Abstract
Background
Sexual dysfunction is one of several factors related to medication compliance in patients taking antipsychotic medication but the magnitude of this problem is unknown.
Aim
Compare the self-reported sexual functioning of clinically stable patients with schizophrenia taking antipsychotic medication to that of healthy controls using the Turkish version of the 5-item Arizona Sexual Experience Scale (ASEX). This scale, which has previously been validated in Turkey, assesses 5 components of sexual function: sex drive, sexual arousal, vaginal lubrication/penile erection, ability to achieve orgasm, and satisfaction with orgasm.
Methods
The Scale for the Assessment of Positive Symptoms, the Scale for Assessment of Negative Symptoms, and ASEX were administered to 101 clinically stable outpatients with schizophrenia (38 females and 63 males). The ASEX was also administered to 89 control subjects (41 females and 48 males) without a history of mental illness. Respondents were classified as having sexual dysfunction if ASEX total score (range 5-30) >18, if any ASEX item score (range 1-6) ≥ 5, or if 3 or more ASEX items ≥4.
Results
Male patients with schizophrenia have significantly more self-reported sexual dysfunction than healthy controls (46% vs. 8%). The prevalence of sexual dysfunction is higher in female patients than in male patients (68% vs. 46%), but it was also very high in healthy female controls (68%), so the sexual dysfunction of female patients cannot be attributed to their illness or to the medications they are taking. Within the patient group, there was no significant relationship between the severity of positive or negative symptoms and the severity of sexual dysfunction, and the severity of sexual function was not different between patients taking first-generation or second-generation antipsychotic medications.
Conclusions
The very different findings by gender in Turkey highlights the importance of assessing location-specific and gender-specific sexual norms when trying to assess the role of mental illness and medications on sexual functioning. Prospective studies are needed to distinguish the relative importance of cultural norms, the schizophrenic illness, and the use of antipsychotic medication in the etiology and course of sexual dysfunction among individuals with schizophrenia.
Keywords: antipsychotic medication, schizophrenia, sexual dysfunction, sexual norms, Turkey
Abstract
背景
性功能障碍是影响患者对抗精神病药物服药依从性的因素之一,但尚不清楚这个问题的严重程度。
目的
比较服用抗精神病药物且临床症状稳定的精神分裂症患者和健康对照者自我报告的性功能状况。评估工具采用土耳其语版的5个条目的亚利桑那性体验量表(Arizona Sexual Experience Scale ,ASEX)。该量表的效度已在土耳其得到验证。量表评估性欲、性唤起、阴道润滑/阴茎勃起、达到高潮的能力以及对高潮的满意度等5个方面的性功能。
方法
采用阳性症状量表、阴性症状量表和ASEX分别对101例临床症状稳定的门诊精神分裂症患者(女性38例、男性63例)进行评估。对89例无精神疾病史的对照者(女性41名、男性48名)也进行了ASEX量表评估。如果ASEX总分(范围5-30)>18,或者任一条目得分(范围1-6)≥5,或者至少有3个条目得分都大于4,则被视为存在性功能障碍。
结果
自我报告有性功能障碍的男性精神分裂症患者多于健康对照者(46%对8%)。虽然女性患者性功能障碍的患病率显著高于男性患者(68%对46%),但是对照组中健康女性的性功能障碍患病率也非常高(68%),因而女性患者的性功能障碍不能归咎于她们的疾病或是正在服用的药物。患者组中,阳性症状和阴性症状的严重程度与性功能障碍无相关性,服用第一代抗精神病药的患者与服用第二代抗精神病药的患者之间的性功能障碍严重程度无明显差异。
结论
在土耳其,不同性别的精神分裂症患者性功能状况的研究结果不同,这凸显了在评估精神障碍和药物对性功能的影响时制定特定区域、特定性别的性行为社会规范的重要性。今后需要采用前瞻性研究来区分文化规范、精神疾病以及使用的抗精神病药物在精神分裂症患者性功能障碍的病因和病程中的相对作用。
1. Introduction
Sexual life is a natural component of human behavior, so treatments for persons with schizophrenia that aim to go beyond the narrow focus of reducing symptoms and, rather, focus on quality of life issues will necessarily include consideration of patients’ sexual functioning.[1],[2],[3],[4] The various components of sexual functioning – libido, arousal, ejaculation, and orgasm – can all be impaired in schizophrenia for both psychological and pathophysiological reasons.[5] The peak age of onset of schizophrenia is during the reproductive period,[6] so impaired sexual functioning among persons with schizophrenia can affect their ability to have a family, and, thus, to fulfill traditional social expectations.
The role of antipsychotic medication in the sexual dysfunction of individuals with schizophrenia has become a recent interest of researchers because this side-effect is one of several issues that may decrease adherence to medication regimens, particularly among males (who tend to be more concerned about sexual dysfunction than females).[7],[8],[9],[10],[11],[12] Clinicians interested in enhancing patients’ adherence to medication need to consider antipsychotic medication-induced sexual dysfunction, particularly in patients taking second-generation antipsychotic medications. These medications may restore sexual desire in patients but can also impair ejaculation and orgasm.[12],[13],[14],[15],[16],[17],[18] Secondgeneration antipsychotic medications are believed to cause sexual dysfunction via a number of different mechanisms, including hyperprolactinemia, sedation, and antagonism of dopaminergic, histaminic, α-adrenergic, and muscarinic receptors.[19],[20],[21],[22] Some studies suggest that elevations in prolactin indirectly affect sexual function via down-regulation of testosterone or estradiol, but other studies find that sexual dysfunction and hyperprolactinemia are independent of testosterone and estradiol levels.[23],[24],[25]
Identifying the specific role of antipsychotic medications in the sexual dysfunction of persons with schizophrenia is difficult because there are many other potential confounding factors, including the concurrent use of other medications, the effect of the disease itself, comorbidity with other psychiatric disorders, and comorbidity with various chronic medical illnesses.[26],[27] Negative symptoms (avolition, anhedonia, and blunted affect), recurrent psychotic episodes, obesity, and low self-esteem all have negative effects on the sexual life of patients with schizophrenia.[28]
The main aims of our study are: (a) to assess the validity of the Turkish version of the Arizona Sexual Experience Scale (ASEX)[29] as a measure of sexual functioning in patients with schizophrenia living in Turkey; and (b) to assess the frequency of sexual dysfunction in a sample of outpatients with schizophrenia currently being treated with antipsychotic medications.
2. Methods
2.1. Participants
The identification of cases and controls is shown in Figure 1. Eligible individuals were: (a) clinically stable outpatients in the Training and Research Hospital at Recep Tayyip Erdogan University; (b) diagnosed with schizophrenia according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, American Psychiatric Association, 1994)[30] assessed by administering the Structured Clinical Interview of DSM-IV (SCID-CV)[31] ; (c) had been ill for at least 2 years; (d) were 18 to 65 years of age; (e) had received at least 6 weeks of treatment with an antipsychotic; and (f) provided informed consent to participate in the study. Exclusion criteria included: (a) hypertension; (b) diabetes mellitus; (c) gonadal injury; (d) cardiovascular disease; (e) endocrine disorder; (f) substance abuse; (g) inability to give informed consent or answer questions; and (h) current use of psychoactive medications other than antipsychotic medications (e.g., antidepressants, anticonvulsants, lithium, or beta-blockers).
Figure 1. Flowchart of the study.
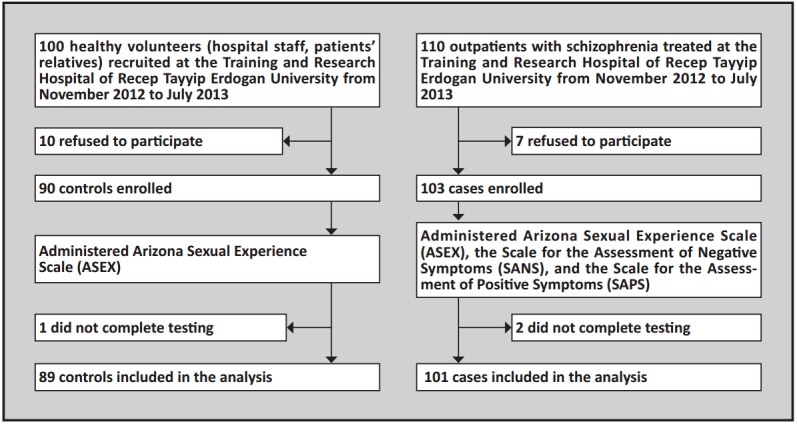
The control group was selected from among hospital workers (n=46) andhealthy relatives of patients (n=43) who accompanied patients to the outpatient department. These individuals were selected so that the gender, age, and level of education in the control group were similar to that of persons in the patient group (but there was no case-control 1-to-1 matching). Persons with current or prior mental health problems or serious physical health problems were excluded. They were administered the SCID-CV[31] to confirm the absence of a current or prior mental disorder.
Overall, 101 patients (38 females and 63 males) and 89 healthy controls (41 females and 48 males) completed the study. All participants were administered a comprehensive demographic data form and the ASEX.[29] Individuals in the patient group were also administered the Scale for the Assessment of Negative Symptoms (SANS)[32] and the Scale for the Assessment of Positive Symptoms (SAPS)[33] at the time of enrollment. All participants provided written informed consent. The study protocol was approved by the Ethics committee of the Faculty of Medicine, University of Recep Tayyip Erdogan, Rize, Turkey.
2.2. Measures
The socio-demographic data form used in the study was prepared by the authors; it assessed the age, gender, education, marital status, economic status, occurrence of other medical disorders, and family psychiatric history of all participants. Persons who stated that they had smoked tobacco daily for the last month were classified as ‘smokers’; women who stated that they drink one or more standard drinks daily and men who stated they had drink two or more standard drinks daily were classified as ‘drinks alcohol’. The patient group was also asked about the duration of illness and their medication history.
SCID-CV is a structured clinical interview for DSMIV Axis 1 psychiatric disorders developed by First and colleagues in 1996.[31] The validity and reliability of the Turkish version of SCID-CV were shown to be satisfactory by Ozkurkcugil and colleagues in 1999.[32]
The Scale for the Assessment of Negative Symptoms (SANS) was developed by Andreasen in 1990 and its Turkish version was validated by Erkoc and colleagues.[33] SANS consists of five subscales: (a) flattened affect, (b) impoverished thought, (c) involuntariness (apathy, anhedonia), (d) social withdrawal, and (e) attention deficit.
The Scale for the Assessment of Positive Symptoms (SAPS) was developed by Andreasen in 1990 and its Turkish version was validated by Erkoc and colleagues.[34] SAPS consists of five sub-scales containing: (a) hallucinations, (b) delusions, (c) bizarre behavior, (d) positive formal thought disorder, and (e) inappropriate affect.
The Arizona Sexual Experience Scale (ASEX) was developed by McGahuey and colleagues[29] in response to the need to evaluate medication-induced sexual dysfunction. The scale was first used to assess sexual dysfunction among persons treated with selective serotonin reuptake inhibitors (SSRIs).Later studies[13] used ASEX to assess sexual functioning in persons with schizophrenia. This is an easy-to-administer, 5-item questionnaire designed to measure different aspects of sexual functioning over the prior week: sexual drive, arousal, penile erection/vaginal lubrication, ability to reach orgasm, and satisfaction with orgasm. Items are rated on a 6-point Likert scale from 1 (hyperfunction) to 6 (hypofunction). This results in a total score that ranges between 5 and 30. A total score of >18, a score of ≥ 5 (very difficult) on any single item, or scores of ≥4 on any 3 of the 5 items are considered indicative of clinically significant sexual dysfunction. The Turkish version of ASEX was validated by Soykan[35] in a sample of 43 patients with end-stage renal disease: the internal consistency of the 5 items was good (alpha=0.89); the test-retest reliability of the total score was satisfactory (r=0.88); and the criterion validity of the total score compared with psychiatrists’ assessment of the presence of sexual dysfunction using a receiver operator characteristics analysis was good (area under the curve=0.86, 95% confidence interval=0.73-0.90). In the current study this scale was used as a self-completion questionnaire but a few subjects needed help from the investigator (who was female) to understand specific items.
2.3. Statistical analysis
SPSS 18.0 for Windows was used for data analysis. The student’s t-test was used to compare normally distributed continuous measures between cases and controls, the Mann Whitney U-test was used to compare non-normally distributed continuous variables, and Chi-square tests or Fisher’s exact tests were used to compare dichotomous or categorical variables. The relationship of the total ASEX score and the total scores for SANS and SAPS is assessed using correlation coefficients. The level of statistical significance was set at p<0.05.
3. Results
The characteristics of participants are shown in Table 1. The mean (sd) age of patients and controls were 38.9 (10.2) years and 39.3 (11.9) years, respectively (t=0.24, p=0.809). There were no significant differences in the gender distribution or educational attainment of patients versus controls, but patients were less likely to be married, more likely to be unemployed, more likely to live in a rural community, and more likely to be smokers.
Table 1.
Characteristics of participants
| Characteristices | patients n=101 n (%) |
controls n=89 n (%) |
statistic | p-value |
|---|---|---|---|---|
| Male | 63 (62.4) | 48 (53.9) | χ2=1.39 | 0.239 |
| Age | ||||
| 18-25 26-35 36-45 46-55 56 and over |
6 (5.9) 32 (31.7) 34 (33.7) 22 (21.8) 7 (6.9) |
11 (12.4) 22 (24.7) 28 (31.5) 14 (15.7) 14 (15.7) |
χ2=7.29 | 0.122 |
| Education | ||||
| no formal education primary school secondary school college university |
1 (1.0) 53 (52.5) 13 (12.9) 24 (23.8) 10 (9.9) |
4 (4.5) 39 (43.8) 15 (16.9) 20 (22.5) 11 (12.4) |
χ2=3.74 | 0.442 |
| Marital status | ||||
| single married divorced |
62 (61.4) 27 (26.7) 12 (11.9) |
16 (18.0) 71 (79.8) 2 (2.2) |
χ2=53.48 | <0.001 |
| Unemployed | 62 (61.4) | 12 (13.5) | χ2=45.66 | <0.001 |
| Rural resident | 73 (72.3) | 42 (47.2) | χ2=12.46 | <0.001 |
| Smokera | 55 (54.5) | 28 (31.5) | χ2=10.17 | <0.001 |
| Drinks alcoholb | 7 (6.9) | 4 (4.5) | χ2=0.52 | 0.473 |
asmoked tobacco daily in prior month
bwomen who drink one or more standard drinks daily or men who drink two or more standard drinks daily
The range in the duration of illness among the 101 patients was 2 to 37 years; the mean (sd) duration of illness was 15.0 (9.6) years. The majority of patients were single and unemployed. Most of the patients (86%) had taken antipsychotic medications regularly throughout their illness; 36.6% had used a single medication, 63.4% had used multiple medications. At the time of enrollment 66% were using secondgeneration antipsychotic medications and the rest were using first-generation antipsychotic medications. At the time of enrollment, the mean (sd) total SAPS score was 35.7 (23.1) and the mean (sd) total SANS score was 47.8 (25.1).
The internal consistency of the 5 items of the ASEX was excellent (Cronbach alpha=0.91), both for patients (alpha=0.92) and for controls (alpha=0.89). The results for ASEX are shown in Table 2. With the exception of the sexual arousal item, the other four components of sexual functioning (sex drive, vaginal lubrication or penile erection, achieving orgasm, and satisfaction with orgasm) were significantly worse in patients than in controls. The overall ASEX score also indicated greater sexual dysfunction in patients than in controls. However, stratified results by gender indicated that all of these differences were driven by differences between male patients and male controls; the total ASEX score and the five ASEX subscale scores were not significantly different between female patients and female controls.
Table 2.
Comparison of the mean (sd) scores on the five items of the Arizona Sexual Experience Scale (ASEX) between patients with schizophrenia and controls (higher scores represent greater dysfunction)
| Item | both genders | females | males | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| patients (n=101) mean (sd) |
controls (n=89) mean (sd) |
t-test (p-value) |
patients (n=38) mean (sd) |
controls (n=41) mean (sd) |
t-test (p-value) |
patients (n=63) mean (sd) |
controls (n=48) mean (sd) |
t-test (p-value) |
|||
| Sexual drive | 3.53 (1.37) |
3.09 (1.36) |
2.09 (0.038) |
3.79 (1.20) |
3.95 (1.25) |
0.52 (0.623) |
3.40 (1.44) |
2.36 (0.99) |
4.10 (<0.001) |
||
| Sexual arousal | 3.61 (1.28) |
3.44 (1.21) |
0.86 (0.390) |
4.04 (1.23) |
3.95 (1.27) |
0.29 (0.776) |
3.40 (1.27) |
3.02 (1.00) |
1.69 (0.094) |
||
| Vaginal lubrication/ penile erection |
3.48 (1.30) |
3.07 (1.34) |
1.99 (0.048) |
3.75 (1.46) |
3.57 (1.41) |
0.51 (0.612) |
3.35 (1.22) |
2.66 (1.14) |
2.91 (0.004) |
||
| Achieve orgasm | 3.56 (1.39) |
3.14 (1.37) |
2.00 (0.047) |
4.18 (1.09) |
3.89 (1.37) |
0.91 (0.366) |
3.26 (1.43) |
2.50 (1.00) |
3.15 (0.002) |
||
| Satisfaction with orgasm |
3.61 (1.24) |
2.68 (1.51) |
4.37 (<0.001) |
3.86 (1.27) |
3.32 (1.63) |
1.42 (0.158) |
3.49 (1.21) |
2.14 (1.15) |
5.69 (<0.001) |
||
| Total score (range 5-30) |
17.80 (5.70) |
15.42 (5.69) |
2.69 (0.008) |
19.61 (5.52) |
18.58 (6.11) |
0.63 (0.528) |
16.91 (5.62) |
12.68 (3.50) |
4.64 (<0.001) |
||
Table 3 presents data on the proportion of participants that meet criteria for sexual dysfunction based on the ASEX results (described in the methods section). Given the huge difference in the prevalence of self-reported sexual dysfunction by gender in control subjects (68% for females and 8% for males), it is necessary to consider the genders separately. Sexual dysfunction occurred in 68% of female patients and in 68% of female controls (χ2=1.79, p=0.181) while it occurred in 46% of male patients and 8% of male controls (χ2=15.86, p<0.001). Considering the five components of sexual functioning separately, there was a lower prevalence of dysfunctional sexual drive in female patients than in female controls (53% vs. 68%), but dysfunction in the other four components of sexual functioning was more prevalent in female patients than in female controls. However, none of these differences for females were statistically significant. All five types of sexual dysfunction were more common in male patients than in male controls; these differences were statistically significant for sexual drive, the ability to achieve orgasm, and satisfaction with orgasm.
Table 3.
Comparison of the proportion of patients with schizophrenia and controls who report sexual dysfunction based on results of the Arizona Sexual Experience Scale (ASEX)
| Item | both genders | females | males | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| patients (n=101) n (%) |
controls (n=89) n (%) |
χ2 (p-value) |
patients (n=38) n (%) |
controls (n=41) n (%) |
χ2 (p-value) |
patients (n=63) n (%) |
controls (n=48) n (%) |
χ2 (p-value) |
|||
| Sexual drivea | 50 (49.5%) |
58 (65.2%) |
3.75 (0.053) |
20 (52.6%) |
28 (68.3%) |
1.32 (0.251) |
30 (47.6%) |
3 (6.3%) |
19.56 (<0.001) |
||
| Sexual arousala | 56 (55.4%) |
40 (44.9%) |
1.95 (0.162) |
27 (71.1%) |
24 (58.5%) |
1.00 (0.318) |
30 (47.6%) |
15 (31.3%) |
2.49 (0.115) |
||
| Vaginal lubrication/ penile erectiona |
51 (50.5%) |
30 (33.7%) |
5.06 (0.024) |
24 (63.2%) |
20 (48.8%) |
1.58 (0.209) |
34 (54.0%) |
10 (20.8%) |
6.09 (0.014) |
||
| Achieve orgasma | 58 (57.4%) |
32 (35.9%) |
7.95 (0.005) |
30 (78.9%) |
25 (61.0%) |
2.02 (0.156) |
30 (47.6%) |
7 (14.6%) |
12.84 (<0.001) |
||
| Satisfaction with orgasma |
51 (50.5%) |
22 (24.7%) |
11.81 (0.001) |
22 (57.9%) |
18 (43.9%) |
1.23 (0.267) |
30 (47.6%) |
4 (8.3%) |
17.10 (<0.001) |
||
| Overall sexual dysfunctionb |
56 (55.4%) |
25 (28.1%) |
10.33 (0.001) |
26 (68.4%) |
28 (68.3%) |
1.79 (0.181) |
29 (46.0%) |
4 (8.3%) |
15.86 (<0.001) |
||
ascores of 4 or greater on the 1-6 Likert scale were considered ‘dysfunctional’
bindividuals with one or more of the following: total ASEX score >18; score on any of 5 items ≥5; score on 3 or more items ≥4
The correlation between ASEX scores and SAPS and SANS total scores for the 101 patients in the study are shown in Table 4. None of the correlation coefficients were statistically significant, but the magnitude of the correlation between the SANS total score and the total ASEX score and several ASEX item scores exceeded 0.20 for female patients, which suggests that female patients with more prominent negative symptoms experience greater sexual dysfunction. Despite the relatively small magnitude of the correlations, all correlation coefficients between ASEX and SAPS were negative (more prominent positive symptoms were associated with less sexual dysfunction) while all correlation coefficients between ASEX and SANS were positive (more prominent negative symptoms were associated with greater sexual dysfunction).
Table 4.
Correlation between sexual functioning and positive and negative symptoms in outpatients with schizophrenia in Turkey
| ASEX Item | both genders (n=101) |
females (n=38) |
males (n=63) |
|||||
|---|---|---|---|---|---|---|---|---|
| SAPS r (p) |
SANS r (p) |
SAPS r (p) |
SANS r (p) |
SAPS r (p) |
SANS r (p) |
|||
| Sexual drive score | -0.06 (0.581) |
0.11 (0.325) |
-0.19 (0.328) |
0.19 (0.344) |
0.00 (0.984) |
0.05 (0.715) |
||
| Sexual arousalscore | -0.09 (0.408) |
0.17 (0.119) |
-0.01 (0.961) |
0.34 (0.079) |
-0.11 (0.426) |
0.04 (0.760) |
||
| Vaginal lubrication/penile erection score | -0.11 (0.309) |
0.15 (0.189) |
-0.15 (0.440) |
0.10 (0.622) |
-0.08 (0.576) |
0.14 (0.303) |
||
| Achieve orgasm score | -0.18 (0.098) |
0.17 (0.125) |
-0.06 (0.753) |
0.24 (0.210) |
-0.21 (0.125) |
0.08 (0.563) |
||
| Satisfaction with orgasm score | -0.15 (0.168) |
0.13 (0.242) |
-0.10 (0.632) |
0.21 (0.289) |
-0.17 (0.213) |
0.06 (0.671) |
||
| total ASEX score | -0.14 (0.208) |
0.17 (0.128) |
-0.12 (0.550) |
0.24 (0.224) |
-0.13 (0.340) |
0.09 (0.531) |
||
ASEX, Arizona Sexual Experience Scale
SAPS, Scale for the Assessment of Positive Symptoms
SANS, Scale for the Assessment of Negative Symptoms
To address the issue of the differential effects of first-generation and second-generation antipsychotic medications on sexual functioning, we identified patients who had been taking first-generation or secondgeneration medication for at least one year at the time of enrollment and compared their results on the ASEX. As shown in Table 5, the number of patients who met these criteria was small so we used Mann-Whitney rank tests (rather than t-tests) to compare the results. There were no significant differences in the total ASEX score or in any of the subscale scores between patients taking first-generation medications and those taking secondgeneration medications for either males or females. However, these results are based on a relatively small number of patients.
Table 5.
Comparison of the scores on the five items of the Arizona Sexual Experience Scale (ASEX) between patients with schizophrenia taking first-generation antipsychotic medications (FGA) and second-generation antipsychotic medications (SGA)
| Item | both genders | females | males | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FGAa (n=7) mean (sd) |
SGAb (n=30) mean (sd) |
U-testc (p-value) |
FGAa (n=2) mean (sd) |
SGAb (n=11) mean (sd) |
U-testc (p-value) |
FGAa (n=5) mean (sd) |
SGAb (n=19) mean (sd) |
U-testc (p-value) |
|||
| Sexual drive | 3.43 (0.79) |
3.47 (1.33) |
100.50 (0.853) |
4.00 (1.41) |
3.45 (1.04) |
8.00 (0.517) |
3.20 (0.45) |
3.47 (1.50) |
39.50 (0.552) |
||
| Sexual arousal | 3.86 (0.90) |
3.57 (1.22) |
92.50 (0.615) |
4.50 (0.71) |
3.91 (1.14) |
7.00 (0.411) |
3.60 (0.89) |
3.37 (1.26) |
44.50 (0.824) |
||
| Vaginal lubrication/ penile erection |
3.43 (1.40) |
3.53 (1.36) |
101.00 (0.873) |
3.00 (2.83) |
3.73 (1.82) |
9.50 (0.760) |
3.60 (0.89) |
3.42 (1.35) |
46.00 (0.913) |
||
| Achieve orgasm | 4.14 (0.69) |
3.43 (1.45) |
72.50 (0.196) |
4.50 (0.71) |
4.00 (1.10) |
6.50 (0.342) |
4.00 (0.71) |
3.11 (1.56) |
32.00 (0.258) |
||
| Satisfaction with orgasm |
3.57 (0.98) |
3.53 (1.25) |
102.00 (0.903) |
4.00 (1.41) |
3.82 (1.25) |
10.00 (0.837) |
3.40 (0.89) |
3.37 (1.26) |
45.00 (0.852) |
||
| Total score (range 5-30) |
18.43 (4.24) |
17.53 (5.82) |
94.00 (0.668) |
20.00 (7.07) |
18.91 (4.91) |
10.00 (0.842) |
17.80 (3.56) |
16.74 (6.27) |
40.00 (0.593) |
||
amonotherapy with zuclopenthixol, flupentixol, or haloperiodol for at least one year prior to the evaluation
bmonotherapy with risperidone, olanzapine, or quetiapine for at least one year prior to the evaluation
cbased on Mann-Whitney rank test
4. Discussion
4.1. Main findings
Using a simple self-report measure of sexual function that has previously been validated in Turkey (ASEX), we found significantly more sexual dysfunction in male patients with schizophrenia under long-term treatment with antipsychotic medications than in healthy male controls (46% vs. 8%). However, in females the prevalence of self-reported sexual dysfunction was equally high in both patients and controls (both 68%). The same pattern was seen when comparing the five different components of sexual functioning assessed by the ASEX: for females, there were no significant differences between patients and controls in either the ASEX item scores or in the proportion of respondents classified as dysfunctional; for males, both the ASEX item score and the proportion of respondents classified as dysfunctional were greater in patients than in controls for four of the five ASEX subscales (sexual drive, penile erection, ability to achieve orgasm, and satisfaction with orgasm).
The prevalence of sexual dysfunction among males and females with schizophrenia found in this study are comparable to those reported in other studies in Turkey[36],[37] ,[38] and in other counties.[18],[26],[27],[39],[40] Other studies have also reported substantial gender differences in the pattern of sexual dysfunction among patients with schizophrenia.[22],[41],[42],[43],[44] Most studies report higher rates of sexual dysfunction in females and usually explain this in terms of the greater effect of antipsychotic medication on the prolactin levels of women than men.[27],[45],[46],[47] However, in our study the equally high prevalence of self-reported sexual dysfunction of female patients and controls indicates that the dysfunction in female patients cannot be attributed either to their illness or to the medications they are taking. In our country sexual conservatism and traditional sexual double standards[48] appear to result in very high levels of self-reported sexual dysfunction among healthy females (as assessed by the ASEX scale), so any potential additional effect of mental illness or of antipsychotic medication on sexual functioning is muted or absent.
Among these clinically stable patients we did not identify any strong relationship between the severity of positive or negative symptoms and the severity of sexual dysfunction. There was, however, a weak signal suggesting the more prominent positive symptoms are associated with less severe sexual dysfunction and more prominent negative symptoms are associated with more severe sexual dysfunction, particularly in females.
Previous reports suggest differences in the sexual dysfunction associated with first-generation and secondgeneration antipsychotic medications. Approximately 50% percent of patients with schizophrenia report sexual dysfunction during treatment with firstgeneration antipsychotic medications.[49] Some reports suggest that second-generation antipsychotic medications may improve some aspects of sexual functioning such as sexual desire,[9] but they may also cause erectile dysfunction and decreased libido. Few comparative studies on the sexual dysfunction associated with first and second-generation antipsychotic medications have been published.[50],[51] One problem in the available reports is that they primarily use prolactin level as a proxy for the effect of medications on sexual functioning, but the relationship of prolactin with sexual functioning has not been fully established. Moreover, it is probable that the different second-generation antipsychotic medications have different effects on sexual functioning.[52] Our study had limited power to assess differences in sexual functioning between patients taking first and secondgeneration antipsychotic medications, but, after limiting our analysis to patients who had regularly been taking a first-generation or a second-generation antipsychotic medication for at least one year at the time of evaluation, we found no significant differences in the ASEX measures between patients using first-generation or second-generation medications.
Clearly, the level of information available from the available studies on the sexual dysfunction of patients is insufficient to develop evidence-based interventions that could minimize this problem, a problem that seriously affects the quality of life of individuals with schizophrenia and may be related to some patients’ unwillingness to adhere to their medication regimens. Researchers need to conduct prospective studies that will help understand the etiology and course of sexual dysfunction in patients with schizophrenia – which may be different in males and females and may be affected by a wide range of potential confounding factors (e.g., alcohol use, physical health, culture- and genderspecific sexual norms, etc.).[12],[53],[54],[55] Clinicians need to increase their awareness of the problem and develop sensitive ways for regularly obtaining information about the sexual lives of their patients.[56] Only then will it be possible to develop and test methods for dealing with this troubling problem.
4.2. Limitations
There are several potential limitations that should be considered when interpreting these results. (a) Like other studies of sexual functioning in schizophrenia, this is a cross-sectional study, so it is impossible to observe the cause-effect relationship between the onset of illness, the use of antipsychotic medications, and the presence and severity of sexual dysfunction. (b) The very high prevalence of self-reported sexual dysfunction in healthy females in our Turkish sample (68%) made it difficult to identify any additional impact schizophrenia or the use of antipsychotic medication had on the sexual functioning of female patients. (c) Patients included in the study all had at least 2 years of illness and the mean duration of illness was 15 years, so it is not possible to differentiate the relative importance of chronic psychiatric illness and medication in the etiology of the patients’ sexual dysfunction. (d) There were substantial differences in marital status between cases and controls that probably had a major effect on respondents’ sexual activity, but it was not possible to determine whether the differences in marital status were the cause of sexual dysfunction or (partially) the result of sexual dysfunction. (e) The sample size was too small to compare the possible effects of different classes of antipsychotic medications (e.g., between first- and second-generation medications), of specific antipsychotic agents, or of a variety of potential confounders such as alcohol use, tobacco use, adjunctive medications, comorbid physical illness, and so forth. (f) The sample came from a single hospital outpatient setting so they may not be representative of patients (or controls) from other parts of Turkey. (g) Possible unwillingness of respondents to respond to the sensitive questions in ASEX. And (h) The lack of biological markers of sexual functioning such prolactin levels and other sex hormone levels.
4.3. Implications
Male patients with schizophrenia under long-term treatment with antipsychotic medications have significantly more self-reported sexual dysfunction than healthy controls of comparable age and educational attainment (46% vs. 8%). In our Turkish sample, the self-reported prevalence of sexual dysfunction is higher in female patients with schizophrenia than in male patients (68% vs. 46%), but the prevalence of selfreported sexual dysfunction is also very high in healthy female controls (68%), so it is not possible to attribute the sexual dysfunction of female patients to their illness or to the medications they are taking. This difference by gender highlights the importance of assessing locationspecific and gender-specific sexual norms when trying to assess the role of mental illness and medications on sexual functioning.
Almost all of the research about this issue – including the present study – are cross sectional, so it is not possible to assess the relative contribution of the illness itself versus the use of antipsychotic medication to sexual dysfunction. Nor is it possible to determine the extent to which this problem undermines medication adherence. Prospective research starting with an inception cohort (i.e., first-onset patients) is needed to clarify these issues and, subsequently, to develop and test strategies for minimizing the negative effects of sexual dysfunction on the quality of life of individuals with schizophrenia.
Biography

Cicek Hocaoglu earned her medical degree from Uludag University Faculty of Medicine in 1987. She received postgraduate training in psychiatry at the Medical School of Karadeniz Technical University in Trabzon, Turkey between 1993 and 1999. She is currently Professor and Head of the Department of Psychiatry at the Recep Tayyip Erdogan University Medical School in Rize, Turkey. Her research interests include mood disorders, suicide, and schizophrenia.
Funding Statement
This study was not financially supported.
Conflict of interest: The authors report no conflict of interest related to this manuscript.
Ethical review: The study protocol was approved by the Ethics Committee of the Faculty of Medicine, University of Recep Tayyip Erdogan, Rize, Turkey.
Informed consent: Written informed consent was obtained from all participants.
References
- 1.Östman M, Björkman AC. Schizophrenia and relationships: the effect of mental illness on sexuality. Clin Schizophr Relat Psychoses. 2013;7: 20–24. doi: 10.3371/CSRP.OSBJ.012513. [DOI] [PubMed] [Google Scholar]
- 2.Bushong ME, Nakonezny PA, Byerly MJ. Subjective quality of life and sexual dysfunction in outpatients with schizophrenia or schizoaffective disorder. J Sex Marital Ther. 2013;39: 336–46. doi: 10.1080/0092623X.2011.606884. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar S, Thomson JA Jr. Schizophrenia and sexuality: a review and a report of twelve unusual cases--part I. J Clin Psychiatry. 1980;41: 134–142. [PubMed] [Google Scholar]
- 4.Deanna LK, Robert RC. Sexuality and schizophrenia: a review. Schizophr Bull. 2004;30: 767–779. doi: 10.1093/oxfordjournals.schbul.a007130. [DOI] [PubMed] [Google Scholar]
- 5.Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol. 2008;23: 201–209. doi: 10.1002/hup.924. [DOI] [PubMed] [Google Scholar]
- 6.Häfner H, Riecher-Rössler A, An Der Heiden W, Maurer K, Fätkenheuer B, Löffler W. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol Med. 1993;23: 925–940. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- 7.Lambert M, Conus P, Eide P, Mass R, Karow A, Moritz S. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry. 2004;19: 415–422. doi: 10.1016/j.eurpsy.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Seeman MV. Loss of libido in a woman with schizophrenia. Am J Psychiatry. 2013;170: 471–475. doi: 10.1176/appi.ajp.2012.12111475. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud A, Hayhurst KP, Drake RJ, Lewis SW, Barnes TR. The ANNSERS (Antipsychotic Non-Neurological Side Effects Rating Scale): Validation of Sexual Side-Effect Measurement. Ther Adv Psychopharmacol. 2011;1: 97–100. doi: 10.1177/2045125311417041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inder WJ, Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N Z J Psychiatry. 2011;45: 830–7. doi: 10.3109/00048674.2011.589044. [DOI] [PubMed] [Google Scholar]
- 11.Dossenbach M, Dyachkova Y, Pirildar S, Anders M, Khalil A, Araszkiewicz A. Effects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12-month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) study. Eur Psychiatry. 2006;21: 251–258. doi: 10.1016/j.eurpsy.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Haddad PM, Sharma SG. Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs. 2007;21: 911–936. doi: 10.2165/00023210-200721110-00004. [DOI] [PubMed] [Google Scholar]
- 13.Byerly M, Nakonezny P, Fisher R, Magouirk B, Rush AJ. An empirical evaluation of the Arizona sexual experience scale and a simple one-item screening test for assessing antipsychotic-related sexual dysfunction in outpatients with schizophrenia and schizoaffective disorder. Schizophre Res. 2006;81: 311–316. doi: 10.1016/j.schres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Knegtering H, Van der Moolen AE, Castelein S. What are the effects of antipsychotics on sexual dysfunctions and endocrine functioning? Psychoneuroendocrinology. 2003;28: 109–123. doi: 10.1016/S0306-4530(02)00130-0. [DOI] [PubMed] [Google Scholar]
- 15.Atmaca M, Kuloglu M, Tezcan E. A new atypical antipsychotic: quetiapine-induced sexual dysfunctions. Int J Impot Res. 2005;17:201–203. doi: 10.1038/sj.ijir.3901260. [DOI] [PubMed] [Google Scholar]
- 16.Wesby R, Bullimore E, Earle J, Heavey A. A survey of psychosexual arousability in male patients on depot neuroleptic medication. Eur Psychiatry. 1996;11: 81–86. doi: 10.1016/0924-9338(96)84784-5. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Kühn KU, Sträter B, Scherbaum N, Weig W. [Adverse side-effect on sexual function caused by psychotropic drugs and psychotropic substances] Nervenarzt. 2010;81: 1129–1137.German. doi: 10.1007/s00115-010-3074-9. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, O’Keane V, Murray R. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry. 2002;181: 49–55. doi: 10.1192/bjp.181.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry. 2002;63: 56–62. [PubMed] [Google Scholar]
- 20.Koroglu A, Hocaoglu C. Risperidone-induced acromegaly: a case report. Ther Adv Psychopharmacol. 2012;2: 84–89. doi: 10.1177/2045125311433581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes LV, Moreira HC, Razzouk D, Nunes SO, Mari J. Strategies for the treatment of antipsychotic-induced sexual dysfunction and/or hyperprolactinemia among patients of the schizophrenia spectrum: a review. J Sex Marital Ther. 2012;38: 281–301. doi: 10.1080/0092623X.2011.606883. [DOI] [PubMed] [Google Scholar]
- 22.Sch?ttle D, Kammerahl D, Huber J, Briken P, Lambert M, Huber CG. [Sexual problems in patients with schizophrenia] Psychiatr Prax. 2009;36:160–8. German. doi: 10.1055/s-0028-1090206. [DOI] [PubMed] [Google Scholar]
- 23.Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res. 1999;35: 67–73. doi: 10.1016/s0920-9964(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 24.Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 2009;29: 64–73. doi: 10.1592/phco.29.1.64. [DOI] [PubMed] [Google Scholar]
- 25.Konarzewska B, Szulc A, Pop?awska R, Galiska B, Juchnowicz D. [Impact of neuroleptic-induced hyperprolactinemia on sexual dysfunction in male schizophrenic patients] Psychiatry Pol. 2008;42: 87–95.Polish. [PubMed] [Google Scholar]
- 26.Olfson M, Uttaro T, Carson WH, Tafesse E. Male sexual dysfuction and quality of life in schizophrenia. J Clin Psychiatry. 2005;66: 331–338. doi: 10.4088/jcp.v66n0309. [DOI] [PubMed] [Google Scholar]
- 27.Nunes LVA, Dieckmann LHJ, Lacaz FS, Bressan R, Matsuo T, Mari JJ. The accuracy of the Arizona Sexual Experience Scale (ASEX) to identify sexual dysfunction in patients of the schizophrenia spectrum. Revista de Psiquiatria Clínica. 2009;36: 182–189. doi: 10.1590/S0101-60832009000500002. [DOI] [Google Scholar]
- 28.Zemishlany Z, Weizman A. The impact of mental illness on sexual dysfunction. Adv Psychosom Med. 2008;29: 89–106. doi: 10.1159/000126626. [DOI] [PubMed] [Google Scholar]
- 29.Mc Gahuey CA, Gelemberg AJ, Laukes CA, Moreno FA, Delgado PI, Mc Knight KM, Manber R. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26: 25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders(DSM-IV), 4th ed, Text Revision. Washington, DC:American Psychiatric Press. 2000
- 31.First MB, Spitzer RL, Gibbon M, Williams JBW, Koroglu E. Structured clinical interview for DSM-IV Axis I disorders, clinician version (SCID-CV) Washington, DC: American Psychiatric Press Inc. 1996 [Google Scholar]
- 32.Ozkurkçugil A, Aydemir O, Yildiz M, Danaci AE, Koroglu E. [Validity and reliability of Turkish version for Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)] İlaç ve Tedavi Dergisi. 1999;12:233–236. Turkish. [Google Scholar]
- 33.Erkoc S, Arkonac O, Atakli C, Ozmen E. [Reliability and validity of negative symptoms rating scale] Düşünen Adam Journal of Psychiatry and Neurological Sciences. 1991b;4:16–19 . Turkish. [Google Scholar]
- 34.Erkoc S, Arkonac O, Atakli C, Ozmen E. [Validity and reliability of Scale for the Assessment of Positive Symptoms] Düşünen Adam Journal of Psychiatry and Neurological Sciences. 1991;4:20–24. Turkish. [Google Scholar]
- 35.Soykan A. The reliability and validity of Arizona sexual experiences scale in Turkish ESRD patients undergoing hemodialysis. Int J Impot Res. 2004;16: 531–534. doi: 10.1038/sj.ijir.3901249. [DOI] [PubMed] [Google Scholar]
- 36.Ucok A, Incesu C, Aker T, Erkoç S. Sexual dysfunction in patients with schizophrenia on antipsychotic medication. Eur Psychiatry. 2007;22: 328–333. doi: 10.1016/j.eurpsy.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Hariri AG, Karadag F, Gurol DT, Aksoy UM, Tezcan AE. Sexual problems in a sample of the Turkish psychiatric population. Compr Psychiatry. 2009;50: 353–360. doi: 10.1016/j.comppsych.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Çakmak S, Karakus G, Evlice YE. [Sexual dysfunction in schizophrenia: A cross-sectional evaluation. ] Klinik Psikiyatri. 2010;13: 55–64. Turkish. [Google Scholar]
- 39.Malik P. Sexual dysfunction in schizophrenia. Curr Opin Psychiatry. 2008;2: 234–237. doi: 10.1097/YCO.0b013e328017f6c4. [DOI] [PubMed] [Google Scholar]
- 40.Oyekanmi AK, Adelufosi AO, Abayomi O, Adebowale TO. Demographic and clinical correlates of sexual dysfunction among Nigerian male outpatients on conventional antipsychotic medications. BMC Res Notes. 2012;7: 267. doi: 10.1186/1756-0500-5-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang XR, Zhang ZJ, Zhu RX, Yuan YG, Jenkins TA, Reynolds GP. Sexual dysfunction in male schizophrenia: influence of antipsychotic drugs, prolactin and polymorphisms of the dopamine D2 receptor genes. Pharmacogenomics. 2011;12:1127–1136. doi: 10.2217/pgs.11.46. [DOI] [PubMed] [Google Scholar]
- 42.Aizenberg D, Zemishlany Z, Dorfman-Etrog P. Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry. 1995;56: 137 –141. [PubMed] [Google Scholar]
- 43.Rettenbacher MA, Hofer A, Ebenbichler C, Baumgartner S, Edlinger M, Engl J. Prolactin levels and sexual adverse effects in patients with schizophrenia during antipsychotic treatment. J Clin Psychopharmacol. 2010;30: 711–715. doi: 10.1097/JCP.0b013e3181faf0e3. [DOI] [PubMed] [Google Scholar]
- 44.Harley EW, Boardman J, Craig T. Sexual problems in schizophrenia: prevalence and characteristics. A cross sectional survey. Soc Psychiatry Psychiatr Epidemiol. 2010;45:759–766. doi: 10.1007/s00127-009-0119-0. [DOI] [PubMed] [Google Scholar]
- 45.Shah SK. A comparative study of sexual dysfunction in schizophrenia patients taking aripiprazole versus risperidone. Kathmandu Univ Med J (KUMJ) 2013;42: 121– 125. doi: 10.3126/kumj.v11i2.12486. [DOI] [PubMed] [Google Scholar]
- 46.David SR, Taylor CC, Kinon BJ, Breier A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin Ther. 2000;22: 1085–1096. doi: 10.1016/S0149-2918(00)80086-7. [DOI] [PubMed] [Google Scholar]
- 47.Hanssens L, L’Italien G, Loze JY, Marcus RN, Pans N, Kerselaers W. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study ( NCT00237913) BMC Psychiatry. 2008;22(8):95. doi: 10.1186/1471-244X-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A?kun D, Ataca B. Sexuality related attitudes and behaviors of Turkish University students. Arch Sex Behav. 2007;36: 741–752. doi: 10.1007/s10508-007-9186-z. [DOI] [PubMed] [Google Scholar]
- 49.Kelly DL, Conley RR. Sexuality and schizophrenia: a review. Schizophr Bull. 2004;30: 767–779. doi: 10.1093/oxfordjournals.schbul.a007130. [DOI] [PubMed] [Google Scholar]
- 50.Tardieu S, Micallef J, Bonierbale M, Frauger E, Lançon C, Blin O. [Sexual behaviour in schizophrenic patients: the impact of antipsychotics] L’Encephale. 2006;32: 697–704. doi: 10.1016/s0013-7006(06)76221-2. French. [DOI] [PubMed] [Google Scholar]
- 51.Iagubov MI, Shtark LN. [Sexual disturbances during the treatment with neuroleptics in patients with schizophrenia and schizophrenia spectrum disorders] Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111: 57–60.Russian. [PubMed] [Google Scholar]
- 52.Bobes J, Garc A-Portilla MP, Rejas J, Hern Ndez G, GarciaGarcia M, Rico-Villademoros F, Porras A. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. Sex Marital Ther. 2003;29: 125–147. doi: 10.1080/713847170. [DOI] [PubMed] [Google Scholar]
- 53.Katarina Melo ChavesKM , Serrano-Blanco A, Ribeiro SB, Soares LAL, et al. Quality of life and adverse effects of olanzapine versus risperidone therapy in patients with schizophrenia. Psychiatr Q. 2013;84: 125–135. doi: 10.1007/s11126-012-9233-3. [DOI] [PubMed] [Google Scholar]
- 54.Fan X, Henderson DC, Chiang E, Briggs LB, Freudenreich O, Evins AE, Cather C, Goff DC. Sexual functioning, psychopathology and quality of life in patients with schizophrenia. Schizophr Res. 2007;94: 119–127. doi: 10.1016/j.schres.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 55.Meyer JM. Sexual dysfunction in patients treated with atypical antipsychotics. J Clin Psychiatry. 2008;69: 26–32. doi: 10.4088/jcp.0908e26. [DOI] [PubMed] [Google Scholar]
- 56.Ucok A, Incesu C, Aker T, Erkoc S. Do psychiatrists examine sexual dysfunction in schizophrenia patients? . J Sex Med. 2008;5:2000–2001. doi: 10.1111/j.1743-6109.2008.00890.x. [DOI] [PubMed] [Google Scholar]


