Abstract
Background
The current study is the first to examine the effectiveness and toxicity of postoperative intensity-modulated radiotherapy (IMRT) in the treatment of intrahepatic cholangiocarcinoma (ICC) abutting the vasculature. Specifically, we aim to assess the role of IMRT in patients with ICC undergoing null-margin (no real resection margin) resection.
Methods
Thirty-eight patients with ICC adherent to major blood vessels were included in this retrospective study. Null-margin resection was performed on all patients; 14 patients were further treated with IMRT. The median radiation dose delivered was 56.8 Gy (range, 50-60 Gy). The primary endpoints were overall survival (OS) and disease-free survival (DFS).
Results
At a median follow-up of 24.6 months, the median OS and DFS of all patients (n=38) were 17.7 months (95% CI, 13.2-22.2) and 9.9 months (95% CI, 2.8-17.0), respectively. Median OS was 21.8 months (95% CI, 15.5-28.1) among the 14 patients in the postoperative IMRT group and 15.0 months (95% CI, 9.2-20.9) among the 24 patients in the surgery-only group (P=0.049). Median DFS was 12.5 months (95% CI, 6.8-18.2) in the postoperative IMRT group and 5.5 months (95% CI, 0.7-12.3) in the surgery-only group (P=0.081). IMRT was well-tolerated. Acute toxicity included one case of Grade 3 leukopenia; late toxicity included one case of asymptomatic duodenal ulcer discovered through endoscopy.
Conclusions
The study results suggest that postoperative IMRT is a safe and effective treatment option following null-margin resections of ICC. Larger prospective and randomized trials are necessary to establish postoperative IMRT as a standard practice for the treatment of ICC adherent to major hepatic vessels.
Keywords: Radiotherapy, intensity-modulated, intrahepatic cholangiocarcinoma (ICC), null-margin resection, postoperative radiotherapy
Introduction
Cholangiocarcinoma is a relatively rare heterogeneous malignancy, accounting for approximately 3% of all gastrointestinal tumors (1). The majority (>90%) of cholangiocarcinomas are adenocarcinomas and are classified based on anatomical location as intrahepatic and extrahepatic. Intrahepatic cholangiocarcinoma (ICC) arises in the epithelia of the small bile ducts in the liver (2), and are commonly well-demarcated mass-forming lesions within the liver parenchyma (3). There has been a 3-fold increase in the diagnosis of ICC from 1975 to 1999 (4) and a similar trend was observed in an independent study detailing the rise in ICC incidence from 1990 to 2006 (5).
Surgery remains the only curative treatment option. However, ICC resectability remains poor at 50% due to the advanced nature of the disease at presentation (1,6), and the 5-year survival rate is less than 5% in patients with unresectable disease (7). Furthermore, tumors close to or adherent to major hepatic vessels often do not permit wide-margin resections. Consequently, achieving a negative-margin resection (R0) remains a challenge, which, as a result, greatly compromises survival (6).
Traditionally, tumors involving the central regions of the liver (Couinaud segments IV, V, VIII) with vascular adherence were deemed unresectable (8,9). These patients also make poor candidates for locoregional treatment options such as radiofrequency ablation, which is not suitable for tumors abutting major vasculature (10). While surgeries are performed with curative intent, R1 resections frequently occur, especially in patients with advanced disease or tumors juxtaposed to large vessels, and are associated with high operative morbidity; for these reasons, several studies have advised against R1 resections (11-13). Advances in surgical techniques and preoperative planning have led to the development of null-margin (no real resection margin) resections, a special type of R1 resection, where surgeons carefully dissect and peel the tumor away from the vascular surface (14,15). Null-margin resections of centrally located liver lesions have been reported to be as safe as conventional R0 surgeries (14,15). We have also begun performing null-margin resections of ICC at our hospital and have found it to be a safe procedure. Given the lack of free resection margin and the presence of microscopic residual tumor, the present study was designed to investigate the role of postoperative intensity-modulated radiotherapy (IMRT) in ICC patients undergoing null-margin resections with the goal of improving outcome.
Methods
Patient selection
A retrospective cohort study was conducted using data from the Cancer Hospital and Institute of the Chinese Academy of Medical Science. The inclusion criteria for the study were as follows: (I) patients with centrally located ICC that has adhered to major blood vessels (e.g., the inferior vena cava, the portal vein and associated branches, and trunks of the hepatic veins) and has undergone null-margin resection; (II) histological confirmation of ICC; (III) complete macroscopic removal of tumor and an absence of gross tumors confirmed by intraoperative ultrasonography; (IV) absence of ICC related treatments prior to surgery; (V) absence of a second primary malignancy; (VI) Eastern Cooperative Oncology Group Performance Status ≤1. Written informed consent in accordance with the Declaration of Helsinki was obtained from each patient. The study was approved by the institutional ethics committee.
Between June 2008 and December 2013, 38 patients were enrolled into this study. Fourteen patients received IMRT as the only postoperative treatment; the remaining 24 patients did not receive any postoperative therapy. The decision to enroll patients into radiotherapy was based on the physician’s discretion, influenced by the patient’s performance status, and the patient’s own willingness. All patients were managed by the same surgical team or radiotherapy team to maintain consistency in treatment quality.
Surgery
The same principles that guide surgical resections of hepatocellular carcinoma also apply to ICC (16). Intraoperative ultrasonography was used to map the tumor location relative to the vasculature. ICC size and location determined the extent of tumor resection. Null-margin hepatectomy with exposure of the tumor and vascular surface was performed as described in (15). In brief, selective and dynamic region-specific vascular occlusion was performed on all patients. In areas where the tumor adhered to major blood vessels, surgeons carefully peeled lesions away from the vascular surface, resulting in a macroscopic 0-mm resection margin (14,15,17). A Cavitron ultrasonic surgical aspirator (CUSA®, Valleylab, Boulder, CO, USA) was used to avoid damage to the major vessels and to prevent postoperative liver failure. Four to six silver markers were stitched into the tumor bed to provide accurate orientation for postoperative radiotherapy. An example of an ICC tumor that has adhered to the portal vein is presented in Figure 1. Postoperative death was measured as death within 30 days of surgery.
Figure 1.
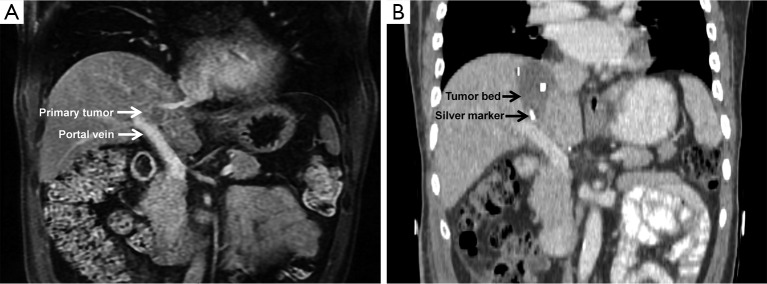
Representative case of an ICC patient undergoing null-margin resection. (A) Magnetic resonance imaging showed a 2 cm primary tumor located in Couinaud segment VIII that is adherent to the portal vein; (B) contrast computed tomography (CT) scan after surgery revealed silver markers stitched onto the tumor cutting bed used to orient the clinical tumor volume for IMRT planning. ICC, intrahepatic cholangiocarcinoma; IMRT, intensity-modulated radiotherapy.
Postoperative radiotherapy
IMRT-based treatment delivery was performed four to six weeks following surgical resection. Thermoplastic mask immobilization of the upper body was performed with the patient in supine position. Patients were scanned on a 16-slice computed tomography (CT) scanner (Brilliance 16, Philips Medical Systems, Cleveland, OH, USA). Preoperative magnetic resonance image (MRI) scans were used to optimize target and normal structure delineation using the Pinnacle3 9.0 treatment planning systems (Philips Healthcare, Andover, MA, USA).
The tumor cutting bed was indicated by silver markers applied during the operation. The clinical target volume (CTV) was defined as the tumor bed plus a 1.0 cm margin, or 1.5 cm margin in regions where the tumor adhered to major vascular structures. In patients with positive lymph node involvement, CTV also included the adjacent lymph node drainage stations in the porta hepatis, celiac axis, and pancreaticoduodenal ligament. To account for respiratory liver motion and set-up variations in 4-dimensional CT, the CTV was expanded by 0.5 cm in the anterior-posterior and medial-lateral directions, and by 1.0 cm in the craniocaudal direction to form the planning target volume (PTV). IMRT planning was used to ensure that the PTV was covered by the 95% isodose envelope while radiation fields were kept to a minimum to spare normal liver tissue. All treatments were delivered through three to four fields of 6 MV photons with the step-and-shoot technique on Elekta Synergy Linac (Elekta, Stockholm, Sweden).
Normal tissue dose-volume constraints were applied to minimize damage. Normal liver (total liver volume minus gross tumor volume) received a mean dose of ≤30 Gy. The maximum allowable point dose to the stomach and duodenum was set to ≤54 Gy, with the volume of organ receiving >45 Gy be <15%. If one kidney received >20 Gy, then >90% of the contralateral kidney would be excluded from radiation. The range of the prescribed dose was 50-60 Gy (median, 56.8 Gy) in 25-30 fractions of 2 Gy. Cone beam CT was used to assist online repositioning prior to treatment.
Follow-up and definition of toxicity
Patients were assessed every three months during the first two years, then every six months thereafter. Laboratory tests included liver function tests, routine blood and coagulation tests, chest X-Ray, CT and/or MRI of the abdomen. Patients were followed until death or until the censoring date (May, 2014).
Acute toxicity of IMRT was evaluated weekly during treatment and the first month following treatment. Late toxicity was defined as morbidity occurring at least one month after the completion of radiotherapy. Toxicities were scored according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
Statistical analysis
Overall survival (OS) was calculated as the number of months from the date of surgical resection to the date of death from any cause or last follow-up. Disease-free survival (DFS) was calculated as the number of months from the date of surgical resection to the date of intrahepatic or extrahepatic recurrence of ICC. Survival rates were calculated using the Kaplan-Meier analysis. Univariate analysis was performed on potential prognostic factors using the log-rank test. Categorical variables between the types of treatment (surgery-only vs. surgery plus IMRT) and clinical and demographic factors were assessed using the Chi-square test. Statistical significance was defined as P<0.05. All statistical analyses were performed using SPSS software, version 17 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
Thirty-eight (18 males and 20 females) patients with a median age of 55 years (range, 36-76 years) underwent null-margin resection. Nine patients were previously infected with hepatitis B virus (HBV) and one patient with hepatitis C virus (HCV). Patients mainly presented with moderately differentiated (47.4%) and poorly differentiated (44.7%) tumors, with the majority being solitary (89.5%). Tumors ≤5.0 cm in diameter were observed in 63.2% of patients. Positive lymph nodes were found in 34.2% of the cases.
Survival
The median follow-up time was 24.6 months (range, 5.1-65.6 months); 14 of 38 patients were still alive at the end of this follow-up, eight from the surgery-only group and six from the surgery plus IMRT group. Twenty of the 24 deaths were due to ICC recurrence; of the remaining four patients, one died three days after surgery due to operative hemorrhagic shock, two died of myocardial infarction, and one died of unknown cause. Overall, null-margin resection was well-tolerated, and postoperative mortality was 2.6%.
All patients (n=38) experienced a median OS of 17.7 months (95% CI, 13.2-22.2) and a median DFS of 9.9 months (95% CI, 2.8-17.0) (Figure 2). Univariate analysis revealed several factors to impact survival for all patients undergoing null-margin resections. Patient age ≤60 (P=0.023), carcinoembryonic antigen (CEA) ≤5.0 ng/mL prior to surgery (P=0.011), and postoperative IMRT (P=0.049) were all positively correlated with increased median OS. Likewise, preoperative CEA ≤5.0 ng/mL also improved median DFS (P=0.04). Other factors that prolonged DFS include tumor size ≤5.0 cm (P=0.011), early tumor stage (P=0.001), and negative lymph node status (P=0.002).
Figure 2.
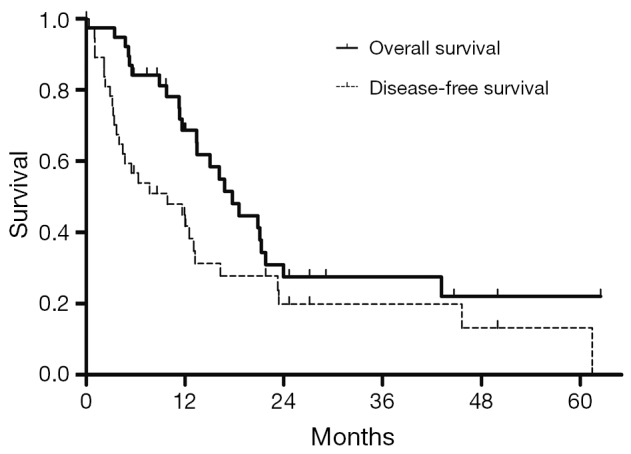
Overall survival (OS) and disease-free survival (DFS) of all patients (n=38).
Fourteen of the 38 patients received postoperative IMRT. All patients shared similar demographic and clinical features regardless of postoperative treatment (Table 1). The follow-up period ranged from 5.1 to 65.6 months (median, 19.0 months) in the surgery-only group and from 5.8 to 62.5 months (median, 25.4 months) in the surgery plus IMRT group. IMRT treatment was associated with a significant improvement in survival (P=0.049): median OS for the surgery-only and surgery plus IMRT groups were 15.0 months (95% CI, 9.2-20.9) and 21.8 months (95% CI, 15.5-28.1) (Figure 3A). Tumor recurrence occurred in 29 of 38 patients (76.0%), 19 from the surgery-only group and 10 from the surgery plus IMRT group (Table 2). Overall, recurrence appeared to occur earlier in the surgery-only group than the surgery plus IMRT group (P=0.081): median DFS for the surgery-only and surgery plus IMRT group were 5.5 months (95% CI, 0.7-12.3) and 12.5 months (95% CI, 6.8-18.2) (Figure 3B). While no patient receiving postoperative IMRT developed in-field failure, tumor recurrence at the resection margin occurred in a small portion of surgery-only patients (three cases, 12.5%). More than three quarters of intrahepatic recurrences in both groups were diffuse (≥2 lesions) in pattern. Intrahepatic recurrences (58.3%) dominated in the surgery-only group, while disease progression outside the liver (57.1%) was the main cause of recurrences in the surgery plus IMRT group.
Table 1. Patient characteristics based on type of treatment.
| Clinical characteristic | Surgery-only, n=24 (%) | Surgery plus IMRT, n=14 (%) | P |
|---|---|---|---|
| Age (years) | |||
| ≤60 | 16 (66.7) | 12 (85.7) | 0.268 |
| >60 | 8 (33.3) | 2 (14.3) | |
| Sex | |||
| Men | 10 (41.7) | 8 (57.1) | 0.357 |
| Women | 14 (58.3) | 6 (42.9) | |
| Hepatitis status | |||
| HBV (+) | 6 (25.0) | 3 (21.4) | 1 |
| HCV (+) | 0 (0) | 1 (7.1) | 0.368 |
| Pretreatment CEA (ng/mL) | |||
| ≤5.0 | 15 (62.5) | 10 (71.4) | 0.379 |
| >5.0 | 6 (25.0) | 1 (7.1) | |
| Unknown | 3 (12.5) | 3 (21.4) | |
| Tumor distribution | |||
| Solitary | 22 (91.7) | 12 (85.7) | 0.616 |
| Multifocal | 2 (8.3) | 2 (14.3) | |
| Tumor size (cm) | |||
| ≤5.0 | 14 (58.3) | 10 (71.4) | 0.42 |
| >5.0 | 10 (41.7) | 4 (28.6) | |
| Tumor grade | |||
| Well differentiated | 0 (0) | 0 (0) | 0.313 |
| Moderately differentiated | 12 (50.0) | 6 (42.9) | |
| Poorly differentiated | 9 (37.5) | 8 (51.7) | |
| Unknown | 3 (12.5) | 0 (0) | |
| Tumor stage (AJCC, 7th ed) | |||
| Stage I | 5 (35.7) | 8 (33.3) | 0.794 |
| Stage II | 1 (7.1) | 2 (8.3) | |
| Stage III | 4 (28.6) | 4 (16.7) | |
| Stage IVA | 4 (28.6) | 10 (41.7) | |
| Lymph node involvement | |||
| Negative | 14 (58.3) | 11 (78.6) | 0.294 |
| Positive | 10 (41.7) | 3 (21.4) |
HBV, hepatitis B virus; HCV, hepatitis C virus; CEA, carcinoembryonic antigen; AJCC, American Joint Committee on Cancer; IMRT, intensity-modulated radiotherapy.
Figure 3.
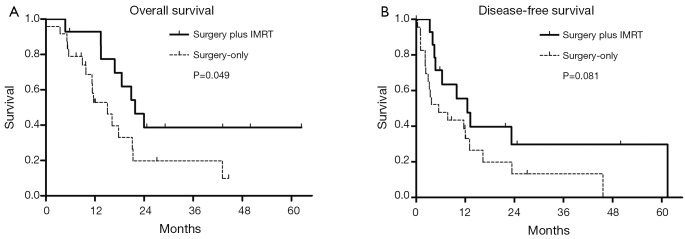
(A) Overall survival (OS) and (B) disease-free survival (DFS) of patients undergoing surgery-only (n=24) or surgery plus IMRT (n=14). IMRT, intensity-modulated radiotherapy.
Table 2. Incidence and patterns of recurrence.
| Pattern of recurrence | Surgery-only, n=24 (%) | Surgery plus IMRT, n=14 (%) | P |
|---|---|---|---|
| Intrahepatic | 14 (58.3) | 6 (42.9) | 0.478 |
| Extrahepatic | 12 (50.0) | 8 (57.1) | 0.357 |
| Total | 19 (79.2) | 10 (71.4) | 0.699 |
IMRT, intensity-modulated radiotherapy.
Toxicities
Radiotherapy was well-tolerated in general (Table 3). Three patients experienced grade 2 hematological toxicities (21.4%) and four patients experienced Grade 2 liver dysfunction (28.6%). Only one case of grade 3 hematological toxicity (leukopenia) was observed, and there were no grade 4 clinically relevant toxicities. All patients recovered from acute adverse reactions within three weeks following completion of IMRT. One case of late toxicity involving an asymptomatic duodenal ulcer discovered through endoscopy was observed.
Table 3. Acute toxicity of IMRT.
| Adverse event | N (%) |
||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Fatigue | 9 (64.3) | 4 (28.6) | 0 (0) | 0 (0) | 0 (0) |
| Dermatitis | 8 (57.1) | 5 (35.7) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal toxicities | 6 (42.9) | 7 (50.0) | 0 (0) | 0 (0) | 0 (0) |
| Liver dysfunction | 3 (21.4) | 6 (42.9) | 4 (28.6) | 0 (0) | 0 (0) |
| Hematological toxicities | 5 (35.7) | 4 (28.6) | 3 (21.4) | 1 (7.1) | 0 (0) |
IMRT, intensity-modulated radiotherapy.
Discussion
To our knowledge, this represents the first report of null-margin resection followed with IMRT in the treatment of ICC patients with tumors adherent to major vasculature. Compared to the surgery-only group, postoperative IMRT increased the median OS from 15.0 to 21.8 months (P=0.049), and appeared to improve the median DFS from 5.5 to 12.5 months (P=0.081). IMRT was well-tolerated with only one incident of grade 3 leukopenia.
Surgical resection is the only curative option in the treatment of ICC. However, owing to the frequently advanced nature of the disease at presentation and/or the tumor’s proximity to vasculature, a R0 resection is achieved in only 30% of ICC patients (6,12,18,19). In a retrospective analysis of 50 patients with locally advanced disease, R1 resection produced similar results as palliative treatment, and both were associated with poor survival (12). This report stressed the importance of preoperative assessment of resectability to avoid non-curative resections. Our study is aligned with this approach as we identified patients with tumors that were traditionally difficult to achieve a R0 resection due to tumor location, and implemented a technically demanding intraoperative procedure in conjunction with postoperative radiotherapy for thorough tumor removal. While patients were not cured of their disease, our results suggest that the addition of postoperative IMRT to null-margin resection provided a measurable improvement in survival over surgery alone.
Given the low incidence of ICC, few studies have examined postoperative radiotherapy as a treatment option (20,21). The largest analysis to date is a retrospective study of 3,839 patients from 1973 to 2003 based on the Surveillance, Epidemiology, and End Results (SEER) database (21). Median OS was 11 months in contrast to 6 months (P=0.014) for ICC patients treated with surgery and radiotherapy versus surgery alone (21). However, information regarding radiation dose, radiation technique, and surgical characteristics (e.g., resection margin status) were not recorded. The longer survival observed in the present cohort compared to the SEER report (21) is likely due to the utilization of IMRT, which allows effective doses of radiation to be delivered specifically to the tumor area while minimizing the volume of normal liver irradiated.
Very few studies have examined the role of combined chemotherapy and radiotherapy in the postoperative setting in the treatment of ICC. A study by Serafini et al. (20) of 92 patients with either extrahepatic cholangiocarcinoma (ECC) or ICC demonstrated a survival benefit from chemoradiotherapy (CRT) in distal ECC patients compared to observational control (median OS, 41 vs. 21 months, P=0.04); a similar trend was observed for ICC patients, but was not statistically significant.
Despite the uncertain role of adjuvant CRT in the treatment of ICC, more evidence exists to support its benefit in ECC. A single-institution, small retrospective study of 34 patients with adenocarcinoma of the distal bile duct treated with pancreaticoduodenectomy and adjuvant CRT (5-fluorouracil and external beam RT, median dose 50.4 Gy) showed improved survival compared to the historical control of surgery alone (median OS, 37 vs. 22 months, P<0.05) (22). Another retrospective study that reviewed 45 patients receiving fluoropyrimidine-based chemotherapy concurrently with external beam radiotherapy (median dose 50.4 Gy) before (n=12) or after (n=33) surgical resection recorded a high median OS of 34 months (23). Additionally, a retrospective study of 65 ECC patients from Borghero et al. (24) reported that administration of adjuvant CRT improved the survival outcome of high-risk R1 resections to that of standard-risk R0 resection (medial OS, 32 vs. 31 months, P=0.59). Given the benefit of postoperative radiotherapy in ICC patients shown in this study and the reported effectiveness of adjuvant CRT in the treatment of ECC, more studies are needed to further investigate whether the addition of chemotherapy to postoperative radiotherapy has the potential to improve survival in ICC patients.
There are a number of limitations in this report. As a retrospective study, patients were not randomized to the type of treatment. Patients with a satisfactory recovery from surgery may be unintentionally selected for radiotherapy treatment. Further, the rarity of the disease and even lower incidence of ICC abutting the vasculature resulted in a small patient sampling size. The effect of these drawbacks on outcome is not known and could possibly explain the lack of statistical significance of postoperative IMRT on DFS. These results should be prospectively validated in a larger study. Lastly, given the encouraging results in ECC and promising preliminary results in ICC, postoperative radiotherapy concurrent with chemotherapy should be further explored in the treatment of ICC.
Conclusions
The current study is the first to examine postoperative radiotherapy in the context of ICC tumors abutting major hepatic vessels. Our findings suggest that null-margin resection in combination with IMRT could prolong survival and may be appropriate in the treatment of ICC tumors that are centrally located and juxtaposed to large hepatic vascular structures. This provides support to conduct prospective and randomized trials to further determine the role of postoperative radiotherapy in the treatment of ICC adherent to major vasculature.
Acknowledgements
Authors’ contributions: AYJ designed the study, carried out the treatment planning, performed data analysis and prepared the manuscript. JXW designed the study and carried out the treatment planning. YTZ performed statistical analysis and manuscript revision. YXL, ZW, WQR, LMW, JJ, SLW, YWS, YPL, HR, HF, WQW, XFL, and ZHY participated in study design, patient treatment, data acquisition, data analysis and manuscript revision. WHW designed the study, carried out the treatment planning, supervised patient treatment, performed data analysis, and prepared the manuscript. All authors read and approved the final manuscript.
Disclosure: The authors declare no conflict of interest.
References
- 1.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994;14:109-14. [DOI] [PubMed] [Google Scholar]
- 2.Sia D, Tovar V, Moeini A, et al. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene 2013;32:4861-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sempoux C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis 2011;31:49-60. [DOI] [PubMed] [Google Scholar]
- 4.Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [DOI] [PubMed] [Google Scholar]
- 5.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [DOI] [PubMed] [Google Scholar]
- 6.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-73; discussion 473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [DOI] [PubMed] [Google Scholar]
- 8.Choi TK, Edward CS, Fan ST, et al. Results of surgical resection for hepatocellular carcinoma. Hepatogastroenterology 1990;37:172-5. [PubMed] [Google Scholar]
- 9.Nagorney DM, van Heerden JA, Ilstrup DM, et al. Primary hepatic malignancy: surgical management and determinants of survival. Surgery 1989;106:740-8; discussion 748-9. [PubMed] [Google Scholar]
- 10.Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9. [DOI] [PubMed] [Google Scholar]
- 11.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-29; discussion 830. [DOI] [PubMed] [Google Scholar]
- 12.Lang H, Sotiropoulos GC, Frühauf NR, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg 2005;241:134-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SY, Cherqui D. Operative management of cholangiocarcinoma. Semin Liver Dis 2013;33:248-61. [DOI] [PubMed] [Google Scholar]
- 14.Matsui Y, Terakawa N, Satoi S, et al. Postoperative outcomes in patients with hepatocellular carcinomas resected with exposure of the tumor surface: clinical role of the no-margin resection. Arch Surg 2007;142:596-602; discussion 603. [DOI] [PubMed] [Google Scholar]
- 15.Yu W, Rong W, Wang L, et al. R1 hepatectomy with exposure of tumor surface for centrally located hepatocellular carcinoma. World J Surg 2014;38:1777-85. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal S, Belghiti J.Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656-65. [DOI] [PubMed] [Google Scholar]
- 17.Miao XY, Hu JX, Dai WD, et al. Null-margin mesohepatectomy for centrally located hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology 2011;58:575-82. [PubMed] [Google Scholar]
- 18.Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Yuan L, Wang Y, et al. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg 2014;18:562-72. [DOI] [PubMed] [Google Scholar]
- 20.Serafini FM, Sachs D, Bloomston M, et al. Location, not staging, of cholangiocarcinoma determines the role for adjuvant chemoradiation therapy. Am Surg 2001;67:839-43; discussion 843-4. [PubMed] [Google Scholar]
- 21.Shinohara ET, Mitra N, Guo M, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2008;72:1495-501. [DOI] [PubMed] [Google Scholar]
- 22.Hughes MA, Frassica DA, Yeo CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys 2007;68:178-82. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JW, Ghafoori AP, Willett CG, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2009;73:148-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borghero Y, Crane CH, Szklaruk J, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol 2008;15:3147-56. [DOI] [PubMed] [Google Scholar]


