Abstract
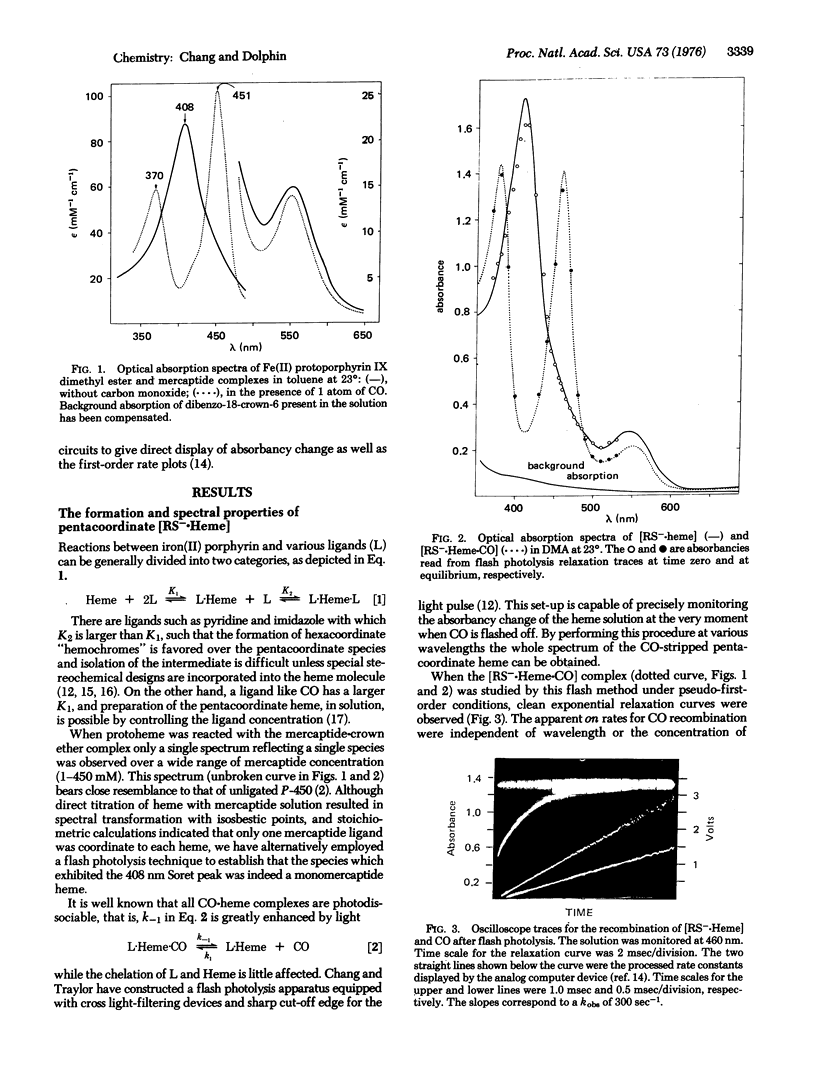
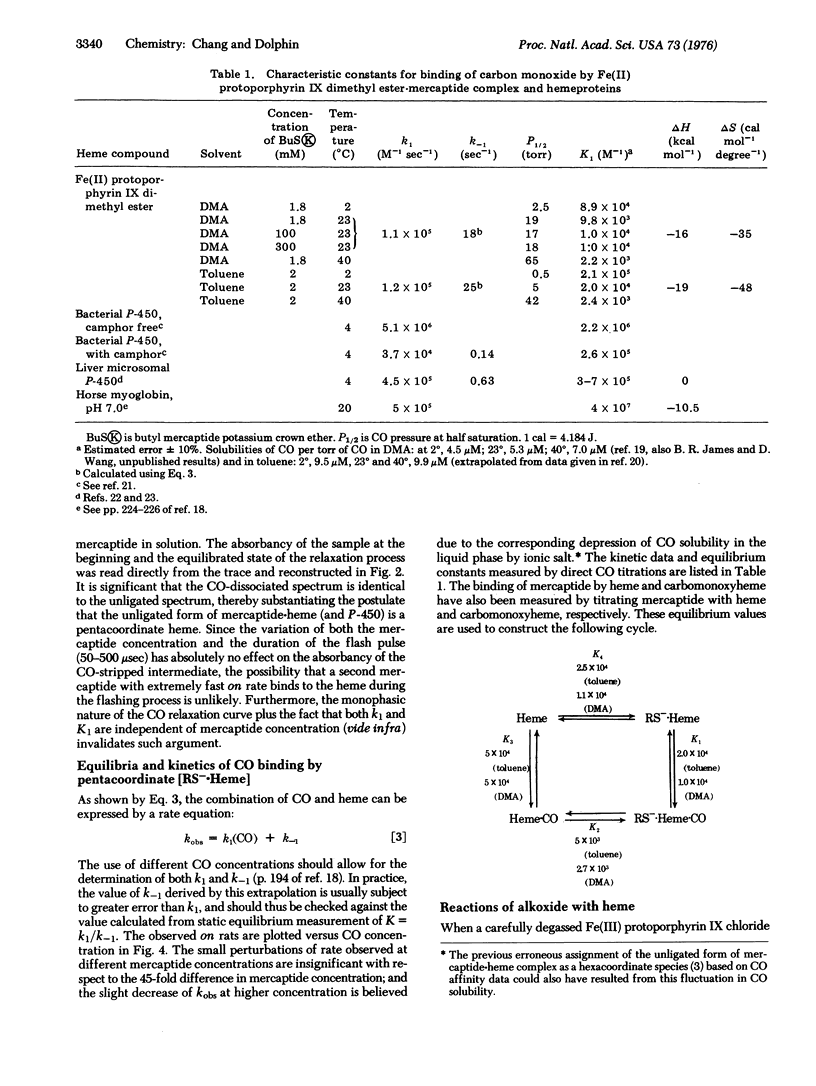
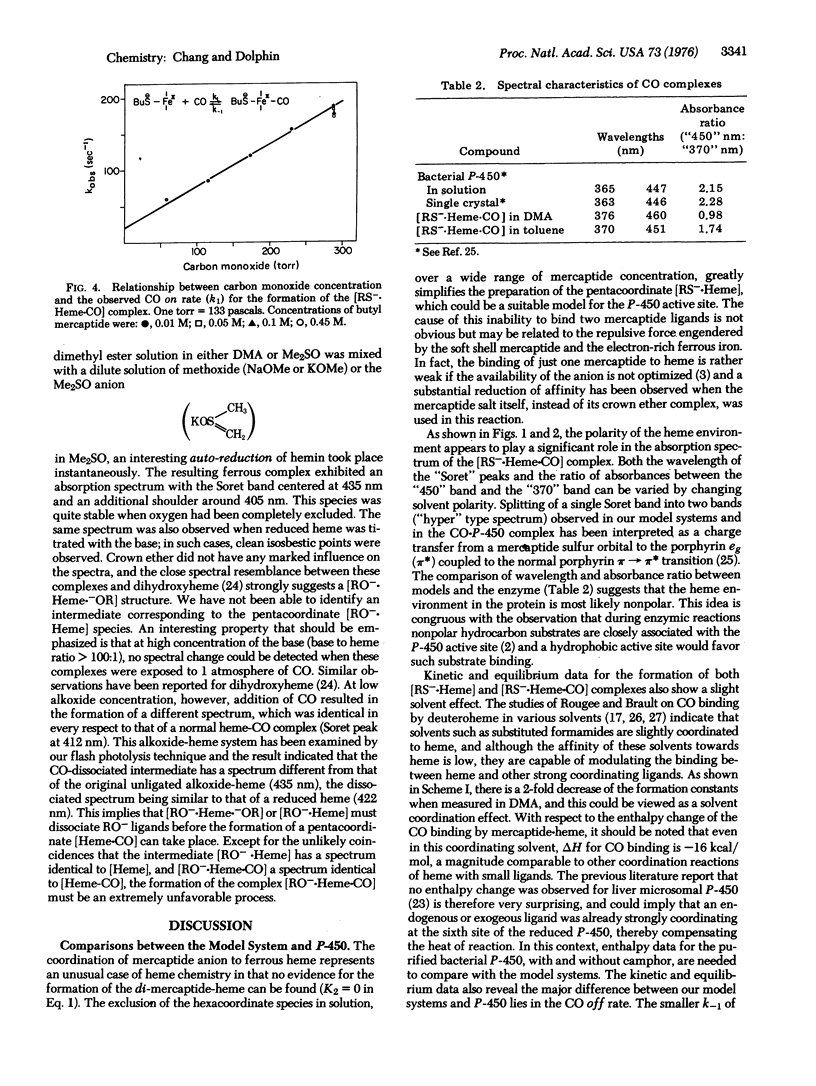
Mercaptide anions form exclusively penta-coordinate heme complexes [RS-heme] in polar and nonpolar solution over a wide range of mercaptide concentration. These complexes have a Soret peak at 408 nm and a formation constant of about 2.5 X 10(4) M(-1), and combine with CO to give a CO-cytochrome P-450 type spectrum. Kinetics of CO binding to mercaptide-heme complexes [RS-heme] have been studied by the flash photolysis method. Characteristic constants for this reaction suggest close similarities between [CH3-(CH2)3-S-heme] and cytochrome P-450. The reaction of alkoxide anion with heme has also been examined but no evidence was found for the existence of the [RO-heme-CO[ species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brault D., Rougee M. Ferrous porphyrins in organic solvents. II. Optical spectra and paramagnetic susceptibilities. Biochemistry. 1974 Oct 22;13(22):4598–4602. doi: 10.1021/bi00719a020. [DOI] [PubMed] [Google Scholar]
- Brinigar W. S., Chang C. K. Simple dioxygen heme complexes formed in N,N-dimethylformamide. J Am Chem Soc. 1974 Aug 21;96(17):5595–5597. doi: 10.1021/ja00824a060. [DOI] [PubMed] [Google Scholar]
- Caughey W. S. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970 Oct 5;174(1):148–153. doi: 10.1111/j.1749-6632.1970.tb49781.x. [DOI] [PubMed] [Google Scholar]
- Champion P. M., Münck E., Debrunner P. G., Hollenberg P. F., Hager L. P. Mössbauer investigations of chloroperoxidase and its halide complexes. Biochemistry. 1973 Jan 30;12(3):426–435. doi: 10.1021/bi00727a011. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Dolphin D. Letter: Ferrous porphyrin-mercaptide complexes. Models for reduced cytochrome P-450. J Am Chem Soc. 1975 Oct 1;97(20):5948–5950. doi: 10.1021/ja00853a069. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Dolphin D. Letter: Oxygen binding to mercaptide-heme complexes. Models for reduced cytochrome P-450. J Am Chem Soc. 1976 Mar 17;98(6):1607–1609. doi: 10.1021/ja00422a069. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Traylor T. G. Kinetics of oxygen and carbon monoxide binding to synthetic analogs of the myoglobin and hemoglobin active sites. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1166–1170. doi: 10.1073/pnas.72.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. K., Traylor T. G. Reversible oxygenation of protoheme-imidazole complex in aqueous solution (1, 2) Biochem Biophys Res Commun. 1975 Feb 3;62(3):729–735. doi: 10.1016/0006-291x(75)90460-x. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Traylor T. G. Synthesis of the myoglobin active site. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2647–2650. doi: 10.1073/pnas.70.9.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang R., Makino R., Spomer W. E., Hager L. P. Chloroperoxidase: P-450 type absorption in the absence of sulfhydryl groups. Biochemistry. 1975 Sep 23;14(19):4166–4171. doi: 10.1021/bi00690a003. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Reed C. A., Halbert T. R., Lang G., Robinson W. T. "Picket fence porphyrins." Synthetic models for oxygen binding hemoproteins. J Am Chem Soc. 1975 Mar 19;97(6):1427–1439. doi: 10.1021/ja00839a026. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Sorrell T. N., Hoffman B. M. Letter: Models for cytochrome P-450. J Am Chem Soc. 1975 Feb 19;97(4):913–914. doi: 10.1021/ja00837a050. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Sorrell T. N. Letter: A model for the carbonyl adduct of ferrous cytochrome P450. J Am Chem Soc. 1975 Jul 9;97(14):4133–4134. doi: 10.1021/ja00847a046. [DOI] [PubMed] [Google Scholar]
- Coughey W. S., Alben J. O., McCoy S., Boyer S. H., Charache S., Hathaway P. Differences in the infrared stretching frequency of carbon monoxide bound to abnormal hemoglobins. Biochemistry. 1969 Jan;8(1):59–62. doi: 10.1021/bi00829a009. [DOI] [PubMed] [Google Scholar]
- Debey P., Hui-Bon-Hoa G., Douzou P. Low temperature studies of microsomal cytochrome P450. I. Stopped-flow experiments. FEBS Lett. 1973 Jun 1;32(2):227–230. doi: 10.1016/0014-5793(73)80838-5. [DOI] [PubMed] [Google Scholar]
- Hanson L. K., Eaton W. A., Sligar S. G., Gunsalus I. C., Gouterman M., Connell C. R. Letter: Origin of the anomalous Soret spectra of carboxycytochrome P-450. J Am Chem Soc. 1976 Apr 28;98(9):2672–2674. doi: 10.1021/ja00425a050. [DOI] [PubMed] [Google Scholar]
- Hollenberg P. F., Hager L. P. The P-450 nature of the carbon monoxide complex of ferrous chloroperoxidase. J Biol Chem. 1973 Apr 10;248(7):2630–2633. [PubMed] [Google Scholar]
- KEILIN J. On the properties and nature of dihydroxyl-haem. Biochem J. 1949;45(4):448–455. doi: 10.1042/bj0450448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEILIN J. The reactions of haems with cyanides and isocyanides. Biochem J. 1949;45(4):440–448. doi: 10.1042/bj0450440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S., Tang S. C., Holm R. H., Frankel R. H., Ibers J. A. Letter: Ferric porphyrin thiolates. Possible relationship to cytochrome P-450 enzymes and the structure of (p-nitrobenzenethiolato)iron(III) protoporphyrin IX dimethyl ester. J Am Chem Soc. 1975 Feb 19;97(4):916–918. doi: 10.1021/ja00837a052. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Peterson J. A., Griffin B. W. Carbon monoxide binding by Pseudomonas putida cytochrome P-450. Arch Biochem Biophys. 1972 Aug;151(2):427–433. doi: 10.1016/0003-9861(72)90518-8. [DOI] [PubMed] [Google Scholar]
- Rougee M., Brault D. Binding of carbon monoxide by deuteroheme in organic solvents. Evidence for the formation of dicarbonyldeuteroheme in non-coordinating solvents. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1364–1369. doi: 10.1016/s0006-291x(73)80044-0. [DOI] [PubMed] [Google Scholar]
- Stern J. O., Peisach J. A model compound study of the CO-adduct of cytochrome P-450. J Biol Chem. 1974 Dec 10;249(23):7495–7498. [PubMed] [Google Scholar]
- Tsai R., Yu C. A., Gunsalus I. C., Peisach J., Blumberg W., Orme-Johnson W. H., Beinert H. Spin-state changes in cytochrome P-450cam on binding of specific substrates. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1157–1163. doi: 10.1073/pnas.66.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]



