Abstract
OBJECTIVES:
To investigate an unusual outbreak of tetrodotoxin poisoning in Leizhou, southeast China, a case series analysis was conducted to identify the source of illness.
METHODS:
A total of 22 individuals experienced symptoms of poisoning, including tongue numbness, dizziness, nausea and limb numbness and weakness. Two toxic species, Amoya caninus and Yongeichthys nebulosus, were morphologically identified from the batches of gobies consumed by the patients. Tetrodotoxin levels in the blood and Goby fish samples were detected using liquid chromatography-tandem mass spectrometry.
RESULTS:
The tetrodotoxin levels in the remaining cooked Goby fish were determined to be 2090.12 µg/kg. For Amoya caninus, the toxicity levels were 1858.29 µg/kg in the muscle and 1997.19 µg/kg in the viscera and for Yongeichthys nebulosus, they were 2783.00 µg/kg in the muscle and 2966.21 µg/kg in the viscera.
CONCLUSION:
This outbreak demonstrates an underestimation of the risk of Goby fish poisoning. Furthermore, the relationships among the toxic species, climates and marine algae present should be clarified in the future.
Keywords: Tetrodotoxin, Poisoning, Gobies, LC-MS/MS
INTRODUCTION
Tetrodotoxin (TTX) is a potent neurotoxin that blocks sodium channels in nerve cells and skeletal muscles when ingested by humans 1-7, resulting in perioral paresthesia, nausea, vomiting, ataxia, weakness of the limbs and respiratory failure, and it can even cause death in severe cases 8. TTX is widely distributed in marine animals, including newts 9-15, frogs 16-20, crabs 21-23, gastropods 24-26, starfish 27 and gobies 1,.
In the 1970s, Noguchi and Hashimoto identified the Goby fish toxin as TTX and extracted it from Gobius criniger 1. Several cases of poisoning resulting from the ingestion of gobies have been reported in Taiwan 30. In subsequent research, more than 300 specimens from 12 species of Goby fish have been collected from 15 locations and examined for toxicity 29,30. Three species of Goby fish, including Yongeichthys kaohsiung and two other species, have been identified as toxic 29,30,32.
The seas of Mainland China include coral reefs, rocky shores, rubble shores, mudflats and thousands of islands and rivers, which form ideal Goby habitats. Mainland China is home to 307 Goby species within 106 genera, 5 subfamilies and 9 families. Among the 2211 Goby species worldwide, 13.9% are found in China, making China one of the most Goby species-rich countries in the world 33. Because of their abundance, gobies are a popular food along the coast. In this paper, we describe an outbreak of 22 cases of TTX poisoning from the consumption of gobies. Liquid chromatography-tandem mass spectrometry (LC-MS/MS), which is the most powerful and sensitive method at present for toxin detection 34,35, was used to detect the toxin in patient blood samples and in uneaten gobies.
METHODS
Epidemiologic investigation
The Leizhou CDC officer conducted a retrospective study in three towns (Tandou, Yingli and Longmen) and three farms (Happy Farm, Dongfanghong Farm and Jinxing Farm), which comprised all locations with reported cases. Historical data on this outbreak were collected using an epidemiological case sheet designed by the local Center for Disease Control and Prevention (CDC) and this form included personal information, the time at which the meal in question was consumed, the foods consumed during the meal, the presenting symptoms, the time of symptom onset and the medical care provided. Because gobies are small fish, the patients were unable to easily recall how many fish they had eaten. However, because the patients cooked the fish at home, the amount of fish brought home by each family could be accurately reported; thus, the proportion that each person consumed was estimated according to the number of members in each family. During this outbreak, individuals who experienced an onset of dizziness, nausea, vomiting, tongue numbness and limb weakness from March 13th-15th were considered to have experienced poisoning.
Specimen identification
Uncooked gobies from the same batch responsible for the poisoning of 22 individuals were collected from the markets and their sizes, fin morphologies, body colors and arrangements of stripes and spots were studied for morphological identification by experts at Fudan University.
Patient blood and Goby samples
During this outbreak, available samples of the remaining cooked and uncooked gobies were scarce; thus, we only examined one specimen for each species. The remaining cooked gobies were collected from the kitchen associated with one case on March 15th and blood samples were collected from seven available patients on March 18th, as well as two uncooked gobies from the associated batch. All samples were frozen at -20°C and sent to the laboratory at the Luohu CDC in Shenzhen.
Extraction and purification of toxin from blood samples
Each 0.1 ml sample of heparinized whole blood was thawed and mixed with 0.4 ml of a 1% acetic acid methanol solution. The samples were immediately centrifuged at 13000 rpm for 10 min. The supernatant was evaporated under a nitrogen stream using a TurboVap II automated evaporation system (Biotage, Charlotte, NC USA), dissolved in 1% acetic acid and reconstituted to a volume of 1 ml. The solution was liquid-extracted with diethyl ether and subsequently centrifuged at 8000 rpm for 10 min. The liquid-liquid extraction step was repeated 3 times and the underlying solution was then collected for subsequent analysis.
Extraction and purification of toxin from Goby samples
Each 1.0 g sample of homogenated Goby muscle was mixed with a 1% acetic acid methanol solution until the weight of the solution was 11.0 g. After the sample was shaken for 10 min, 1.0 g of the extract was mixed with 20 ml of 1% acetic acid and reconstituted to a volume of 21 ml. The solution was then ultrasonically extracted for 30 min. 1 ml of the extracted solution was mixed with 3 ml of acetonitrile and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was then collected for subsequent analysis.
LC-MS/MS analysis of TTX
LC-MS/MS data were obtained using an Agilent 6410 Triple Quadrupole LC-MS/MS system equipped with a liquid Chromatograph Agilent 1200 and electrospray ion source (ESI) (Agilent Technologies, Santa Clara, CA, USA). The extracted solutions were separated using a liquid chromatograph HP1200, which consisted of a quaternary pump and Merck seQuant™ ZIC-HILIC (150 mm×2.1 mm, 5 µm) Column (Merck Sequant, Umea, Sweden). Mobile phase A contained 10 mM ammonium formate and 0.1% formic acid and mobile phase B contained acetonitrile. The injection volume was 10 µl and the flow rate was 0.2 ml/min. The column temperature was maintained at 25°C. The mass spectrometer was operated in MS/MS mode with a multiple reaction monitor (MRM) to detect specific precursor ions and produce ion transitions for each analyte. The optimum ion source parameters were as follows: temperature = 350°C; capillary voltage = 4000 V; drying gas flow = 11.0 l/min; and nebulizer pressure = 38 psi. For multiple reaction monitoring, Q1/Q3 ion transitions of m/z 320.0/162.1 and m/z 320.0/302.1 were used to quantitatively and qualitatively monitor TTX. The detection limit (LOD) for the blood samples was 0.3 ng/ml and the LOD for the Goby samples was 0.7 ng/ml.
RESULTS
Epidemiologic investigation
On March 13th-14th 2012, a fish dealer purchased gobies from fishermen in Xiahai village, Yingli, Guangdong province. The gobies were caught from Leizhou Bay and the dealer sold the gobies to markets in three towns (Tandou, Yingli and Longmen) and to three farms (Happy Farm, Dongfanghong Farm and Jinxing Farm). The individuals who consumed the gobies in these towns successively suffered dizziness, nausea, vomiting, tongue numbness and limb weakness. Seven individuals on March 14th, twelve individuals on March 15th and three individuals on March 16th required hospitalization. In total, 26 individuals ingested gobies by the end of March 15th and 22 met the case definition for the outbreak and required hospitalization, which indicated an attack rate of 84%.
After being admitted to the hospital, the patients with mild symptoms were treated with emetic or lavage with the oral administration of water. Patients with moderate to severe symptoms were subjected to gastric lavage with a gastric tube and then to catharsis with 250 ml of 20% mannitol. Conventional treatments were applied as follows: intravenous injection of dexamethasone and scopolamine, intramuscular injection of vitamins B1 and B12, intravenous infusion, diuresis treatment and maintenance treatment of the water electrolyte balance. Following 3-5 days of supportive treatment, all patients recovered and were discharged.
The 22 cases included 9 males and 13 females with a median age of 54 years and ages ranging from 31-78 years. Most patients experienced illness from March 13th-14th. The first patient consumed gobies at 18:00 on March 13th and became ill within half an hour. The last patient consumed gobies at 12:00 on March 15th and reported illness at 14:00. The shortest and longest incubation periods were 30 min and 3 h, respectively (mean of 1.8 h).
The patients were distributed in the towns and farms where the gobies had been sold. Among the exposed individuals, five of the six who consumed gobies in Xiahai, Tandou (5/6) became ill, as well as eight of nine in Yingli (8/9), two of two at Dongfanghong Farm (2/2), five of six at Happy Farm (5/6), one in Longmen (1/1) and one at Jinxing Farm (1/1).
These patients belonged to thirteen families, all of whom had prepared the gobies at home in a traditional way to make fish soup, in which the fish were cleaned and only the liver and eggs were removed and they were then cooked in soup broth for 2-3 min. In the soup, different species of gobies were mixed and cooked with the viscera. Each family bought approximately 500 g of fish and the amount of fish eaten by each patient ranged from 100 g to 300 g per meal. Two patients consumed the fish three times and the other 20 consumed it only once. One patient consumed a bowl of soup but did not eat the fish.
Most of the patients claimed that they had eaten gobies before but were unaware of their toxicity. These individuals could not distinguish the difference between various Goby species or even the difference between gobies and mudskippers, which is another edible species that is morphologically similar to gobies.
Specimen identification
The cooked fish could be identified as gobies and their lengths were measured; however, they could not be identified morphologically because their skins were missing and their surface patterns were altered. Thus, uncooked gobies were collected for morphological identification and two toxic species were identified, Amoya caninus and Yongeichthys nebulosus.
Laboratory analysis
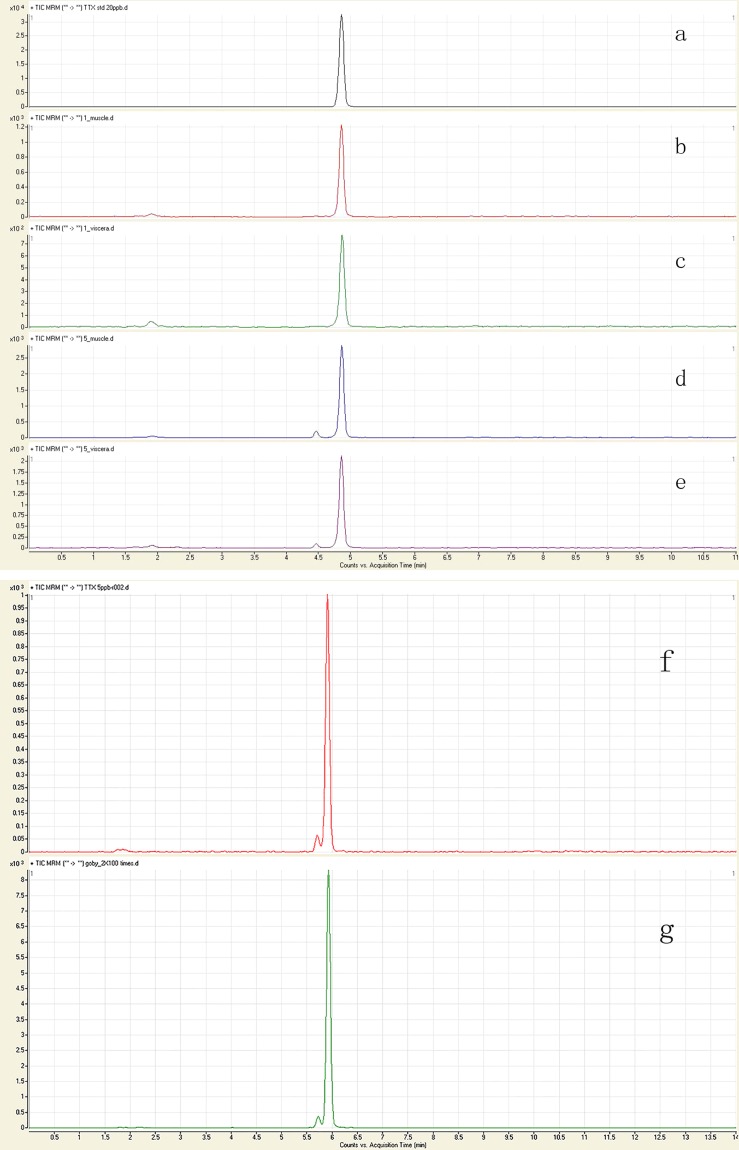
Because the patients' symptoms indicated TTX poisoning, we qualitatively and quantitatively tested for TTX in blood and food samples. No TTX was detected in the blood samples of seven patients. MRM chromatograms of the samples matched the standard chromatograms and confirmed that TTX was the etiologic agent (Figure 1). The TTX in the remaining food samples of the prepared Goby muscles amounted to 2090.12 µg/kg. TTX was detected in Amoya caninus and Yongeichthys nebulosus specimens collected from the market. The TTX toxicity levels in the muscle and viscera of Amoya caninus were 1858.29 µg/kg and 1997.19 µg/kg, respectively and those in the muscle and viscera of Yongeichthys nebulosus were 2783.00 µg/kg and 2966.21 µg/kg, respectively. In humans, 10,000 mouse units (MUs) are equivalent to 2 mg of TTX 8. Thus, for Amoya caninus, the toxicity levels of the tissues were 9.69 MUs/g in the muscle and 10.42 MUs/g in the viscera and for Yongeichthys nebulosus, they were 14.51 MUs/g in the muscle and 15.47 MUs/g in the viscera.
Figure 1.
The figure MRM chromatogram of TTX standard and Goby samples. (a) and (f): TTX standard; (b): muscle of Amoya caninus; (c): viscera of Amoya caninus; (d): muscle of Yongeichthys nebulosus; (e): viscera of Yongeichthys nebulosus; (g): remaining food sample of the prepared Goby muscles.
Poisoning diagnosis
Although TTX was not detected in the blood samples due to late sampling, the relationship of the poisoning outbreak to the consumption of Goby was confirmed by the field and laboratory data. Clinical manifestations included tongue numbness, dizziness, nausea, vomiting and limb numbness, which are typical neurological symptoms of TTX poisoning. The incubation period was 0.5-3 h, which is within the window observed in previous cases of food-induced TTX poisoning. These cases were not clustered spatially but were associated with Goby consumption. No other substance that might induce similar symptoms was detected in the foods that the patients had ingested. Using LC-MS/MS, which is the accepted technique for the accurate and effective detection of TTX, we detected 2090.12 µg/kg of TTX in the remaining gobies, confirming food-induced TTX poisoning in these individuals.
DISCUSSION
Although several TTX poisoning incidents have occurred in Taiwan and Japan as a result of Goby ingestion 1,30, such outbreaks are rare in Mainland China. Mainland China regulates TTX in puffer fish and forbids the consumption of these species; however, the sale and consumption of gobies are unregulated. Individuals who live along the coast, including those involved in the outbreak, were familiar with gobies and had frequently consumed them in the past; however, they were not aware of their toxicity. This is the first detailed report of TTX poisoning caused by gobies in Mainland China, as shown by the epidemiological investigation and LC-MS/MS detection. This incident involved 22 individuals from three towns and three farms. This wide distribution indicates the potential importance of this problem. These findings should alert health authorities to the need for potential preventive measures.
To date, an antidote for TTX is unavailable; thus, our treatments were supportive. The reasons that the patients were treated with dexamethasone, scopolamine and B-complex vitamins were as follows: 1) dexamethasone was used because high doses of this drug during the early stage of poisoning can enhance the stress response of the body and its tolerance to poison and hypoxia, reduce cell inflammation and provide protection; 2) scopolamine was used because it can counteract the inhibition of striated muscles caused by TTX by a mechanism similar to the antagonistic effects of neostigmine against tubocurarine; and 3) B-complex vitamins were used because vitamins B1 and B12 serve to nourish peripheral nerves.
Following this outbreak, we morphologically identified Amoya caninus and Yongeichthys nebulosus. These two species may have been present in China for many years but have remained undiscovered because of insufficient monitoring efforts. Most of the 2000 species of gobies are not poisonous. However, Yongeichthys nebulosus and Amoya caninus have been reported to be poisonous in Japan, Taiwan and mainland China 29,30. Many experimental studies have supported the hypothesis of an exogenous TTX source and research in Japan has indicated a close relationship between the intake of benthic copepods as food and the toxicity of Yongeichthys nebulosus. These findings indicate that feeding habits may play an important role in TTX infestation. However, the particular organisms involved in Goby fish intoxication are still unknown. To educate the public, TTX sources, such as marine algae and the specified foods consumed by Goby fish should be further clarified.
People in coastal China generally prepare small fishes, such as gobies, by mixing various species and cooking them with the viscera, including the gonads. In Yongeichthys nebulosus, TTX accumulates at relatively high levels during the spawning period, which usually occurs from March to June and this toxin is concentrated primarily in the ovaries 28. This information partially explains why this incident occurred in March. To avoid future incidents, coastal residents should be educated to discard all viscera, as well as the gonads, especially during the spawning period.
In our study, the toxicity of Amoya caninus during this outbreak was similar to that determined in a previous study performed in Taiwan and that of Yongeichthys nebulosus was lower compared with previous reports. We could not determine whether the studied gobies accumulated TTX in the skin alone or in both the skin and muscle because they were analyzed together. A previous study that assessed the toxicity of gobies in Taiwan detected TTX not only in the skin, viscera and gonads but also in the muscles, head and fins and revealed that the toxicity levels in the muscle and skin were approximately equal.
No TTX was detected in the blood samples collected from the patients. There are two potential explanations for these results: 1) according to the patients' self-reports, they each consumed approximately 100 g to 300 g of gobies. TTX was detected at 2090.12 µg/kg in the remaining gobies; thus, each patient was estimated to have ingested more than 0.63 mg of TTX. This amount may cause symptoms to appear, but it has not been found to be lethal in adults 8; and 2) we obtained the blood samples at 3 days after the appearance of clinical symptoms. According to previous research, most ingested TTX is eliminated in the urine and becomes undetectable at 24 h following poisoning 36. This finding likely explains why TTX was not detected in some patients even though they were still considered to be cases. Because early diagnosis and treatment are essential in cases of TTX intoxication, biological samples, such as blood and urine, should be collected as early as possible during similar acute poisoning incidents.
In a previous study, fish species of unknown samples were successfully identified using DNA sequencing 37. However, in this investigation, following the detection of Goby toxicity, we did not retain the samples for subsequent DNA detection, which would have been especially suitable for identifying the species in the cooked Goby samples because morphological identification was impossible after they had been cooked. Currently, Goby species are primarily identified by their surface patterns; thus, they are easily misidentified as non-toxic and edible mudskippers or other species by individuals without special training. As a result, the development of rapid genomic techniques to effectively identify gobies during the investigation of poisoning outbreaks should be included in future studies.
ACKNOWLEDGMENTS
We are grateful to Dr. F Li of Fudan University for assisting with the identification of the Goby species and to Dr. Q Huang of the Guangdong Province Center for Disease Control and Prevention for providing guidance during our investigation.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Noguchi T, Hashimoto Y. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon. 1973;11(3):305–7. doi: 10.1016/0041-0101(73)90060-3. [DOI] [PubMed] [Google Scholar]
- 2.Lee CH, Ruben PC. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels (Austin) 2008;2(6):407–12. doi: 10.4161/chan.2.6.7429. [DOI] [PubMed] [Google Scholar]
- 3.Guzman A, Fernandez de Henestrosa AR, Marin AP, Ho A, Borroto JI, Carasa I, et al. Evaluation of the genotoxic potential of the natural neurotoxin Tetrodotoxin (TTX) in a battery of in vitro and in vivo genotoxicity assays. Mutat Res. 2007;634(1-2):14–24. doi: 10.1016/j.mrgentox.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Duch DS, Levinson SR. Neurotoxin-modulated uptake of sodium by highly purified preparations of the electroplax tetrodotoxin-binding glycopeptide reconstituted into lipid vesicles. J Membr Biol. 1987;98(1):43–55. doi: 10.1007/BF01871044. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JC, Catterall WA. Tetrodotoxin-insensitive sodium channels. Ion flux studies of neurotoxin action in a clonal rat muscle cell line. J Biol Chem. 1981;256(12):6213–22. [PubMed] [Google Scholar]
- 6.Sheumack DD, Howden ME, Spence I, Quinn RJ. Maculotoxin: a neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science. 1978;199(4325):188–9. doi: 10.1126/science.619451. [DOI] [PubMed] [Google Scholar]
- 7.Mosher HS, Fuhrman FA, Buchwald HD, Fischer HG. Tarichatoxin--Tetrodotoxin: A Potent Neurotoxin. Science. 1964;144(3622):1100–10. doi: 10.1126/science.144.3622.1100. [DOI] [PubMed] [Google Scholar]
- 8.Hwang DF, Noguchi T. Tetrodotoxin poisoning. Adv Food Nutr Res. 2007;52:141–236. doi: 10.1016/S1043-4526(06)52004-2. [DOI] [PubMed] [Google Scholar]
- 9.Mebs D, Arakawa O, Yotsu-Yamashita M. Tissue distribution of tetrodotoxin in the red-spotted newt Notophthalmus viridescens. Toxicon. 2010;55(7):1353–7. doi: 10.1016/j.toxicon.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Gall BG, Stokes AN, French SS, Brodie ED, 3rd, Brodie ED., Jr Female newts (Taricha granulosa) produce tetrodotoxin laden eggs after long term captivity. Toxicon. 2012;60(6):1057–62. doi: 10.1016/j.toxicon.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Mebs D, Yotsu-Yamashita M, Seitz HM, Arakawa O. Tetrodotoxin does not protect red-spotted newts, Notophthalmus viridescens, from intestinal parasites. Toxicon. 2012;60(1):66–9. doi: 10.1016/j.toxicon.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Gall BG, Stokes AN, French SS, Schlepphorst EA, Brodie ED, 3rd, Brodie ED., Jr Tetrodotoxin levels in larval and metamorphosed newts (Taricha granulosa) and palatability to predatory dragonflies. Toxicon. 2011;57(7-8):978–83. doi: 10.1016/j.toxicon.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Yotsu-Yamashita M, Mebs D, Kwet A, Schneider M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp. Urodela, Salamandridae) from southern Germany. Toxicon. 2007;50(2):306–9. doi: 10.1016/j.toxicon.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Yotsu M, Iorizzi M, Yasumoto T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon. 1990;28(2):238–41. doi: 10.1016/0041-0101(90)90419-8. [DOI] [PubMed] [Google Scholar]
- 15.Wakely JF, Fuhrman GJ, Fuhrman FA, Fischer HG, Mosher HS. The occurrence of tetrodotoxin (tarichatoxin) in amphibia and the distribution of the toxin in the organs of newts (taricha) Toxicon. 1966;3(3):195–203. doi: 10.1016/0041-0101(66)90021-3. [DOI] [PubMed] [Google Scholar]
- 16.Pires OR, Jr, Sebben A, Schwartz EF, Morales RA, Bloch C, Jr, Schwartz CA. Further report of the occurrence of tetrodotoxin and new analogues in the Anuran family Brachycephalidae. Toxicon. 2005;45(1):73–9. doi: 10.1016/j.toxicon.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, Brown GB, Mosher FA. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science. 1975;189(4197):151–2. doi: 10.1126/science.1138374. [DOI] [PubMed] [Google Scholar]
- 18.Ujii R, Novales RR. Tetrodotoxin: effects on fish and frog melanophores. Science. 1968;160(3832):1123–4. doi: 10.1126/science.160.3832.1123. [DOI] [PubMed] [Google Scholar]
- 19.Aceves J, Erlij D. Effects of norepinephrine on tissues of the frog heart atrium poisoned by tetrodotoxin. Nature. 1967;215(5106):1178–9. doi: 10.1038/2151178b0. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara S, Nakajima S. Tetrodotoxin and manganese ion: effects on action potential of the frog heart. Science. 1965;149(3689):1254–5. doi: 10.1126/science.149.3689.1254. [DOI] [PubMed] [Google Scholar]
- 21.Tsai YH, Hwang DF, Chai TJ, Jeng SS. Toxicity and toxic components of two xanthid crabs, Atergatis floridus and Demania reynaudi, in Taiwan. Toxicon. 1997;35(8):1327–35. doi: 10.1016/s0041-0101(97)00005-6. [DOI] [PubMed] [Google Scholar]
- 22.Baker PF, Rubinson KA. Chemical modification of crab nerves can make them insensitive to the local anaesthetics tetrodotoxin and saxitoxin. Nature. 1975;257(5525):412–4. doi: 10.1038/257412a0. [DOI] [PubMed] [Google Scholar]
- 23.Konosu S, Inoue A, Noguchi T, Hashimoto Y. Comparison of crab toxin with saxitoxin and tetrodotoxin. Toxicon. 1968;6(2):113–7. doi: 10.1016/0041-0101(68)90029-9. [DOI] [PubMed] [Google Scholar]
- 24.Yin HL, Lin HS, Huang CC, Hwang DF, Liu JS, Chen WH. Tetrodotoxication with nassauris glans: a possibility of tetrodotoxin spreading in marine products near Pratas Island. Am J Trop Med Hyg. 2005;73(5):985–90. [PubMed] [Google Scholar]
- 25.Hwang DF, Lin LC, Jeng SS. Occurrence of a new toxin and tetrodotoxin in two species of the gastropod mollusk Nassariidae. Toxicon. 1992;30(1):41–6. doi: 10.1016/0041-0101(92)90500-5. [DOI] [PubMed] [Google Scholar]
- 26.Hwang DF, Tai KP, Chueh CH, Lin LC, Jeng SS. Tetrodotoxin and derivatives in several species of the gastropod Naticidae. Toxicon. 1991;29(8):1019–24. doi: 10.1016/0041-0101(91)90084-5. [DOI] [PubMed] [Google Scholar]
- 27.Lin SJ, Hwang DF. Possible source of tetrodotoxin in the starfish Astropecten scoparius. Toxicon. 2001;39(4):573–9. doi: 10.1016/s0041-0101(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 28.Tatsuno R, Shikina M, Soyano K, Ikeda K, Takatani T, Arakawa O. Maturation-associated changes in the internal distribution of tetrodotoxin in the female goby Yongeichthys criniger. Toxicon. 2013;63:64–9. doi: 10.1016/j.toxicon.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Chen CY, Chou HN, Chen YM, Lee TH. Detection of tetrodotoxin by HPLC in shellfishes and goby from south Taiwan. J Nat Toxins. 2002;11(1):63–8. [PubMed] [Google Scholar]
- 30.Lin SJ, Chen JB, Hsu KT, Hwang DF. Acute goby poisoning in southern Taiwan. J Nat Toxins. 1999;8(1):141–7. [PubMed] [Google Scholar]
- 31.Hashimoto Y, Noguchi T. Occurrence of a tetrodotoxin-like substance in a goby Gobius criniger. Toxicon. 1971;9(1):79–84. doi: 10.1016/0041-0101(71)90046-8. [DOI] [PubMed] [Google Scholar]
- 32.Huang C, Chen QL, Luo Z, Shi X, Pan YX, Song YF, et al. Time-dependent effects of waterborne copper exposure influencing hepatic lipid deposition and metabolism in javelin goby Synechogobius hasta and their mechanism. Aquat Toxicol. 2014;155:291–300. doi: 10.1016/j.aquatox.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Zeng WW, Li KB, Chang OQ, Liu C, Wu GH, et al. Outbreaks of an iridovirus in marbled sleepy goby, Oxyeleotris marmoratus (Bleeker), cultured in southern China. J Fish Dis. 2011;34(5):399–402. doi: 10.1111/j.1365-2761.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 34.Hayashida M, Hayakawa H, Wada K, Nihira M, Ohno Y. Sensitive determination of tetrodotoxin using column-switching liquid chromatography-mass spectrometry with electrospray ionization in mouse serum. J Anal Toxicol. 2004;28(1):46–9. doi: 10.1093/jat/28.1.46. [DOI] [PubMed] [Google Scholar]
- 35.Shoji Y, Yotsu-Yamashita M, Miyazawa T, Yasumoto T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal Biochem. 2001;290(1):10–7. doi: 10.1006/abio.2000.4953. [DOI] [PubMed] [Google Scholar]
- 36.O′Leary MA, Schneider JJ, Isbister GK. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon. 2004;44(5):549–53. doi: 10.1016/j.toxicon.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Wu YJ, Cheng YJ, Jen HC, Pan CH, Lin TC, Lin SJ, et al. Liquid chromatography-tandem mass spectrometry determination of the toxicity and identification of fish species in a suspected tetrodotoxin fish poisoning. J Food Prot. 2011;74(5):789–95. doi: 10.4315/0362-028X.JFP-10-435. [DOI] [PubMed] [Google Scholar]