Abstract
Background
Since the advent and the success of adjuvant medical therapy for human epidermal growth factor receptor 2 (HER2)-positive breast cancer in the form of trastuzumab there has been increasing interest in the development of similar therapies in other solid organ malignancies including gastric cancer and oesophageal cancer. Over the years, multiple observational studies have been inconsistent. Several meta-analyses have been published looking at the association between HER2 and gastric cancer and oesophageal cancer. This review aims to summarize the meta-analytic evidence for the association between HER2 in gastric and oesophageal cancer.
Methods
A systematic search was conducted using MEDLINE, PubMed, EMBASE, Current Contents Connect, Cochrane Library, Google Scholar, Science Direct, and Web of Science.
Results
Of the articles selected, only nine studies met full criteria. Six of them reviewed the role of HER2 in gastric cancer and the remaining three reviewed its role in oesophageal cancer.
Conclusions
The current evidence regarding the role of HER2 is unclear. However, it clearly plays a key role in the pathogenesis of gastric and oesophageal carcinomas. Targeted therapy towards this subgroup (despite variable frequency and association with survival) would offer a mortality benefit and improve survival.
Keywords: Human epidermal growth factor receptor 2 (HER2), gastric cancer, esophageal cancer
Introduction
Recently, several meta-analyses have been published regarding the role of human epidermal growth factor receptor 2 (HER2) oncogene in gastric and oesophageal cancer. HER2 encrypts for a 185 KD transmembrane glycoprotein receptor with intracellular tyrosine kinase activity and is positioned at the long arm of human chromosome 17 (17q12) (1), and was first discovered in breast cancer and has become an important prognostic factor (2,3). Since the advent and the success of adjuvant medical therapy for HER2-positive breast cancer in the form of trastuzumab there has been increasing interest in the development of similar therapies in other solid organ malignancies. It has been assessed in various other solid organ malignancies including gastric cancer (4), oesophageal cancer (5), colorectal cancer (6-8), osteosarcoma (9), ovarian cancer (10,11), prostate cancer (12), lung cancer (13), pancreatic cancer (14), bladder cancer (15), and uterine cancer (16). In gastric and oesophageal cancer the incidence of HER positive tumours range from 4% to 53% (17) and 9% to 64% (18). However, multiple observational studies have been inconsistent with regards to its correlation with survival. A major breakthrough in targeted therapy in gastric cancer was the ToGA trial (19). This multicentre randomized trial of 594 gastric cancer patients demonstrated an increase of 2.7 months in the median overall survival (OS) with trastuzumab and a recent meta-analysis (20) of randomized control trials suggested an improvement in overall and progression free survival with the addition of trastuzumab to chemotherapy. The most important issue is that some of the subsequent meta-analyses published produce conflicting results. This review aims to summarize the meta-analytic evidence for the association between HER2 in gastric and oesophageal cancer.
Methods
Search strategy
The search strategy involved the major computer databases, including Medline, PubMed, EMBASE and Current Contents (January 1983 to November 2014). The search methodology involved using combinations of the following keywords: HER2, gastric cancer/carcinoma/ adenocarcinoma, stomach cancer, meta-analysis, systematic review, oesophageal cancer/carcinoma/adenocarcinoma/squamous cell carcinoma. Additional manual searches were made using the reference lists from the selected articles to retrieve other papers relevant to the topic. No language restriction was placed on any of the literature searches.
Inclusion criteria
We included studies that met the following inclusion criteria:
❖ Meta-analyses and systematic reviews on the role of HER2 in gastric and oesophageal cancer.
Results
Overall
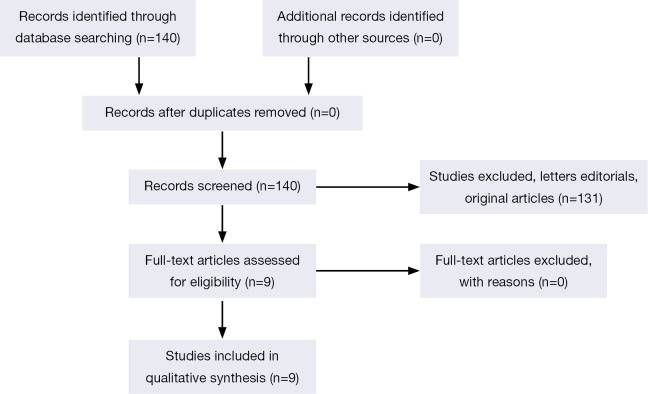
The original search strategy retrieved 140 citations (Figure 1). The abstracts were reviewed and after applying the inclusion and exclusion criteria, articles were selected for full-text evaluation. Of the articles selected, only 9 studies (4,5,17,18,21-25) met full criteria for analysis and are summarised in Table 1. The years of publication ranged from 2011 to 2014.
Figure 1.
Flow of included studies.
Table 1. Summary of all systematic review/meta-analyses regarding the association between HER2 and gastric cancer oesophageal cancer.
| Author | Journal | Cancer | Country | Literature search | Inclusion criteria | Language | Software | Publication bias | Model | Number of studies | Sample size | Correlation of HER2 with survival | Correlation of HER2 with clinicopathological parameters | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jørgensen et al. 2012 (25) | Journal of Cancer | Gastric | Denmark | PubMed from 1986 to August 2011 | (I) The number of patients studied should be ≥100 and the HER2 status should have been determined by IHC or ISH such as FISH or CISH; (II) the selected articles should include an analysis of the association between the HER2 status and survival, e.g., OS and/or DFS and/or relevant clinicopathological characteristics such as serosal invasion, vascular invasion, lymph node involvement, distant metastases, disease stage, etc. |
English articles only | Systematic review only | Systematic review only | Systematic review only | 42 | 12,749 | HER2-postive status was associated with poor survival | Serosal invasion, lymph node metastases, disease stage, or distant metastases | HER2 as a negative prognostics factor in gastric cancer |
| Chen et al. 2013 (22) | Archives of Medical Research | Gastric | China | PUBMED (2007 May 1, 2013), MEDLINE (2007-May 1, 2013) and EMBASE (2007-May 1, 2013), Cochrane Library (no date restriction) |
(I) Proven diagnosis of GC in humans; (II) HER2 evaluation using special methods (e.g., PCR, IHC and FISH); and (III) correlation of HER2 with OS |
English articles only | STATA 11.0 | Egger’s test | Fixed effects model | 8 | 2,376 | HR 1.43 (95% CI: 1.09-1.88) | Not performed | HER2 have significant predictive ability for estimating OS |
| Gu et al. 2014 (23) | Tumor Biology | Gastric | China | EMBASE (Jan 2008 to Nov 2013), Medline (Jan 2008 to Nov 2013), PubMed (Jan 2008 to Nov 2013), and Scopus (Jan 2008 to Nov 2013) |
(I) patients had a diagnosis of GC; (II) OS and/or RFS were analyzed/ stratified by HER2 status; (III) The HER2 expression was detected by IHC and ISH approaches, with HER2 expression (positivity) defined as IHC score of 3+ or IHC score of 2+ plus HER2 gene amplification as detected by FISH or other ISH methods |
English articles only | RevMan 5.2 analysis software (Cochrane Collaboration, Copenhagen, Denmark) | Funnel plot | Random effects model | 11 | 4,569 | HR 0.97; (95% CI: 0.84-1.12) univariate analysis, 1.01; (95% CI: 0.75-1.35) multivariate analysis | ORs (95% CIs) for HER2 positivity were 2.31 (95% CI: 1.59-3.36) for male patients, 1.80 (95% CI: 1.38-2.35) for tumor location at upper location or GEJ adenocarcinoma, 5.32 (95% CI: 3.95-7.17) for well/moderately differentiated tumor, and 5.55 (95% CI: 4.01-7.67) for intestinal-type tumor | HER2 expression based on ToGA criteria is not related to the survival in patients with gastric cancer |
| Liang et al. 2014 (21) | Tumor Biology | Gastric | China | PubMed, Ovid, Web of Science, and Cochrane from 1990 to July 31, 2013 | (I) proven diagnosis of gastric cancer; (II) HER2 expression evaluation using special methods such as IHC, FISH or other methods; (III) provided information on clinicopathological results and OS; and (IV) either one of the higher quality or the most recent study was included when two studies were published by the same institution or authors | English articles only | STATA 12.0 | Begg’s funnel plot and Egger’s test | Random effects model | 15 | 5,290 | HR, 1.56; 95% CI: 1.05-2.07 | Bormann type (OR: 1.76; 95% CI: 1.19-2.59; Z=2.85; P=0.004), tumor differentiation (OR: 3.14; 95% CI: 1.91-5.17; Z=4.49; P= 0.000), Lauren’s classification (OR: 6.25; 95% CI: 4.29-9.10; Z=9.54; P=0.000), lymph node metastasis (OR: 1.43; 95% CI: 1.15-1.77; Z=3.23; P=0.001), venous invasion (OR: 1.69; 95% CI: 1.15-2.48; Z=2.67; P=0.008), and lymphovascular invasion (OR: 1.57; 95% CI: 1.21-2.04; Z=3.4; P=0.001) | HER2 overexpression had an unfavourable prognostic role for patients with gastric cancer. HER2-positive expression was associated with Bormann type, Lauren’s classification, tumour differentiation, lymph node status, venous invasion, and lymphovascular invasion |
| Chua et al. 2012 (17) | International Journal of Cancer | Gastric | Australia | MEDLINE and PubMed databases January 1990 to January 2011 | HER2 protein expression evaluation in primary gastric cancer tissue (surgical or biopsy specimens) as opposed to serum or metastatic tissue. Metastatic tissues were excluded as there are insufficient data in the literature correlating the overexpression of metastases versus the primary tumor and the potential effects of chemotherapy on augmentation of HER2 expression. HER2 protein expression evaluated by any method, gastric cancer evaluated using conventional histopathological diagnosis, correlation of HER2 protein overexpression with clinicopathologic markers and the association of HER2 protein overexpression on DFS and OS | English articles only | Systematic review only | Systematic review only | Systematic review only | 49 | 11,337 | In patients with and without HER2 overexpression, the median 3-year DFS rate was 58% (range, 50-88%) and 86% (range, 62-97%), respectively | Of the 35 studies reporting the impact of HER2 overexpression on survival, 20 studies (57%) reported no difference in OS, two studies (6%) reported significantly longer OS in patients with HER2 overexpression and 13 studies (37%) reported significantly poorer OS in patients with HER2 overexpression. The median OS and 5-year survival rate was 21 (range, 10-57) months and 42%, and 33 (range, 13-80) months and 52% in patients with and without HER2 overexpression, respectively | HER2 overexpression appears to be associated with poorer survival and with intestinal-type gastric cancer in this group of patients for whom majority undergone curative gastrectomy |
| Wang et al. 2011 (4) | Asian Pacific Journal of Cancer Prevention | Gastric | China | MEDLINE, PubMed, EMBASE and Chinese database of National Knowledge Infrastructure (CNKI) and WANFANG DATA search | (I) Patients included had surgery and their disease was identified as gastric cancer by postoperative pathological check; (II) the endpoint investigated was disease specific or OS; (III) the study reported a HR survival rate or data sufficient to estimate the HR; (IV) all publications are limited to using English and Chinese |
English and Chinese | STATA 11.0 | Egger’s and Begg’s test | Random effects model | 19 | 4,342 | HR, 1.58, 95% CI: 1.20-2.12 | Not performed | HER-2/neu over-expression is related to poor prognosis of gastric cancer but has a modest effect on survival in gastric cancer as an independent prognosis factor |
| Gowryshankar et al. 2014 (5) | Journal of Gastrointestinal Oncology | Oesophageal | Australia | MEDLINE (from 1950), PubMed (from 1946), EMBASE (from 1949), PubMed (from 1950), and Current Contents Connect (from 1980) through to 2013 |
(I) HER2 positivity was measured in subjects with BE; (II) HER2 positivity was measured in subjects with EC; (III) diagnostic method was reported; (IV) prevalence of HER2 in BE or EC was reported |
No language restrictions | Comprehensive Meta-analysis (version 2.0) | Egger’s test | Random effects model | 23 | 2,319 | HR, 1.45; 95% CI: 0.85-2.48 | Not performed | HER2+ appears to decrease the survival time of EC patients |
| Chan et al. 2012 (18) | Journal of Gastrointestinal Surgery | Oesophageal | United Kingdom | Medline and Embase (January 1990 to November 2011) | Translational studies comparing OS outcomes in patients with operable oesophageal cancer with and without HER2 overexpression or gene amplification | English articles only | ReviewManager 5.1 | Funnel plot | Random effects model | 14 | 1,464 | Five-year mortality was significantly higher in HER2-positive patients (OR: 1.43; 95% CI: 1.04-1.95; P=0.03. Analysis related to histological cell type demonstrated significantly higher 5-year mortality in HER2-positive squamous cell carcinoma (OR: 2.88; 95% CI: 1.34-6.17; P=0.006) and adenocarcinoma (OR: 1.91; 95% CI: 1.15-3.17; P=0.01) on sensitivity analysis of higher-quality studies | Not performed | HER2 overexpression and gene amplification in operable oesophageal cancer was an indicator of poor prognosis |
| Chen et al. 2013 (24) | BMC Cancer | Oesophageal | China | PubMed | Criteria used to determine study eligibility were as follows: (I) a prospective or retrospective cohort design with a well-defined study population and justification for all excluded eligible cases; (II) assay of the primary EC specimens; (III) a clear description of methods for specimen handling and testing, including selection and preparation of reagents or kits, as well as visualization techniques; (IV) clear statements on the choice of positive/present and negative/absent controls and on assay validation; (V) statistical analysis using multivariable proportional hazards modeling that adjusted for clinical prognostic factors; and (VI) reporting of the resultant adjusted HRs and their 95% CIs, or provision of data available for statistical estimation of HRs |
No language restrictions | STATA SE 11.0 software | Begg’s adjusted rank correlation test and by Egger’s regression asymmetry test | Random effects model | 6 | 1,162 | HER2 expression in adenocarcinoma and had a pooled HR of 2.15 (95% CI: 1.39-3.33) with no evidence of heterogeneity. Two studies assessed HER2 expression in an oesophageal cancer setting with a pooled HR of 0.91 (95% CI: 0.73-1.12) | Not performed | HER2 potential predictor of outcome |
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; FISH, fluorescence in situ hybridization; CISH, chromogenic in situ hybridization; DFS, disease-free survival; OS, overall survival; RFS, relapse-free survival; HR, hazard ratio; GEJ, gastroesophageal junction; OR, odds ratio.
HER2 and gastric cancer
There have been six systematic reviews published so far regarding the role of HER2 in gastric cancer (4,17,21-23,25). Most of the studies were from China (Table 1). A recent meta-analysis of eight studies (22) reported that HER2 had significant predictive ability for estimating OS with a hazard ratio (HR: 1.43; 95% CI: 1.09-1.88) and was associated with moderate heterogeneity (I2=52.90, P=0.038) and no publication bias (P=0.256). Similarly, Wang et al. (4) included 4,342 gastric cancer cases which suggested that HER2 was poor prognostic feature with a HR of 1.59 (95% CI: 1.20-2.12) and was associated with moderate heterogeneity (I2=48.10, P=0.019) and no publication bias (P=0.081). Liang et al. (21) suggested that HER2 overexpression was linked with Bormann type (I + II), well differentiated, intestinal type, lymph node metastasis, venous invasion, and lymphovascular invasion. Nevertheless, it had no relationship with depth of invasion, tumour size and stage. On the contrary, HER2 was significantly associated with patients’ OS. A recent multicentre study consisting of 1,148 gastric cancer patients who underwent gastrectomy in eleven institutes across Japan found HER2 overexpression to be an important predictive factor in patients with any stage of operable gastric cancer (26).
A robust publication from Gu et al. (23) included only publications that classified HER2 expression based on ToGA criteria (27). This study demonstrated that relapse-free survival (RFS) as well as OS was not related to HER2 expression. The heterogeneity among the studies was low to moderate. The pooled odds ratio (OR) for HER2 positivity was linked to being male (OR: 2.31; 95% CI: 1.59-3.36), well/moderately differentiated tumour (OR: 5.32; 95% CI: 3.95-7.17), and for intestinal-type tumour (OR: 5.55; 95% CI: 4.01-7.67). Jørgensen et al. (25) published a systematic review with 12,749 patients which suggested HER2 positive tumours were associated with poor survival, serosal invasion, lymph node metastases and distant metastases. Finally, Chua et al. (17) published an excellent review of 49 studies. Out of which 35 studies reported the influence of HER2 overexpression on survival. Among these only two studies stated considerably longer OS in patients with HER2 overexpression and 13 studies reported significantly poorer OS in patients with HER2 overexpression.
HER2 and oesophageal cancer
The role of HER2 has also been well investigated in oesophageal cancer. Chan et al. (18) reviewed the effect of HER2 expression in surgically resectable oesophageal carcinoma which included 1,464 patients. The 5-year mortality rate was considerably greater in HER2-positive patients with an OR of 1.43 (95% CI: 1.04-1.95). However, there was significant heterogeneity among the studies and publication bias was evident. The effect of HER2 positivity was greater in squamous cell carcinoma (OR: 2.88; 95% CI: 1.34-6.17; I2=0.00; P=0.52) compared to adenocarcinoma (OR: 1.91; 95% CI: 1.15-3.17; I2=78.00; P=0.001) with respect to 5-year mortality. A recent meta-analysis of 2,319 oesophageal cancer patients of all stages demonstrated that HER2 positive tumours survived 7 months less than HER2 negative tumours (5), however, this was not statistically significant.
Discussion
Gastric and oesophageal cancers result in 700,000 and 386,000 deaths every year respectively (28). HER2 plays an important role in the aggressiveness and progression of gastric (21,29) and oesophageal cancer (18). The overall direction of the meta-analyses for gastric cancer (4,17,21-23) suggests HER2 as a poor prognostic factor and is associated with poor OS. However, Gu et al. (23) published that RFS and OS were not related to HER2 expression. Gu et al. (23) included only publications that classified HER2 expression based on ToGA criteria making the analysis robust (27). As per Hofmann et al. (27) the definition for HER2 positivity was reclassified from IHC 2+ or 3+ or amplification in FISH to IHC 3+ or IHC 2+ and hence the conclusion from Gu et al. (23) were significantly different from the other meta-analyses. HER2 positive tumours are more likely to be well differentiated, intestinal-type, with lymphovascular invasion and more common in men. As far as oesophageal cancer is concerned (5,18), HER2 positive tumours have negative impact on survival and have a higher 5-year mortality rate.
Strengths and limitations
Gu et al. (23) searched the multiple databases including PubMed, EMBASE, Scopus, Medline between the dates Jan 2008 to Nov 2013 only, a significant drawback of the meta-analysis. Similarly, Chen et al. (22) searched only from 2007-May 1, 2013. Chen et al. (24), Chua et al. (17), and Chan et al. (18) explored only one or two databases for studies. The search strategy could explain the variability in the number of studies included among the meta-analyses and the inconsistency of the ORs for the various outcomes and heterogeneity among the meta-analyses. Gu et al. (23) did not describe a search strategy was and manual searches were not stated openly. Publication language in these meta-analyses was restricted to English only which introduces a language bias (17,21-23,25).
A strict inclusion/exclusion criterion was lacking in Gu et al. (23). The publication bias in these meta-analyses (18,23) was assessed by visual examination of the funnel plot which was unsuitable as the number of studies were less than ten and this was acknowledged only by Chan et al. (18). Inverse variance (IV) random-effects model was utilized in this meta-analysis which does not consider both within- and between-study variations (23) and Chen et al. (22) used fixed effects model. The authors suggest that the DerSimonian and Laird random-effects model (30) should be utilised to obtain a more accurate estimate and the confidence interval. Lastly, only three meta-analyses (17,21,23) performed stratified analysis according to the clinicopathological parameters of tumours this could be due to insufficient information acquired from the included studies.
Conclusions
It is essential for a good meta-analysis to have a through database search (preferably multiple) and avoid language bias. With the available data the authors suggest that future prospective studies should use ToGA criteria (27) to assess the HER2 status and large studies are the need of the hour to confirm the evidence. HER2 status clearly plays a key role in the pathogenesis of gastric and oesophageal carcinomas. Targeted therapy towards this subgroup (despite variable frequency and association with survival) would offer a mortality benefit and improve survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985;229:974-6. [DOI] [PubMed] [Google Scholar]
- 2.Walker RA, Bartlett JM, Dowsett M, et al. HER2 testing in the UK: further update to recommendations. J Clin Pathol 2008;61:818-24. [DOI] [PubMed] [Google Scholar]
- 3.Penault-Llorca F, Bilous M, Dowsett M, et al. Emerging technologies for assessing HER2 amplification. Am J Clin Pathol 2009;132:539-48. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Zheng G, Chen L, et al. Effect of HER-2/neu over-expression on prognosis in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2011;12:1417-23. [PubMed] [Google Scholar]
- 5.Gowryshankar A, Nagaraja V, Eslick GD. HER2 status in Barrett's esophagus & esophageal cancer: a meta analysis. J Gastrointest Oncol 2014;5:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han J, Meng QY, Liu X, et al. Lack of effects of HER-2/neu on prognosis in colorectal cancer: a meta-analysis. Asian Pac J Cancer Prev 2014;15:5551-6. [DOI] [PubMed] [Google Scholar]
- 7.Wu SW, Ma CC, Yang Y. The prognostic value of HER-2/neu overexpression in colorectal cancer: evidence from 16 studies. Tumour Biol 2014;35:10799-804. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Liu DR, Ye LY, et al. HER-2 overexpression and survival in colorectal cancer: a meta-analysis. J Zhejiang Univ Sci B 2014;15:582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Ma YH, Sun ZZ, et al. Effect of c-erbB2 overexpression on prognosis in osteosarcoma: evidence from eight studies. Tumour Biol 2014;35:8939-43. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang D, Ren M.Prognostic value of HER-2/neu expression in epithelial ovarian cancer: a meta-analysis. Tumour Biol 2014;35:33-8. [DOI] [PubMed] [Google Scholar]
- 11.de Graeff P, Crijns AP, de Jong S, et al. Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer 2009;101:149-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neto AS, Tobias-Machado M, Wroclawski ML, et al. Her-2/neu expression in prostate adenocarcinoma: a systematic review and meta-analysis. J Urol 2010;184:842-50. [DOI] [PubMed] [Google Scholar]
- 13.Meert AP, Martin B, Paesmans M, et al. The role of HER-2/neu expression on the survival of patients with lung cancer: a systematic review of the literature. Br J Cancer 2003;89:959-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aumayr K, Soleiman A, Sahora K, et al. HER2 gene amplification and protein expression in pancreatic ductal adenocarcinomas. Appl Immunohistochem Mol Morphol 2014;22:146-52. [DOI] [PubMed] [Google Scholar]
- 15.Wülfing C, von Struensee D, Bierer S, et al. Expression of Her2/neu in locally advanced bladder cancer: implication for a molecular targeted therapy. Aktuelle Urol 2005;36:423-9. [DOI] [PubMed] [Google Scholar]
- 16.Teplinsky E, Muggia F.Targeting HER2 in ovarian and uterine cancers: Challenges and future directions. Gynecol Oncol 2014;135:364-70. [DOI] [PubMed] [Google Scholar]
- 17.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer 2012;130:2845-56. [DOI] [PubMed] [Google Scholar]
- 18.Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal cancer. J Gastrointest Surg 2012;16:1821-9. [DOI] [PubMed] [Google Scholar]
- 19.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [DOI] [PubMed] [Google Scholar]
- 20.Luo HQ, Han L, Jiang Y. Meta-analysis of six randomized control trials of chemotherapy plus anti-HER monoclonal antibody for advanced gastric and gastroesophageal cancer. Asian Pac J Cancer Prev 2014;15:5343-8. [DOI] [PubMed] [Google Scholar]
- 21.Liang JW, Zhang JJ, Zhang T, et al. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumour Biol 2014;35:4849-58. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Yang JM, Hu TT, et al. Prognostic role of human epidermal growth factor receptor in gastric cancer: a systematic review and meta-analysis. Arch Med Res 2013;44:380-9. [DOI] [PubMed] [Google Scholar]
- 23.Gu J, Zheng L, Wang Y, et al. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol 2014;35:5315-21. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Huang J, Zhu Z, et al. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer 2013;13:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer 2012;3:137-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurokawa Y, Matsuura N, Kimura Y, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [DOI] [PubMed] [Google Scholar]
- 28.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [DOI] [PubMed] [Google Scholar]
- 29.Hsu JT, Chen TC, Tseng JH, et al. Impact of HER-2 overexpression/amplification on the prognosis of gastric cancer patients undergoing resection: a single-center study of 1,036 patients. Oncologist 2011;16:1706-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]