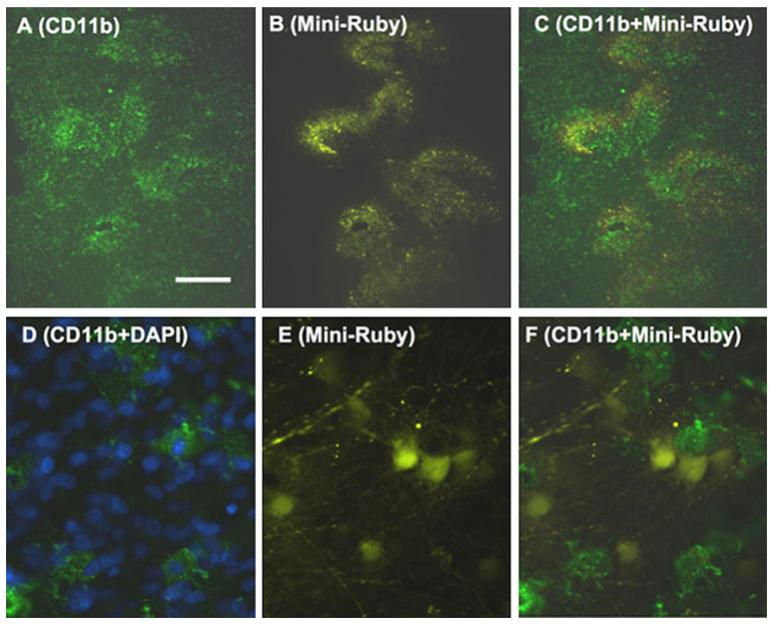
When Mini-ruby was applied onto organotypic nBM brain slices a strong immunoreactive staining was evident for CD11b (Fig. 3a, d) after 24 h, which co-localized with the site of Mini-ruby application (Fig. 3b, c). However, Mini-ruby-positive cells did not co-localize with CD11b-positive microglia (Fig. 3e, f).
Fig. 3.
Effects of Mini-ruby application on CD11b-microglial immunohistochemistry in organotypic brain slices of the basal nucleus of Meynert. Immunohistochemial staining for CD11b (a) clearly showed that sites of Mini-ruby application (b) displayed enhanced microglial stainings (c, merged) after 24 h. Co-stainings did not show that Mini-ruby-positive cells were identical with CD11b positive microglia (e, f). Nuclei were counterstained with DAPI (blue, d). DAPI was visualized with filter A4 (excitation 360/40, emission 470/40), CD11b Alexa 488 with filter L5 (excitation 480/40, emission 527/30) and Mini-Ruby with filter Y3 (excitation 535/50, emission 610/75). Scale bar in a = 500 μm (a–c); 140 μm (d–f)
Dynamic Changes of Mini-Ruby Positive Processes
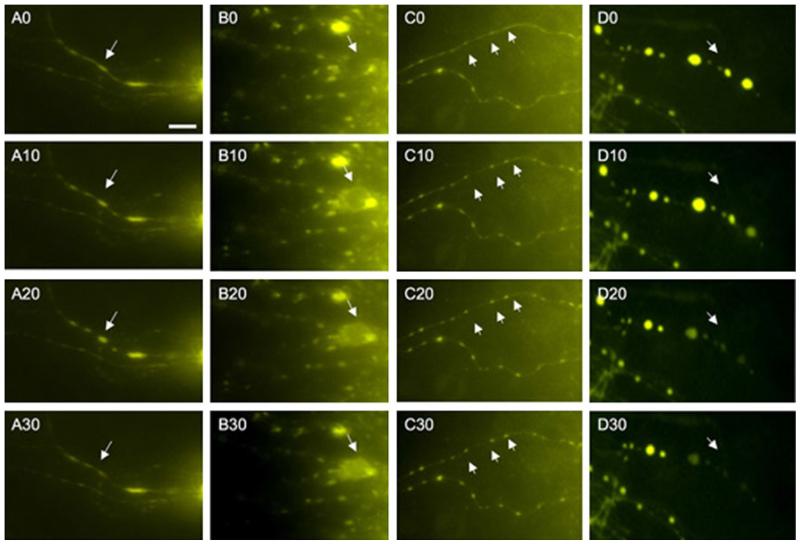
Brain slices of the nBM treated with Mini-ruby displayed several tracer-positive processes which had a rapid turnover within 30 s (Fig. 4). Processes were seen where the tracer was markedly increased in varicosities and rapidly disappeared within seconds (Fig. 4a). In some cases a cell perikaryon was filled with the tracer within seconds either by retrograde transport or uptake (Fig. 4b). In some processes enhanced fragmented Mini-ruby-positive varicosities were found (Fig. 4c). In the majority of cases the Mini-ruby positive varicosities disappeared within seconds (Fig. 4d).
Fig. 4.
Fast dynamic turn-over of labeled processes after Mini-ruby application visualized all 10 s (0-10-20-30). Mini-Ruby was visualized with filter Y3 (excitation 535/50, emission 610/75) and photographed all 10 s. Different forms of dynamic changes were found: increased fluorescence at a specific spot representing active transport (a, arrow), loading of a cell with Mini-ruby representing retrograde transport or uptake (b, arrow), fragmentation of a cellular process into varicosities (c, arrows) or disappearance of a fluorescence spot (d, arrow). Scale bar in a = 35 μm
Discussion
The present study shows, that the fluorescent tracer Mini-ruby is able to label a few cholinergic neurons but many non-cholinergic neurons and glial cells in brain slices of the nucleus basalis of Meynert. Furthermore, varicosities in nerve fibers demonstrated a tracer-positive labeling and exhibited a dynamic turnover.
Several classes of tracers exist and are divided into fluorescent and non-fluorescent markers [8]. A variety of retrograde and anterograde fluorescent tracers, such as carbocyanine dyes or dextran amines can be visualized under the microscope and provide numerous approaches for analyzing neuronal circuits [14, 15]. Thus, fluorescent markers in combination with techniques such immunohistochemistry or electrophysiological recording provide the opportunity to study various neurobiological questions [14,16]. In contrast to non-fluorescent tracers, fluorescent markers offer the major advantage of the simultaneous examination in vitro and in vivo [8]. It has been demonstrated that tracers are incorporated via diffusion into neurons at the injection site and transported rapidly in a retrograde or anterograde direction [8, 17]. However, it is not consistent if exclusively neurons or additionally glial cells can be labeled. In this study, Mini-ruby, a fluorescently (tetramethylrhodamine) tagged biotinylated dextran amine with a size of 10,000 MW was used to study a tracer-positive cholinergic labeling in organotypic brain slices. Mini-ruby is a widely used neuronal tracer in vitro and in vivo [9, 17] and function efficiently as anterograde or retrograde tracers. Mini-ruby is supplied in a crystaline form and is applied onto the slices very carefully at different spots. Thus this type of application causes a relatively inhomogenous labeling of the slices. In addition, one needs to be very careful not to destruct the slices when the crystals are applied. Further studies are necessary to use dissolved dyes which can be easier applied as a liquid all over the slices. A similar problem was observed with the DiO paste. Another problem is that it looked like that mainly the surface cells in the brain slices were stained. Thus it seems likely that Mini-ruby does not diffuse deeper into the brain slice. It will be necessary to develop and use dyes which can more easily penetrate into the tissue and diffuse at least 100 μm into the slice.
The organotypic brain slice model is well established in our research group [5-7] and provides an easily accessible in vitro model containing all cells of the brain in close interaction. During the preparation of the slices, the processes of the neurons are cut, but these axotomized neurons had 2 weeks to recover and to seal their cut axons and dendrites. In the present study, we used single brain slices of the nBM, which are derived from early postnatal day 7–9 rat brains. Immunohistochemical staining against the key enzyme ChAT exhibits cholinergic neurons of the nBM. Furthermore, astrocytes are visualized by specific glutamine-synthetase labeling and neuronal cells and fibers are shown via MAP-2 staining. Microglia were stained against the selective CD11b antigen using the OX-42 antibody. Our data provide evidence, that only a few cholinergic neurons can be labeled within 1 h with the fluorescent tracer Mini-ruby. However, in addition to cholinergic neurons, non-cholinergic neuN positive neurons and GS-positive astrocytes were labeled. Thus, this shows that cholinergic neurons cannot be studied specifically under these conditions. To analyze cholinergic cells and axonal processes it will be necessary to stain the living brain slice with more selective dyes. Anyhow, this method may allow to visualize growth of nerve fibers in a co-culture system. Indeed anterograde tracing of the entorhinal-hippocampal pathway in organotypic brain slices has already been demonstrated [9]. In addition to the staining of neurons, many astrocytes were labeled by Mini-ruby, which indicates that the uptake of Mini-ruby was not selective for neurons. Furthermore, it was interesting to observe an upregulation and activation of microglial cells in the surrounding area of the Mini-ruby application site. This underlines the viability of the organotypic brain slice model as an in vivo-like model with a fast reaction time to different stimuli. However, we could not identify Mini-ruby-positive microglia, which is an intriguing observation regarding the upregulation of microglia after Mini-ruby application.
Our data clearly showed that Mini-ruby was found in neuronal processes, which were positive for MAP-2. This staining was also very prominent in neuronal varicosities, representing sites of enhanced dynamic activity and interaction between neurons. This could be interpreted, that Mini-ruby is taken up by neurons and transported either retrogradely or anterogradely. Compared to other fluorescent dyes, dextrans are incorporated into injured neurons and are transported rapidly in a predominantly anterograde direction. The nBM slice model represents an axotomized in vitro model, because cholinergic axons are cut during the dissection. However the majority of these axons survive in the brain slice when incubated with nerve growth factor. Anyhow, it is possible that these axons are more sensitive to uptake of Mini-ruby. To further study these processes, we observed the living brain slices under the microscope and found that the fluorescence signal in these varicosities underwent a rapid dynamic change within seconds. This phenomenon is not easy to explain, but we can exclude that the fluorescence was just bleaching or the focus of the microscope was shifting, because these dynamic changes appeared on definite fibers and not in the whole neuron. We observed several different forms of dynamic changes: (1) altered fluorescence at specific nerve fibers representing active transport (2) active loading of Mini-ruby into a cell representing retrograde transport or uptake (3), fragmentation of a cellular process into varicosities representing transport processes and (4) disappearance of a fluorescence spot representing metabolism, transport or release. Thus, our data suggest that the varicosities may display sites of dynamic enhanced activation [18] which alters within the axon. These varicosities may also present sites of neuronal plasticity [19] or changes in the intra-axonal ion concentration may have an impact on the fluorescent signal intensity [20]. However, further investigations will be necessary, especially to block axonal transport and to visualize if the transport occurs to the synapse or to the cell body.
Taken together, our study shows that Mini-ruby application to organotypic brain slices of the basal nucleus of Meynert stains a few cholinergic neurons but mainly non-cholinergic neurons and astrocytes. The application was accompanied by microglial activation. The fluorescent staining rapidly disappeared in varicosities pointing to a rapid dynamic turnover within seconds. In conclusion, Mini-ruby may be a potent neurotracing tool to study neurons and astrocytes in organotypic brain slices.
Acknowledgments
This study was supported by the Austrian Science Fonds (P1911-B05 and SFB F4405-B19). We thank Mag. Nina Daschil and Ursula Kirzenberger-Winkler for their excellent technical help.
References
- 1.Duff K, Noble W, Gaynor K, Matsuoka Y. Organotypic slice cultures from transgenic mice as disease model systems. J Mol Neurosci. 2002;19:317–320. doi: 10.1385/JMN:19:3:317. [DOI] [PubMed] [Google Scholar]
- 2.Gähwiler BH, Hefti F. Guidance of acetylcholinesterase-containing fibers by target tissue in co-cultured brain slices. Neuroscience. 1984;40:235–243. doi: 10.1016/0306-4522(84)90088-5. [DOI] [PubMed] [Google Scholar]
- 3.Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a techniques has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 4.Stoppini L, Buch PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 5.Weis C, Marksteiner J, Humpel C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience. 2001;102:129–138. doi: 10.1016/s0306-4522(00)00452-8. [DOI] [PubMed] [Google Scholar]
- 6.Ullrich C, Pirchl M, Humpel C. Effects of cholesterol and its 24S-OH and 25-OH oxysterols on choline acetyltransferase-positive neurons in brain slices. Pharmacology. 2010;86:15–21. doi: 10.1159/000314333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zassler B, Weis C, Humpel C. Tumor necrosis factor-alpha triggers cell death of sensitized potassium chloride-stimulated cholinergic neurons. Mol Brain Res. 2003;113:78–85. doi: 10.1016/s0169-328x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 8.Köbbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Progr Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 9.Kluge A, Hailer NP, Horvath TL, Bechmann I, Nitsch R. Tracing of the entorhinal-hippocampal pathway in vitro. Hippocampus. 1998;8:57–68. doi: 10.1002/(SICI)1098-1063(1998)8:1<57::AID-HIPO6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Lin S, Huang Y, Lee T. Nuclear receptor unfulfilled regulates axonal guidance and cell identity of Drosophila mushroom body neurons. PLoS One. 2009;4:8392. doi: 10.1371/journal.pone.0008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Turco D, Deller T. Organotypic entorhino-hippocampal slice cultures—a tool to study the molecular and cellular regulation of axonal regeneration and collateral sprouting in vitro. Methods Mol Biol. 2007;399:55–66. doi: 10.1007/978-1-59745-504-6_5. [DOI] [PubMed] [Google Scholar]
- 12.Prang P, Del Turco D, Deller T. Associational sprouting in the mouse fascia dentata after entorhinal lesion in vitro. Brain Res. 2003;978:205–212. doi: 10.1016/s0006-8993(03)02836-1. [DOI] [PubMed] [Google Scholar]
- 13.Stavridis SI, Dehghani F, Korf H-W, Hailer NP. Cocultures of rat sensorimotor cortex and spinal cord slices to investigate corticospinal tract sprouting. SPINE. 2009;34:2494–2499. doi: 10.1097/BRS.0b013e3181b4abd8. [DOI] [PubMed] [Google Scholar]
- 14.Schofield BR. Retrograde axonal tracing with fluorescent markers. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0117s43. Chapter 1. Unit 1.17. [DOI] [PubMed] [Google Scholar]
- 15.Matsubayashia Y, Iwai L, Kawasakia H. Fluorescent double-labeling with carbocyanine neuronal tracing and immunohistochemistry using a cholesterol-specific detergent digitinon. J Neurosci Meth. 2008;174:71–81. doi: 10.1016/j.jneumeth.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Novikova L, Novikov L, Kellerth J-O. Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods. 1997;74:9–15. doi: 10.1016/s0165-0270(97)02227-9. [DOI] [PubMed] [Google Scholar]
- 17.Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Meth. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 18.Zeng B-Y, Iravani MM, Jackson MJ, Rose S, Parent A, Jenner P. Morphological changes in serotoninergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol Dis. 2010;40:599–607. doi: 10.1016/j.nbd.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Bennett CB, Muschol M. Large neurophysial varicosities amplify action potentials: results from numerical simulations. Neuroendocrinology. 2009;150:2829–2836. doi: 10.1210/en.2008-1636. [DOI] [PubMed] [Google Scholar]
- 20.Brain KL, Trout SJ, Jackson VM, Dass N, Cunnane TC. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. doi: 10.1016/s0306-4522(01)00280-9. [DOI] [PubMed] [Google Scholar]