ABSTRACT
Salt-inducible kinase 2 (SIK2) is an AMP-activated protein kinase (AMPK) related kinase abundantly expressed in adipose tissue. Our aim was to identify molecular targets and functions of SIK2 in adipocytes, and to address the role of PKA-mediated phosphorylation of SIK2 on Ser358. Modulation of SIK2 in adipocytes resulted in altered phosphorylation of CREB-regulated transcription co-activator 2 (CRTC2), CRTC3 and class IIa histone deacetylase 4 (HDAC4). Furthermore, CRTC2, CRTC3, HDAC4 and protein phosphatase 2A (PP2A) interacted with SIK2, and the binding of CRTCs and PP2A to wild-type but not Ser358Ala SIK2, was reduced by cAMP elevation. Silencing of SIK2 resulted in reduced GLUT4 (also known as SLC2A4) protein levels, whereas cells treated with CRTC2 or HDAC4 siRNA displayed increased levels of GLUT4. Overexpression or pharmacological inhibition of SIK2 resulted in increased and decreased glucose uptake, respectively. We also describe a SIK2–CRTC2–HDAC4 pathway and its regulation in human adipocytes, strengthening the physiological relevance of our findings. Collectively, we demonstrate that SIK2 acts directly on CRTC2, CRTC3 and HDAC4, and that the cAMP–PKA pathway reduces the interaction of SIK2 with CRTCs and PP2A. Downstream, SIK2 increases GLUT4 levels and glucose uptake in adipocytes.
KEY WORDS: SIK2, Adipocyte, CRTC2, CRTC3, HDAC4, PP2A, Glucose uptake
INTRODUCTION
The AMPK-related kinase salt-inducible kinase 2 (SIK2) is abundantly expressed in adipocytes and both protein and activity levels have been found to be increased in an obese mouse model (Du et al., 2008; Horike et al., 2003). In spite of this, no substrates of SIK2 have so far been described in adipocytes. The kinase activity of SIK2, similar to AMPK and other AMPK-related kinases, is dependent on its phosphorylation at Thr175 by the upstream kinase LKB1 (also known as STK11) (Lizcano et al., 2004). SIK2 is also phosphorylated at other sites and this might further control its cellular function (Dentin et al., 2007; Katoh et al., 2004; Sasaki et al., 2011). In adipocytes, we recently demonstrated that SIK2 is regulated by the cAMP–PKA pathway through phosphorylation of Ser358, resulting in the binding of SIK2 to 14-3-3 proteins and a subsequent relocalisation to the cytosol with no effect on intrinsic kinase activity (Henriksson et al., 2012). Insulin, which has been suggested to regulate SIK2 activity and Ser358 phosphorylation (Dentin et al., 2007; Küser-Abali et al., 2013), was found not to alter SIK2 Ser358 phosphorylation or activity in adipocytes (Henriksson et al., 2012). In HEK293T cells, SIK2 has also been reported to be regulated by its binding to protein phosphatase 2A (PP2A), which acts to preserve SIK2 kinase activity (Lee et al., 2014).
In contrast to AMPK, the targets of which include many metabolic enzymes (Hardie et al., 2012), SIK isoforms have so far mainly been suggested to regulate gene expression by phosphorylating transcriptional regulators like the CREB-regulated transcription co-activators (CRTCs) (Dentin et al., 2007; Horike et al., 2010; Koo et al., 2005; Patel et al., 2014; Screaton et al., 2004). This regulation has been described in non-adipose cells, including pancreatic beta cells, melanocytes and hepatocytes. CRTCs, when phosphorylated, bind to 14-3-3 proteins and are sequestered in the cytoplasm where they are unable to activate CREB-induced gene transcription (Bittinger et al., 2004; Jansson et al., 2008; Screaton et al., 2004). In response to elevations in cAMP, CRTCs are dephosphorylated, leading to nuclear translocation and binding to CREB (Altarejos and Montminy, 2011). Several sites have been described to take part in the phosphorylation-dependent nucleo-cytoplasmic shuttling of CRTCs. Ser171 and Ser307 have been demonstrated to regulate CRTC2 in hepatocytes (Koo et al., 2005; Screaton et al., 2004; Uebi et al., 2010), and phosphorylation of Ser275 by the AMPK-related kinase MARK2 was found to be important for the exclusion of CRTC2 from the nucleus in beta cells (Jansson et al., 2008). Through activation of CREB target genes, CRTCs have been linked to several important metabolic processes such as gluconeogenesis (Dentin et al., 2007; Koo et al., 2005; Wang et al., 2010), mitochondrial biogenesis (Than et al., 2011; Wu et al., 2006) and beta cell survival (Jansson et al., 2008). In adipocytes, CRTCs and CREB have been suggested to attenuate β-adrenergic signalling as well as the expression of adiponectin and GLUT4 (also known as SLC2A4) (Qi et al., 2009; Song et al., 2010).
Similar to CRTCs, the localisation and activity of class IIa histone deacetylases (HDACs) are also regulated by phosphorylation and interaction with 14-3-3 proteins (Bassel-Duby and Olson, 2006). Following their dephosphorylation, class IIa HDACs translocate to the nucleus, where they modulate gene expression by associating with transcription factors. Class IIa HDACs are implicated in a wide array of biological processes, including the control of GLUT4 expression during adipogenesis and the activity of FOXO transcription factors (Mihaylova et al., 2011; Weems and Olson, 2011). Interestingly, they are suggested to be targets for AMPK or SIK phosphorylation in Drosophila, Caenorhabditis elegans and in mammals (Berdeaux et al., 2007; van der Linden et al., 2007; Walkinshaw et al., 2013; Wang et al., 2011). In Drosophila, the regulation of HDAC4 by a SIK isoform is important for lipid accumulation in response to feeding and starvation (Wang et al., 2011).
Although it is not mechanistically clear how, cAMP–PKA-mediated phosphorylation of SIKs has been suggested to inhibit SIK function, leading to CRTC and class IIa HDAC dephosphorylation and activation (Screaton et al., 2004; Takemori and Okamoto, 2008). In adipocytes, the cAMP-induced binding to 14-3-3 proteins and relocalisation of SIK2 that we described previously (Henriksson et al., 2012) could potentially restrict the ability of SIK2 to phosphorylate its substrates. This view is supported by our recent findings in hepatocytes, in which we demonstrated that SIK2, like in adipocytes, is subject to multi-site phosphorylation in response to cAMP induction, and that overexpression of a phosphorylation-resistant version of SIK2 prevented cAMP-induced reduction in the phosphorylation of CRTC2 (Patel et al., 2014). The identity of substrates and thus the biological function of SIK2 in adipocytes, however, remain to be investigated. In this study, we present evidence that CRTC2, CRTC3 and HDAC4 are direct molecular targets of SIK2 in both rodent and human adipocytes. Using these proteins as read-outs of SIK2 cellular activity, we also evaluate the functional importance of cAMP-mediated regulation of SIK2 in these cells. Moreover, we demonstrate that SIK2 is involved in the regulation of GLUT4 levels and glucose uptake in adipocytes, potentially through its action on CRTCs and HDAC4.
RESULTS
Phosphorylation of CRTC2 and class IIa HDACs is reduced following LKB1 silencing in adipocytes
When silencing the expression of LKB1 in 3T3-L1 adipocytes using retrovirally introduced short hairpin RNA (shRNA), we observed that the phosphorylation of CRTC2 at Ser275, a site known to regulate the localisation of CRTC2 (Jansson et al., 2008), was significantly reduced (Fig. 1A, left panel). The multiple bands detected in these cells using the Ser275-specific antibody most likely represent other similarly regulated CRTC isoforms. We also monitored the phosphorylation of class IIa HDACs 4, 5 and 7 at Ser246, Ser259 and Ser155, respectively, and similarly to CRTC2, the phosphorylation of class IIa HDACs was reduced in LKB1-shRNA-treated adipocytes (Fig. 1A, right panel). Based on its clear responsiveness to cAMP (Figs 2–4), along with recent findings suggesting that HDAC4 is of particular relevance in mature adipocytes (Weems et al., 2012), we focused on this class IIa HDAC isoform in our further analysis. Only low amounts of residual LKB1 protein and mRNA expression were detected in adipocytes expressing LKB1 shRNA, as shown by western blotting and qPCR (Fig. 1B). The LKB1 antibody detects a doublet, out of which the lower band is absent in LKB1-shRNA-expressing cells and hence represents LKB1. The upper band represents a nonspecific target of the antibody.
Fig. 1.
LKB1-shRNA-expressing adipocytes display reduced phosphorylation of CRTC2 and HDAC4. (A, B) 3T3-L1 preadipocytes were lentivirally transduced with scrambled (Scr) or LKB1 shRNA and differentiated into adipocytes. Lysates from fully differentiated cells were analysed with antibodies against Ser275 phosphorylation of CRTC2 (A, left) and Ser246, Ser259 and Ser155 phosphorylation of class IIa HDAC4, HDAC5 and HDAC7, respectively (A, right). The bar graphs represent quantified phosphorylation western blot signals, corrected for total protein levels (CRTC2 and HDAC4). The triplicates shown in the western blots constitute three independent experiments. (B) LKB1 levels in LKB1-shRNA-expressing cells were compared to those of Scr-treated control cells by western blotting (B, left) and qPCR analysis (B, right). ERK1/2 was used as a loading control. The data are presented as the mean±s.e.m. (two to five individual experiments); **P<0.01; ***P<0.001.
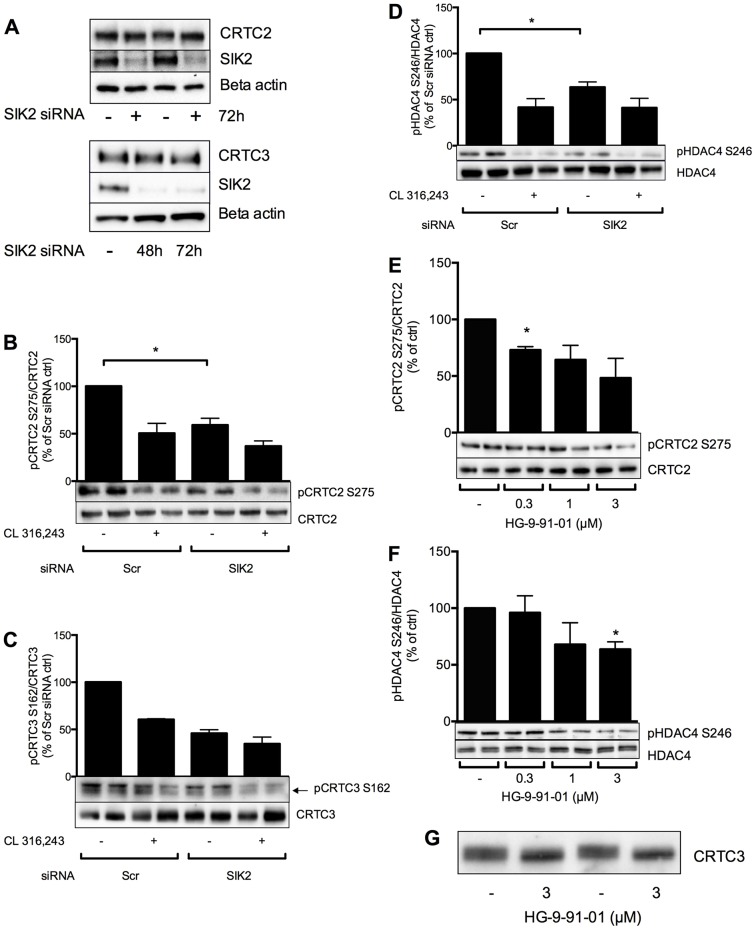
Fig. 2.
Silencing or inhibition of SIK2 results in reduced phosphorylation of CRTC2, CRTC3 and HDAC4 in adipocytes. (A–D) The expression of SIK2 was reduced by electroporation of scrambled (Scr) or SIK2 siRNA into differentiated 3T3-L1 adipocytes. The effect of SIK2 silencing for 48 h (A) or 72 h (B–D) on the phosphorylation of potential molecular targets was monitored by investigating CRTC2 and CRTC3 electrophoretic mobility shifts (A) and by using phosphospecific antibodies against Ser275 of CRTC2 (B), Ser162 of CRTC3 (C) or Ser246 of HDAC4 (D). Bar graphs represent the mean±s.e.m. of quantified western blot signals from two to four individual experiments in which the data were normalized to those of Scr-treated non-stimulated cells (set as 100%). (E–G) Primary rat adipocytes were treated with increasing doses of HG-9-91-01 for 1 h and the phosphorylation of CRTC2 and HDAC4 was analysed by western blotting using phosphospecific antibodies against Ser275 of CRTC2 (E) or Ser246 of HDAC4 (F), or by monitoring the electrophoretic mobility of CRTC3 (G). In E, western blotting was performed on CRTC2 immunoprecipitates. Bar graphs represent the mean±s.e.m. of quantified western blot signals from three or four individual experiments in which the data were normalized to those of non-treated cells (set as 100%). *P<0.05.
Fig. 3.
Adenoviral expression of SIK2 results in increased phosphorylation of CRTC2, CRTC3 and HDAC4 in adipocytes – the role of SIK2 kinase activity. Primary rat adipocytes were transduced overnight with adenoviral vectors encoding GFP or wild-type (wt) or Thr175Ala SIK2, and then treated with CL 316,243 (100 nM, 30 min). The effect on CRTC2 (A,B,E), CRTC3 (C,D) and HDAC4 (F) phosphorylation was evaluated by monitoring their migration pattern on SDS-PAGE gels (A–D) or by western blotting using phosphospecific antibodies against Ser275 of CRTC2 (E) and Ser246 of HDAC4 (F). Bar graphs represent the mean±s.e.m. of quantified western blot signals from three individual experiments in which the data were normalized to those of non-treated cells expressing wild-type SIK2 (set as 100%). *P<0.05.
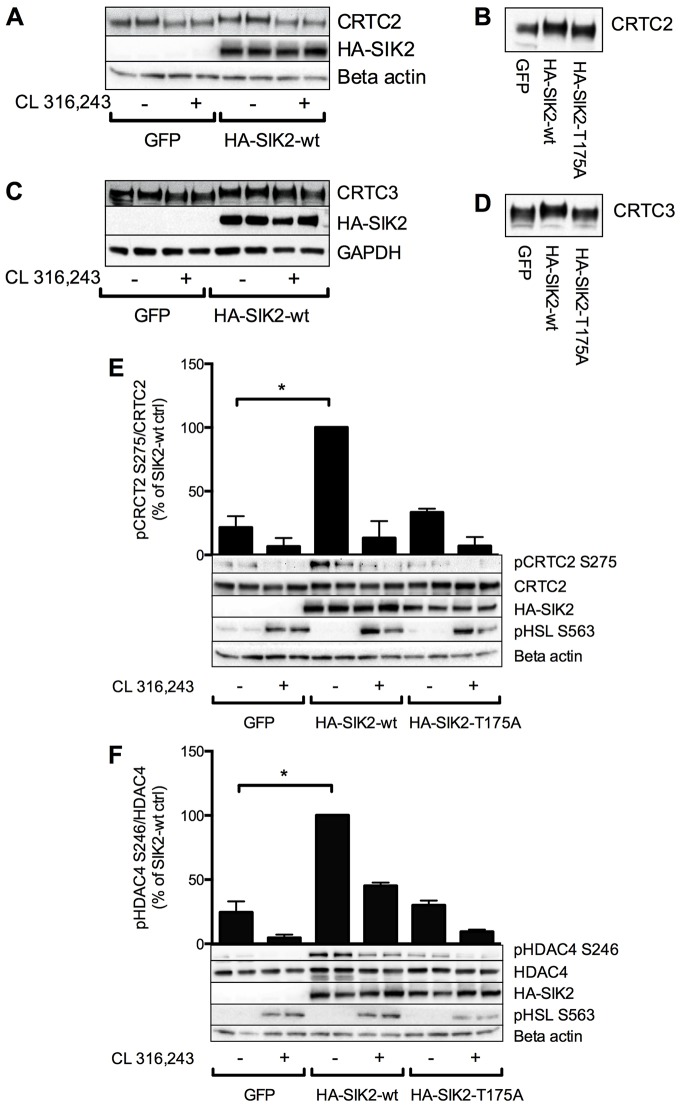
Fig. 4.
Role of PKA phosphorylation sites in SIK2 for the regulation of CRTC2 and HDAC4 dephosphorylation by cAMP. (A) Adenoviral vectors encoding wild-type (wt), Ser343Ala, Ser358Ala, Thr484Ala or Ser587Ala SIK2 were introduced into primary adipocytes followed by treatment with CL 316,243 (100 nM, 30 min). The phosphorylation of SIK2 was analysed by western blotting using PKA consensus antibodies (PKA cons), anti-SIK2-pSer358 or anti-SIK2-pSer343 antibodies (upper panel). HA immunoprecipitates (IP) of SIK2 were analysed for the presence of 14-3-3 proteins (lower panel). Similar results were obtained in two individual experiments. (B,C) Adenoviral vectors encoding wild-type or Ser358Ala SIK2 were introduced into primary adipocytes, followed by stimulation of the cells as in A. The phosphorylation of CRTC2 on Ser275 (B) or HDAC4 on Ser246 (C) was analysed by western blotting using phosphospecific antibodies. Bar graphs represent the mean±s.e.m. of quantified western blot signals from four to six individual experiments in which the data were normalized to those of non-treated cells for each construct (set as 100%). *P<0.05; **P<0.01; ***P<0.001.
Endogenous SIK2 is required for the phosphorylation of CRTC2, CRTC3 and HDAC4 in adipocytes
Our findings in LKB1-shRNA-expressing cells demonstrated that the phosphorylation of CRTC2 and HDAC4 is regulated downstream of LKB1 in adipocytes. Effects of LKB1, including those on CRTC isoforms and class IIa HDACs, have been shown previously to be mediated by members of the AMPK family of kinases, which require LKB1 for their activity (Lizcano et al., 2004). Therefore, we next investigated the requirement for SIK2, one of the most prominently expressed AMPK-related kinases in adipocytes, in the phosphorylation of CRTC2 and HDAC4 in these cells. We also analysed the phosphorylation of CRTC3, another CRTC family member that has been reported to attenuate cAMP signalling in adipose tissue (Song et al., 2010). For this purpose, 3T3-L1 adipocytes were electroporated with scrambled (Scr) small interfering RNA (siRNA) or siRNA targeting SIK2, and the phosphorylation of CRTC2, CRTC3 and HDAC4 was monitored in response to elevated levels of cAMP following stimulation of the cells with the β3-adrenergic receptor agonist CL 316,243 (Fig. 2A–D). The kinase activity of SIK2 in adipocytes treated with SIK2 siRNA was 25% of that in Scr-treated cells, and SIK2 protein levels were similarly reduced (supplementary material Fig. S1). The mRNA level of SIK2 was found to be 45% of that in Scr-treated cells 72 h after electroporation (data not shown). No changes in mRNA or protein expression of AMPK and SIK3, or in the activity of AMPK (as evaluated using phosphospecific antibodies against Thr172), were detected as a result of reduced expression and activity of SIK2 (data not shown). Phosphorylation of hormone-sensitive lipase (HSL) on Ser563 was used as a positive control for the successful induction of cAMP–PKA throughout this paper.
Both CRTC2 and CRTC3 displayed a reduction in overall phosphorylation after silencing of SIK2 for 48 h or 72 h, as demonstrated by a slightly faster migration in 7% SDS-PAGE detected by western blotting (Fig. 2A). This effect was more prominent after 72 h of SIK2 silencing, and this time-point was thus selected for additional experiments. The specific phosphorylation of CRTC2 at Ser275 was clearly reduced in response to increased levels of cAMP in adipocytes, and was also significantly lower in SIK2-siRNA-treated cells as compared with the Scr control (Fig. 2B). Dephosphorylation after cAMP induction or SIK2 siRNA treatment was also observed when analysing the phosphorylation of CRTC3 on Ser162 (Fig. 2C), a site shown to be phosphorylated by SIK isoforms and to control CRTC3 function in macrophages (Clark et al., 2012). The antibody against phosphorylated (p)CRTC3-Ser162 detects two bands, out of which (as determined in immunoprecipitation experiments) the upper one represents phosphorylation of CRTC2 on Ser171 and the lower band represents phosphorylation of CRTC3. Furthermore, as shown in Fig. 2D, phosphorylation of HDAC4 on Ser246 was significantly reduced in cells treated with the β3-adrenergic receptor agonist or with SIK2 siRNA. In conclusion, silencing of SIK2 resulted in a reduction of overall as well as site-specific phosphorylation of CRTC2, CRTC3 and HDAC4 in adipocytes. The specific phosphorylations investigated were in all cases reduced to the same level as in CL 316,243-treated Scr-siRNA-expressing cells, indicating an important role of endogenous SIK2 in maintaining CRTC and HDAC4 phosphorylation in resting cells.
Given that silencing by means of siRNA is not possible in primary adipocytes, we instead used the highly selective pan-SIK inhibitor HG-9-91-01 (Clark et al., 2012) to address the requirement for SIK2 in the phosphorylation of CRTC2, CRTC3 and HDAC4 in this more physiologically relevant model. Treatment of primary rat adipocytes with HG-9-91-01 for 1 h resulted in a dose-dependent reduction in the phosphorylation of CRTC2 and HDAC4 on Ser275 and Ser246, respectively (Fig. 2E,F). The antibody against pCRTC3-Ser162 was not sensitive enough to allow us to confidently detect specific phosphorylation of CRTC3 in primary cells, but a downward shift in the electrophoretic mobility of CRTC3 indicated that HG-9-91-01 induced a dephosphorylation of this CRTC isoform as well (Fig. 2G). These data further support a role for SIK2 in the regulation of CRTCs and HDAC4 in adipocytes, and confirm the efficacy of HG-9-91-01 in these cells.
Overexpression of SIK2 is sufficient to induce phosphorylation of CRTCs and HDAC4 in primary adipocytes
We next employed adenoviral overexpression of HA–SIK2 in primary rat adipocytes to study whether increased SIK2 levels are sufficient to induce CRTC and HDAC4 phosphorylation, as well as the requirement of SIK2 kinase activity for this effect. Primary rat adipocytes were transduced overnight with adenoviral vectors encoding GFP, wild-type SIK2 or a kinase-inactive version of SIK2, in which the T-loop Thr175 residue has been converted to alanine (Thr175Ala SIK2). As observed in 3T3-L1 adipocytes, both CRTC2 and CRTC3 were dephosphorylated in response to cAMP elevation in primary adipocytes, as indicated by an electrophoretic mobility gel shift downwards in response to stimulation with CL 316,243 (Fig. 3A,C). Expression of wild-type SIK2, but not the Thr175Ala mutant, resulted in a slower migration of CRTC2 and CRTC3 compared with that of the GFP control (Fig. 3B,D), indicating an increased overall phosphorylation status. The phosphorylation of CRTC2 on Ser275 was reduced in response to CL 316,243 (Fig. 3E), and the basal phosphorylation of this site was robustly increased in cells expressing wild-type SIK2 compared with those expressing Thr175Ala SIK2 or GFP (Fig. 3E). Similarly, the phosphorylation of HDAC4 on Ser246 was increased after expression of wild-type SIK2 (Fig. 3F). The ability of wild-type but not kinase-inactive SIK2 to promote the phosphorylation of CRTC2, CRTC3 and HDAC4 further supports a role for SIK2 in the phosphorylation of these transcriptional regulators and demonstrates that SIK2 activity is required for this process.
Role of PKA-mediated phosphorylation of SIK2 for the regulation of CRTCs and HDAC4 by cAMP in adipocytes
SIK2 is regulated by cAMP–PKA signalling in adipocytes through phosphorylation at four sites – Ser343, Ser358, Thr484 and Ser587 – and we have shown previously that this is associated with the binding of SIK2 to 14-3-3 proteins (Henriksson et al., 2012). The total phosphorylation of SIK2 by PKA after CL 316,243-treatment, as measured by the use of PKA consensus antibodies and the binding of 14-3-3, was dramatically reduced when expressing Ser343Ala- or Ser358Ala SIK2 in primary rat adipocytes, but not when mutating Thr484 or Ser587 (Fig. 4A). From blotting with anti-pSer358 or anti-pSer343 antibodies, but also from phosphopeptide mapping performed previously by us (Henriksson et al., 2012), it was clear that the Ser343Ala mutant lacks phosphorylation on both Ser343 and Ser358, whereas the Ser358Ala mutant is still phosphorylated on Ser343. From these data, we conclude that Ser358 appears to be the dominant site in SIK2 mediating its regulation by cAMP–PKA in adipocytes. We therefore focused on Ser358 in our investigation of whether PKA phosphorylation of SIK2 is functionally important for the regulation of CRTC2 and HDAC4 by cAMP. Wild-type or Ser358Ala SIK2 were expressed in primary adipocytes, which were then stimulated with CL 316,243 to increase cAMP levels. As previously observed, the phosphorylation of CRTC2 and HDAC4 was reduced in response to increased levels of cAMP in cells expressing wild-type SIK2 (Fig. 3; Fig. 4B,C). Contrary to our expectation, this cAMP-induced dephosphorylation was not affected when expressing the Ser358Ala mutant SIK2 (Fig. 4B,C). This suggests that PKA phosphorylation of SIK2 on Ser358 does not alone mediate the cAMP-induced dephosphorylation of CRTC2 and HDAC4.
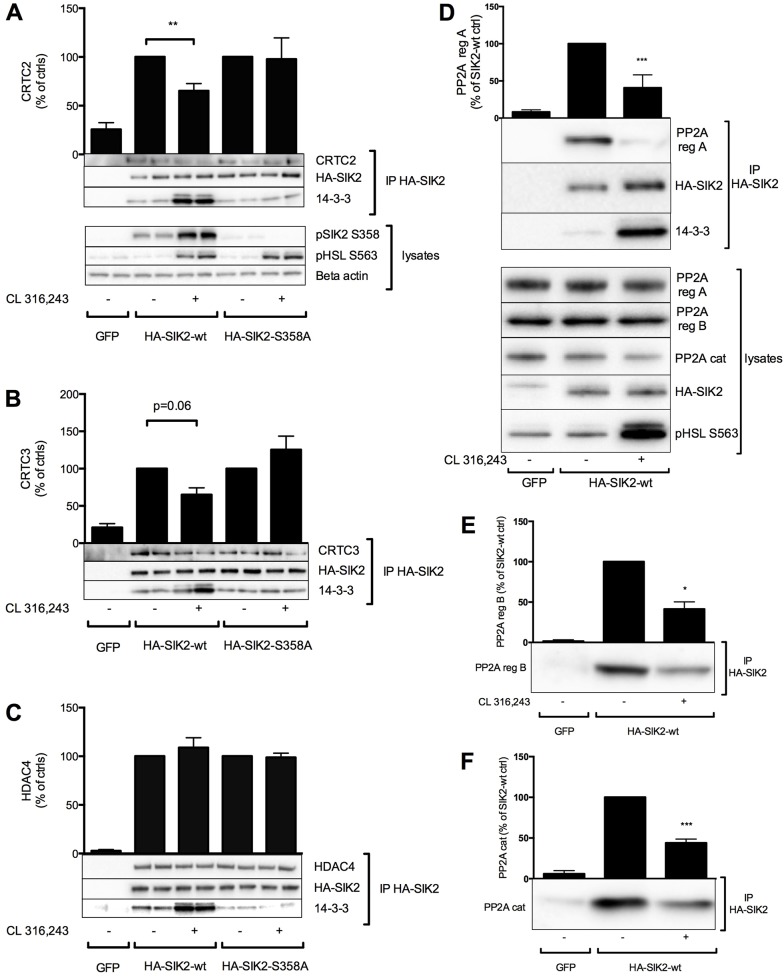
CRTC2, CRTC3, HDAC4 and PP2A interact with SIK2 in primary adipocytes
To identify SIK2-interacting proteins, we employed both focused and more general unbiased strategies. First, to address whether CRTC2, CRTC3 and HDAC4 are indeed direct molecular targets of SIK2 in adipocytes, as well as to further evaluate the functional importance of SIK2 Ser358 phosphorylation, we specifically investigated the ability of CRTC2, CRTC3 and HDAC4 to interact with different variants of SIK2 and the effect of cAMP on these interactions. HA-tagged wild-type or Ser358Ala SIK2 were expressed in primary adipocytes followed by stimulation with CL 316,243. HA–SIK2 was then immunoisolated using anti-HA–agarose, and co-immunoprecipitating proteins were identified by western blotting. Lysates from GFP-expressing cells were used as a negative control. As shown previously (Henriksson et al., 2012; Fig. 4A), CL 316,243 induced the binding of wild-type but not Ser358Ala SIK2 to 14-3-3 proteins (Fig. 5A–C). CRTC2, CRTC3 and HDAC4 all co-immunoprecipitated with both the wild-type and the mutated version of SIK2 (Fig. 5A–C). The interaction of wild-type SIK2 with CRTC2 and CRTC3 decreased in response to increased cAMP levels. This was not observed when analysing the binding of HDAC4 to SIK2, which remained unchanged in the presence of CL 316,243. Interestingly, the interaction of Ser358Ala SIK2 with CRTC2 and CRTC3 was not sensitive to cAMP induction, as was the case for wild-type SIK2 (Fig. 5A,B). This suggests that phosphorylation of Ser358 is important for cAMP-dependent regulation of the interaction of SIK2 with some of its molecular targets.
Fig. 5.
CRTC2, CRTC3, HDAC4 and PP2A interact with SIK2 in adipocytes. Adenoviral vectors encoding GFP or wild-type (wt) or Ser358Ala SIK2 were introduced into primary rat adipocytes, followed by treatment with CL 316,243 (100 nM, 30 min) and immunoprecipitation (IP) of HA-tagged SIK2. Co-immunoprecipitating proteins were detected by western blotting using antibodies against CRTC2 (A), CRTC3 (B), HDAC4 (C), 14-3-3 proteins (A–D), PP2A regulatory (reg) subunits A (PR65) (D) and B (PR55) (E), and PP2A catalytic (cat) subunit (F). Bar graphs represent the mean±s.e.m. of quantified western blot signals from three to seven individual experiments in which the data were normalized to those of non-treated cells for each construct (set as 100%). *P<0.05; **P<0.01; ***P<0.001.
As part of a more unbiased strategy to identify SIK2 targets or regulatory proteins, we stably expressed FLAG–SIK2 in HEK293 cells, from which large-scale immunoisolation of SIK2 was then performed. When identifying co-immunoprecipitated proteins by mass fingerprinting, we noted a large number of peptides originating from the regulatory as well as the catalytic subunit of PP2A (supplementary material Fig. S2). Immunoprecipitates of both wild-type and Ser358Ala SIK2 contained PP2A and, in this setting (using a non-quantitative form of mass spectrometry), we did not observe any marked differences between samples originating from cells stimulated with or without forskolin. Interestingly, HA–SIK2 expressed in primary adipocytes was also associated with different subunits of PP2A, and in these cells the amount of the PP2A regulatory subunits A and B, and PP2A catalytic subunits in the immunoprecipitate was clearly reduced in response to cAMP elevation by CL 316,243 (Fig. 5D–F).
SIK2 signalling regulates GLUT4 expression and glucose uptake in adipocytes
CREB and HDAC4 have been suggested to inhibit GLUT4 mRNA expression in adipocytes (Qi et al., 2009; Weems et al., 2012). Therefore, we silenced SIK2, CRTC2 or HDAC4 in 3T3-L1 adipocytes (supplementary material Fig. S3A–C) and analysed the expression of GLUT4 protein by western blotting. Cells treated with SIK2 siRNA displayed a 40–50% reduction in GLUT4 protein levels (and a 30% reduction in GLUT4 mRNA level; data not shown) compared with levels in Scr-siRNA-transfected cells (Fig. 6A). By contrast, silencing of CRTC2 or HDAC4 resulted in a 30–50% increase in GLUT4 protein expression (Fig. 6B,C). Moreover, adipocytes isolated from SIK2 whole-body-knockout mice displayed a 60% reduction in GLUT4 protein levels (Fig. 6D). These data indicate that GLUT4 levels are positively regulated by SIK2, possibly through its effects on CRTC2 and HDAC4. Considering the important role of GLUT4 in glucose uptake, we next investigated the impact of SIK2 on basal and insulin-stimulated glucose uptake in primary rat adipocytes by monitoring the uptake of 14C glucose in cells expressing GFP or the wild-type or kinase-inactive Thr175Ala SIK2. Adipocytes expressing wild-type SIK2 displayed increased basal glucose uptake compared with that of adipocytes expressing GFP or Thr175Ala SIK2 (Fig. 6E). Glucose uptake in the presence of insulin was not affected by the expression of wild-type SIK2. However, the level of insulin-induced glucose uptake in adipocytes expressing the inactive Thr175Ala HA–SIK2 was found to be significantly lower compared with that of cells expressing GFP or wild-type SIK2, raising the possibility that Thr175Ala SIK2 acts in a dominant-negative manner. The in vitro kinase activity of SIK2 immunoprecipitated from Thr175Ala SIK2-expressing cells was, however, not reduced compared with that of GFP-expressing cells (data not shown), nor was the phosphorylation of downstream targets CRTC2 and HDAC4 (Fig. 3E, F), arguing against this idea.
Fig. 6.
SIK2–CRTC2–HDAC4 signalling regulates GLUT4 protein levels and glucose uptake in adipocytes. The expression of SIK2 (A), CRTC2 (B) and HDAC4 (C) was reduced by electroporation of siRNA into differentiated 3T3-L1 adipocytes, and GLUT4 protein levels were analysed by western blotting 72 h after electroporation. Silencing of the respective proteins was confirmed by western blotting (supplementary material Fig. S2). Bar graphs represent the mean±s.e.m. of quantified western blot signals from three or four individual experiments, in which the data were normalized to those of Scr-treated cells (set as 100%). (D) Adipocyte lysates from 28-week-old male wild-type (wt) and SIK2-knockout (ko) mice were analysed for GLUT4 protein levels by western blotting. The bar graph represents the mean±s.e.m. of quantified western blot signals from three wild-type and two knockout mice. Similar results were obtained when analyzing lysates from adipocytes isolated and pooled from 11–21-week-old mice (seven per genotype, data not shown). (E) Adenoviral vectors encoding GFP or wild-type or Thr175Ala (T175A) HA–SIK2 were introduced into primary rat adipocytes and basal and insulin-stimulated (10 nM, 30 min) 14C glucose uptake was measured. Data presented show the mean±s.e.m. from six individual experiments, in which the data were normalized to those of non-treated GFP-expressing cells (set as 100%). Primary rat adipocytes were treated with increasing concentrations of the SIK inhibitors HG-9-91-01 (F–H) or MRT199665 (I) for 16 h, and 14C glucose uptake was measured in the absence or presence of insulin (10 nM, 30 min). Graphs represent the mean±s.e.m. from three or four individual experiments in which the data were normalized to those of non-treated cells (F,I), cells treated without inhibitor but with insulin (G) or cells treated without insulin for each dose of the inhibitor (H). *P<0.05; **P<0.01.
We also pre-treated rat adipocytes with increasing concentrations of HG-9-91-01, a highly selective pan-SIK inhibitor, or MRT199665, an inhibitor of several AMPK-related kinases including SIKs, albeit with lower potency (Clark et al., 2012), for 16 h (to allow for changes in gene expression). We thereafter analysed glucose uptake in the absence and presence of insulin. As shown in Fig. 6F,G, basal and insulin-stimulated glucose uptake were inhibited by 40–50% as a result of pre-treatment with 1 µM HG-9-91-01. The fold-increase in glucose uptake in response to insulin stimulation was, however, not different in inhibitor-treated cells (Fig. 6H). Pre-treatment with MRT199665 at slightly higher concentrations than that of HG-9-91-01 resulted in a similar inhibition of basal glucose uptake (Fig. 6I) (and uptake in the presence of insulin; data not shown). These data collectively suggest that SIK2 activity is involved in maintaining basal glucose uptake in primary rat adipocytes.
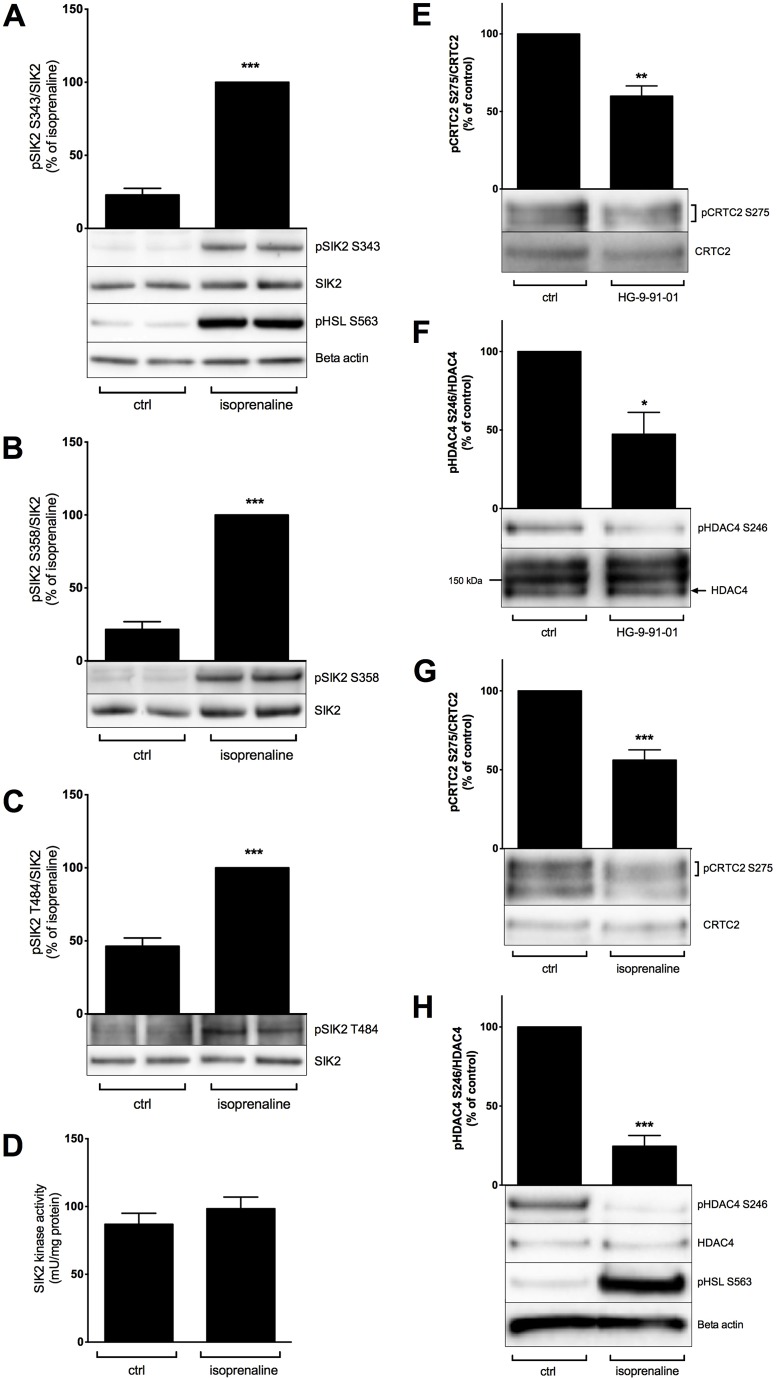
SIK2 signalling in human adipocytes
Our data from rodent adipocytes make SIK2 an interesting protein to study in relation to human adipose tissue function and dysfunction. Because there are no previous studies of SIK2 in humans, we decided as a first step to address the existence of SIK–CRTC2–HDAC4 signalling and its potential regulation in response to cAMP–PKA activation in human adipocytes. To do so, we used human adipocytes isolated from surgical adipose tissue biopsies. Similar to rat adipocytes, stimulation of human adipocytes with the β-adrenergic receptor agonist isoprenaline resulted in the phosphorylation of SIK2 on residues Ser343, Ser358 and Thr484, without altering the specific kinase activity (Fig. 7A–D). Moreover, the phosphorylation of CRTC2 and HDAC4 was significantly lower in isoprenaline- or HG-9-91-01-treated cells compared with that of the control (Fig. 7E–H).
Fig. 7.
Regulation of SIK2 signalling in human adipocytes. Primary adipocytes isolated from human adipose tissue were stimulated with isoprenaline (100 nM, 30 min) (A–D,G,H) or treated with HG-9-91-01 (1 µM, 16 h) (E,F). The phosphorylation of SIK2 on residues Ser343 (A), Ser358 (B) and Thr484 (C), and the phosphorylation of CRTC2 on Ser275 (E,G) and HDAC4 on Ser246 (F,H) was analyzed by western blotting using phosphospecific antibodies. Bar graphs represent the mean±s.e.m of quantified western blot signals from two (E,F), six (B), seven (C,G) or nine (A,H) individual subjects, normalized to total protein levels and expressed as a percentage of signals from isoprenaline-stimulated cells (A–C) or from non-treated cells (E–H). Representative immunoblots are shown. The phosphorylation of HSL on Ser563 was used as a positive control for the stimulation with isoprenaline, and β-actin was used as loading control. The kinase activity of SIK2 (D) was measured by in vitro kinase assay towards the peptide substrate HDAC5tide. The bar graph represents the mean±s.e.m of activity data from six individual subjects. P<0.05; **P<0.01; ***P<0.001.
DISCUSSION
So far very few studies have attempted to identify molecular targets or biological roles of SIK2 in adipocytes – even though SIK2 levels are several-fold higher in adipose tissue than in any other tissue. Here, we demonstrate that the transcriptional regulators CRTC2, CRTC3 and HDAC4 are direct substrates of SIK2 in adipocytes. Importantly, we also show the existence of a SIK–CRTC2–HDAC4 pathway and its regulation by physiologically relevant stimuli in human adipose tissue. This highlights a potential role for SIK2 in human adipose tissue function and in the development of obesity and associated disorders. Furthermore, we show that phosphorylation of SIK2 by PKA is required for some aspects of the regulation of CRTCs by cAMP – a subject that has so far been poorly understood. We also reveal a link between SIK2 signalling and the regulation of GLUT4 levels and glucose uptake in adipocytes.
Having established that CRTCs and HDAC4 are regulated downstream of LKB1 in adipocytes, we employed different strategies to test the hypothesis that SIK2, at least in part, mediates this regulation. Silencing, acute pharmacological inhibition and overexpression experiments performed in primary or cultured adipocytes supported the notion that CRTC2, CRTC3 and HDAC4 are phosphorylated downstream of SIK2. We conclude that CRTC2, CRTC3 and HDAC4 are most likely direct substrates of SIK2 in adipocytes, based on the following: (1) CRTCs and class IIa HDACs are phosphorylated by SIK isoforms in vitro (Henriksson et al., 2012), (2) the sites we found to be regulated conform to a previously described SIK2 consensus sequence (LXBS/TXSXXXL) (Screaton et al., 2004), and (3) SIK2 interacts physically with CRTC2, CRTC3 and HDAC4 in adipocytes (Fig. 5).
To monitor site-specific phosphorylation of CRTC2 and CRTC3, we employed phosphospecific antibodies against Ser275 and Ser162, respectively, both of which are sites previously suggested to be important for the localisation of these proteins (Clark et al., 2012; Jansson et al., 2008; Screaton et al., 2004). Ser275 was shown to be regulated by glucose in beta cells, where it is phosphorylated by the AMPK-related kinase MARK2 and mediates the binding of CRTC2 to 14-3-3 proteins under basal conditions (Jansson et al., 2008). Interestingly, in adipocytes, we found that this site is also a target of regulation by cAMP and SIK2. The shift in electrophoretic mobility that we observed (for example, when overexpressing wild-type SIK2) indicates an effect of SIK2 on overall phosphorylation of CRTC2 and CRTC3 (and not only on Ser275 and Ser162, respectively). In addition to Ser275, CRTC2 activity is controlled by phosphorylation on Ser171 and Ser307 (Koo et al., 2005; Screaton et al., 2004; Uebi et al., 2010). Owing to the lack of commercially available tools of sufficient quality, we were not able to confidently study the effect of SIK2 on these sites in adipocytes. However, based on western blotting of lysates from SIK2-siRNA-treated cells with the pCRTC3-Ser162-specific antibody, which crossreacts with the corresponding Ser171 site on CRTC2, it appears that Ser171 is also phosphorylated by SIK2 in adipocytes. We attempted to directly map the sites phosphorylated in CRTC2 when coexpressed with wild-type SIK2 in primary adipocytes, but this analysis was hampered by the poor yield of CRTC2 and a tryptic cleavage pattern generating several peptides that were too large for detection.
In 3T3-L1 adipocytes expressing SIK2 siRNA, the phosphorylation of CRTC2, CRTC3 and HDAC4 was comparable to the level observed in Scr-siRNA-expressing cells stimulated with CL 316,243, which induced a dephosphorylation of all three proteins. This suggests that SIK2 accounts for the portion of CRTC and HDAC phosphorylation that is inhibited by cAMP. However, the SIK3 isoform is also regulated by cAMP-induced phosphorylation in adipocytes, resulting in reduced kinase activity (Berggreen et al., 2012), and could potentially also phosphorylate CRTCs and class IIa HDACs in adipocytes. Additional kinases involved in the residual phosphorylation of CRTC2 and HDAC4 are still to be determined, but might include MARK2, AMPK and/or SIK1. However, AMPK is not likely to mediate cAMP effects (dephosphorylation) on CRTCs and class IIa HDACs in adipocytes, because AMPK in fact has been shown to be activated by elevated cAMP levels in these cells (Omar et al., 2009).
It is assumed that PKA-dependent phosphorylation and inhibition of SIK2 mediates the net dephosphorylation and nuclear translocation of CRTCs and class IIa HDACs by cAMP; however, activation of the cAMP–PKA pathway does not affect the intrinsic kinase activity of SIK2 (Henriksson et al., 2012; supplementary material Fig. S1). The identification of a direct action of SIK2 on CRTCs and HDAC4 provided a read-out for SIK2 cellular activity in adipocytes. This enabled us to perform a series of experiments in order to address the functional importance of the phosphorylation of SIK2 by PKA in these cells. As we had shown previously in HEK293 cells (Henriksson et al., 2012), phosphorylation of Ser358 was found to have a much stronger impact on total PKA phosphorylation and 14-3-3 binding of SIK2 than any of the other PKA sites. Moreover, we have also shown that Ser358 mediates a cAMP-induced translocation of SIK2 to the cytosol in adipocytes (Henriksson et al., 2012). Based on these data, we focused on the role of Ser358 in the regulation of CRTCs and HDAC4 by cAMP. Contrary to our expectations, expressing the cAMP-resistant Ser358Ala SIK2 mutant did not prevent the dephosphorylation of CRTC2 and HDAC4 in response to cAMP. The possibility remains that the expression of the cAMP-insensitive SIK2 mutant was not sufficient to mask the effect of endogenous SIK2, which would still be responsive to cAMP. We therefore also tested the effect of Ser358Ala SIK2 in our co-immunoprecipitation assay, in which we found that wild-type SIK2 interacts with CRTCs and HDAC4, and that the interaction with CRTCs (but not the one with HDAC4) is reduced by cAMP. Although these results by themselves suggest that the regulation of CRTC2 and CRTC3 by cAMP indeed involves SIK2, this assay also provided an opportunity to selectively analyse the effect of exogenous (HA-tagged) SIK2 on CRTCs and HDAC4. Interestingly, mutation of Ser358 to Ala prevented the cAMP-induced reduction in co-immunoprecipitated CRTC2 or CRTC3 observed in wild-type SIK2-expressing cells. Thus, our data suggest that Ser358 is functionally important for some aspects of CRTC2 and CRTC3 regulation by cAMP, but not for that of HDAC4. Further studies are required to fully understand the mechanism whereby CRTCs and HDAC4 are activated in response to cAMP in adipocytes and other cells, and the role of SIK2 herein. Individual or additional PKA sites might, for example, have subtle effects on SIK2 function that we have so far not recognized, and mutating these sites (possibly in combination) could be required to prevent the regulation of CRTCs and HDAC4 by cAMP. In support of this, we recently demonstrated that expression of a mutant form of SIK2 in which all four PKA sites were substituted for an Ala prevented the dephosphorylation of CRTC2, CRTC3 and HDAC4 in hepatocytes, but the mutation of the sites one by one did not (Patel et al., 2014).
In our search for SIK2-interacting proteins, we also found that subunits of PP2A co-immunoprecipitate with SIK2 in primary adipocytes, and that the interaction was reduced in response to cAMP induction. During the revision of our paper, it was reported that SIK2 interacts with PP2A in HEK293T cells and that this is functionally important to maintain both phosphatase and kinase activities (Lee et al., 2014). Although the exact mechanisms remain to be identified, the reduction of PP2A binding to SIK2 that we observed in response to cAMP–PKA activation could thus be contributing to the inhibition of SIK2 function by cAMP signalling.
Our identification of the transcriptional regulators CRTCs and HDAC4 as molecular targets for SIK2 in adipocytes suggests that SIK2 is involved in the regulation of gene transcription in adipocytes. A previous report demonstrated that SIK2 inhibits expression of the lipogenic genes ACC2 (also known as ACACB), FASN and SCD1 in 3T3-L1 CAR adipocytes (Du et al., 2008). However, the SIK2 substrate(s) mediating this effect was not identified. Moreover, a potential link between these genes and CRTCs and class IIa HDACs, as well as their regulation by cAMP, has not been described to our knowledge. Interestingly, a recent study proposed that regulation of class IIa HDACs by cAMP influences the transcriptional regulation of GLUT4 (Weems et al., 2012), implying a role for SIK2–HDAC4 in controlling glucose uptake in adipocytes. SIK2 could potentially also regulate GLUT4 levels through its effects on CRTCs, given that adipocyte CREB has been associated with the transcription of GLUT4 through regulation of the repressor ATF3 (Qi et al., 2009). With this in mind, we focused our present analysis of SIK2 function on GLUT4 expression and glucose uptake. We indeed found that siRNA-mediated silencing or genetic deletion of SIK2 (whole-body knockout) resulted in reduced GLUT4 levels in adipocytes and that silencing of HDAC4 or CRTC2 had the opposite effect. In relation to this, overexpression of wild-type but not kinase inactive Thr175Ala SIK2, was sufficient to enhance basal glucose uptake. Conversely, two different inhibitors of SIK isoforms reduced glucose uptake. Although the maximal level of glucose uptake in the presence of insulin was also reduced after pre-treating cells with SIK inhibitors, the fold induction by insulin was not affected. Two studies have proposed that SIK2 negatively affects insulin sensitivity in 3T3-L1 adipocytes and Müller cells of the eye, by phosphorylating IRS1 on a serine residue (Ser789 and Ser794 in murine IRS1 and human IRS1, respectively) (Horike et al., 2003; Küser-Abali et al., 2013). However, we have previously failed to detect any phosphorylation of the corresponding IRS1 peptide in vitro (Henriksson et al., 2012), and our present results with regards to insulin-induced glucose uptake also argue against any effects of SIK2 on insulin signalling per se in adipocytes. In our experiments, the 16-h pre-treatment with SIK inhibitors was followed by an incubation period of 2 h in the absence of inhibitors. Any acute effects of SIK2 inhibition were thus most likely reversed before the glucose uptake assay, and we therefore suggest that the observed effects are due to changes in gene expression. Taken together, our data are in line with the hypothesis that SIK2 stimulates the rate of glucose uptake by preventing HDAC4 and CRTC2 from inhibiting GLUT4 transcription. Future experiments should further evaluate this hypothesis, for example, by investigating the direct requirement for CRTC2 and HDAC4 in the regulation of glucose uptake by SIK2. Another area to be explored is the potential role of SIK2 in mediating hormonal effects on the expression of adipocyte genes, for example GLUT4, which has been reported to be downregulated by cAMP (Weems et al., 2012).
The main findings of this paper, and our hypothesis for how these might be linked, are summarized in Fig. 8. In conclusion, we present evidence that the transcriptional regulators CRTC2, CRTC3 and HDAC4 are regulated by cAMP and are molecular targets of SIK2 in adipocytes. We also show that PP2A interacts with SIK2 in adipocytes and that this interaction is regulated by cAMP. The phosphorylation of SIK2 by PKA might restrict SIK2 accessibility and/or binding to some of these targets (CRTC2, CRTC3 and PP2A). This indicates that SIK2 could be involved in controlling gene transcription in adipocytes, in particular those affected by cAMP. Indeed, we found that GLUT4 mRNA and protein levels, as well as glucose uptake, are stimulated downstream of SIK2 in adipocytes.
Fig. 8.
Hypothesis for SIK2 action in adipocytes. In this study, we have shown that CRTC2, CRTC3 and HDAC4 are direct molecular targets of SIK2 in adipocytes. Both SIK2 and its substrates are subjected to regulation by cAMP–PKA (Henriksson et al., 2012; and current data). SIK2 is phosphorylated by PKA on multiple sites, out of which Ser358 mediates 14-3-3 binding and cytosolic translocation. By contrast, CRTC2, CRTC3 and HDAC4 are dephosphorylated in response to cAMP elevation and this has, in other models, been shown to lead to nuclear translocation. Our current data suggest that Ser358 phosphorylation might restrict the binding or accessibility of SIK2 to its substrates and to PP2A, but further studies are warranted in this area. Furthermore, we show that GLUT4 expression is stimulated or inhibited downstream of SIK2 or CRTC2–HDAC4, respectively. Finally, basal glucose uptake is promoted by SIK2, potentially through its effects on CRTC2, HDAC4 and GLUT4.
MATERIALS AND METHODS
Materials
3T3-L1 cells were from American Type Culture Collection and DMEM, FBS, gentamicin, dexamethasone, IBMX, insulin, isoprenaline, CL 316,243 and HA–agarose were all from Sigma. Protein-G–Sepharose was from GE Healthcare. SuperScript™II RNaseH, DNase I, pre-cast Novex SDS polyacrylamide Bis-Tris gels, Tris-acetate gels and LDS sample buffer were all from Invitrogen. SuperSignal West Pico and Femto Chemiluminescent Substrates were from Thermo Scientific. RNeasy® Mini Kit and QIAzol™ lysis reagent were from Qiagen. Taqman® Gene Expression Assays for Stk11 (LKB1), Prkaa1 (AMPKα1), Sik2, Sik3, ribosomal protein S29 (Rps29) and 18S rRNA, Taqman® Universal PCR Master mix and SYBR® Green PCR Master Mix were obtained from Applied Biosystems. The QuantiTect Primer Assays with SYBR® Green detection for Glut4, Rps29 and 18s rRNA were obtained from Qiagen. Penicillin-streptomycin was from VWR. Complete protease inhibitor cocktail and phenylisopropyl adenosine (PIA) were from Roche. [γ32P]-ATP, 14C-glucose and 3H-2D-glucose were from Perkin Elmer, and phosphocellulose P81 paper was from Whatman. siRNAs were from Ambion. HDAC5tide was synthesised by GL Biochem, China. Adenoviral vectors for GFP, human wild-type HA–SIK2 (GenBank accession number XM_041314) and SIK2 mutants Ser343Ala, Ser358Ala, Thr484Ala, Ser587Ala and Thr175Ala were produced by Vector BioLabs Inc. HG-9-91-01 and MRT199665 were kindly provided by Kristopher Clark and Philip Cohen (University of Dundee, UK; Clark et al., 2012).
Antibodies
The following primary antibodies were used for western blotting: anti-HSL-pSer563, anti-ERK1/2, anti-HDAC-4/5/7-pSer246/259/155, anti-AMPK, anti-AMPK-pThr172, anti-CRTC3, anti-PP2A reg A (PR65, detects both α- and β isoforms), anti-PP2A reg B (PR55, might detect α, β, γ and δ isoforms) and consensus antibodies against phosphorylated PKA substrates (PKA cons) were all from Cell Signaling Technology. Anti-PP2A catalytic subunit (α isoform) was from Millipore. Anti-HA, anti-GAPDH and anti-β-actin were from Sigma, and anti-14-3-3 was from Santa Cruz. Anti-CRTC2 was from Calbiochem and anti-HDAC4 and anti-LKB1 were from Abcam. The following antibodies were raised in rabbit and affinity purified by Innovagen (Lund, Sweden); anti-CRTC2-pSer275 (residues 268–282 of human CRTC2, AMNTGGpSLPDLTNLH), anti-SIK2 (residues 906–926 of human SIK2, LFDCEMLDAVDPQHNGYVLVN), anti-SIK2-pSer358 (residues 351–365 of human SIK2, DGRQRRPpSTIAEQTV) and anti-SIK3 (residues 1349–1369 of human SIK3, TDILLSYKHPEVSFSMEQAGV). Anti-GLUT4 antibodies were raised and purified as described previously (Dawson et al., 2001). CRTC2 pSer275 was a kind gift from Robert Screaton (University of Ottawa, Canada). The generation of anti-CRTC2 used for immunoprecipitation, anti-SIK2-pSer343 and anti-SIK2-pThr484 antibodies was described previously (Patel et al., 2014). Anti-CRTC3-pSer162 was a kind gift from Kristopher Clark and Philip Cohen (University of Dundee, UK; Clark et al., 2012), and secondary antibodies conjugated to horseradish peroxidase (HRP) were from Invitrogen (anti-rabbit-IgG), Pierce (anti-sheep-IgG) and GE Healthcare (anti-mouse-IgG).
RNA preparation and quantitative real-time PCR
Analysis of gene expression was assessed by quantitative real-time PCR. 3T3-L1 adipocytes were lysed and homogenized in QIAzol™ lysis reagent. Total RNA was isolated using the RNeasy® Mini Kit according to the manufacturer's recommendations. Total RNA (1 µg) was treated with DNase I and then reverse transcribed using random hexamers (Amersham Biosciences) and SuperScript™II RNaseH reverse transcriptase according to the manufacturer's recommendations. The cDNA was used in quantitative PCR using TaqMan or SYBR®Green chemistry in an ABI 7900 Sequence Detection System. Relative abundance of mRNA was calculated after normalization to the geometric mean of two internal control genes (Rps29 and 18S rRNA) (Ferguson et al., 2010; Vandesompele et al., 2002). Each sample was analysed in duplicate.
Generation of a 3T3-L1 cell line with stable LKB1 shRNA expression
3T3L1 fibroblasts stably expressing scrambled or LKB1 shRNA were generated by means of lentiviral transduction as described previously (Gormand et al., 2014). Differentiation and preparation of cell lysates for analysis by western blot was performed as described below.
Culture, differentiation, electroporation and stimulation of 3T3-L1 adipocytes
3T3-L1 fibroblasts were cultured and differentiated as described previously (Berggreen et al., 2009). All experiments were performed at day 10–14 after differentiation. Between 85% and 95% of differentiated cells were electroporated and/or stimulated with the β-adrenergic receptor agonist CL 316,243. Cells were electroporated with 1–1.5 nmole per 10-cm dish of either scrambled (Scr) siRNA (Ambion, Neg control #1 siRNA) or siRNA targeting SIK2 (Ambion, ID s108118), CRTC2 (Ambion, ID s92672) or HDAC4 (Ambion, ID s101930) using a Bio-Rad Gene Pulser™ with the following settings: 0.18 kV, 960 µFarad, resistance of ∞. 3T3-L1 adipocytes from one 10-cm dish were plated onto two to three wells of a six-well plate following electroporation and were stimulated as described above after 48–72 h of gene silencing. After stimulation, cells were rinsed with PBS and harvested in ice-cold lysis buffer containing 50 mM Tris-HCl pH 7.5, 1 mM EGTA, 1 mM EDTA, 1% (w/v) NP-40, 1 mM sodium orthovanadate, 10 mM sodium-β-glycerophosphate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.27 M sucrose, 1 mM dithiothreitol (DTT) and complete protease inhibitor (one tablet/50 ml). Following centrifugation at 4°C for 15 min at 13,000 g, infranatants were collected and total protein content was determined by Bradford.
Isolation, transduction and stimulation of primary adipocytes
Subcutaneous and/or omental adipose tissue was collected, after written informed consent, from 11 patients (BMI 41±10.3 kg/m2) undergoing abdominal surgery. The human adipose tissue was placed in PBS at room temperature and immediately transported to the laboratory for isolation. Adipocytes were prepared from human adipose tissue or rat epididymal adipose tissue as described previously under a protocol approved by the ethical review committee at Lund University (number M286-10 for animals and 2012/134 for humans) (Berggreen et al., 2009). After isolation, human adipocytes were either stimulated directly with isoprenaline in a 37°C shaking water bath or incubated overnight with HG-9-91-01 in DMEM containing penicillin and streptomycin (100 U/ml and 0.1 mg/ml, respectively), 200 nM phenylisopropyl adenosine (PIA) and 3.5% BSA at 37°C under 5% CO2. Adipocytes were subsequently washed and resuspended in KRH containing 1% BSA, defined in Berggreen et al., 2009, and lysed in lysis buffer containing 50 mM Tris-HCl pH 7.5, 0.27 M sucrose, 1 mM EDTA, 1 mM EGTA, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1 mM DTT and complete protease inhibitor cocktail (one tablet/50 ml). Lysates were centrifuged at 13,000 g for 15 min (4°C) and the supernatant was collected. Rat adipocytes were transduced overnight with adenoviruses encoding wild-type, Ser343Ala, Ser358Ala, Thr484Ala, Ser587Ala or Thr175Ala HA–SIK2 (50–150×106 pfu/ml cells; ∼40–120 MOI) in DMEM containing 1 mg/ml gentamicin, 200 nM PIA and 3.5% BSA at 37°C under 5% CO2. Adipocytes were subsequently washed and resuspended in KRH and stimulated in a 37°C shaking water bath, and lysates were prepared as described previously (Berggreen et al., 2009). The transduction efficiency was ∼90%, as determined by transduction with GFP, and the fold overexpression when using 150×106 pfu/ml cells was ∼50-fold in terms of SIK2 kinase activity level in wild-type SIK2-expressing cells compared to those expressing GFP (data not shown).
SIK2 knockout mice
Whole-body SIK2 knockout mice were generated by crossing SIK2+/lox mice described previously with deleter EIIa-CRE transgenic mice to produce SIK2+/− mice (Holzenberger et al., 2000; Patel et al., 2014). These mice were crossed to generate wild-type (control) and SIK2−/− mice. Routine genotyping was carried out by multiplex PCR on tail DNA with the P1 (5′-GTAGTTTACATTAGCACATTGGTGCCTC-3′), P2 (5′-CCTAGAATGCACTCTGCAAACACTGGACAC-3′) and P3 (5′-TCTACATGGAGGGTGTCGCAGAGCTCCATG-3′) primers, to yield amplification products of 391 bp (wild-type allele) with P1/P2 and 708 bp (knockout allele) with P1/P3. Mice were bred (courtesy of Bente Kiens and Erik Richter, University of Copenhagen) and sacrificed under a protocol approved by the Danish Animal Experimental Inspectorate and that complied with the European Convention for the Protection of Vertebrate Animals used for Experiments and other Scientific Purposes (2010/63/EU). Mice were housed in temperature-controlled (22±1°C) facilities, maintained on a 12∶12 light∶dark cycle and received standard chow (Altromin, cat. no. 1324; Brogaarden, Lynge, Denmark) and water ad libitum. Adipocytes were isolated from epididymal adipose tissue of male 28-week-old or 11–12-week-old mice, as described for rats (Berggreen et al., 2009).
Measurement of SIK2 kinase activity
Cell lysates from 3T3-L1 or human adipocytes (25 or 10 µg of total protein, respectively) were incubated for 1 h on a shaking platform with 5 µg of anti-SIK2 conjugated to 5 µl of packed Protein-G–Sepharose. The immunoprecipitates were then further processed and the phosphotransferase activity of immunoprecipitated SIK2 towards the peptide substrate HDAC5tide (PLRKTASEPNLKRRR, residues 253–267, R265, 266, 267 of human HDAC5) was measured as described previously (Berggreen et al., 2009; Henriksson et al., 2012).
Immunoprecipitation and western blot analysis
Between 800 µg and 1200 µg of total protein from primary rat adipocytes expressing HA-tagged SIK2 was pre-cleared using 5 µl of packed Protein-G–Sepharose and then immunoprecipitated with 5 µl of packed anti-HA–agarose and further processed as described above, with the exception that co-immunoprecipitates were washed in the presence of 0.15 M NaCl (Berggreen et al., 2009). Alternatively, lysates (200 µg) were subjected to immunoprecipitation with 5 µg of anti-CRTC2 conjugated to 5 µl of packed Protein-G–Sepharose. Immunoprecipitates or cell lysates (5–30 µg of total protein) were heated in LDS sample buffer before loading onto pre-cast Novex 4–12% Bis-Tris or 7% Tris-acetate gels. Tris-acetate gels were only used for the detection of electrophoretic mobility shifts of CRTC2 and CRTC3 and were run at 100 V for 1.5 h. Proteins were then transferred to nitrocellulose membrane as described previously (Berggreen et al., 2009). Detection was performed using HRP-conjugated secondary antibodies, ECL reagent and a Bio-Rad Chemidoc XRS+ system. Quantifications were made by using the software Image Lab 4.0.
14C glucose uptake in rat adipocytes
Primary rat or mouse adipocytes were transduced with adenoviruses encoding GFP, wild-type or Thr175Ala HA–SIK2 or, alternatively, were treated with SIK inhibitors. Transductions and inhibitor treatments were performed overnight in DMEM supplemented with gentamicin (1 mg/ml), 3.5% BSA and PIA (200 nM), under 5% CO2 at 37°C, as described above. Cells were then washed three times in a glucose-free incubation buffer (pH 7.4) containing 30 mM HEPES, 0.12 M NaCl, 4 mM KH2OPO4, 1 mM MgSO4, 0.75 mM CaCl2, 10 nM NaHCO3, 200 nM adenosine and 1% BSA. A suspension of 5% cells was prepared in triplicate and incubated for 1 h at 37°C before the addition of insulin (10 nM) and cytochalasin B (10 µM). Adipocytes were stimulated for 30 min followed by addition of 14C glucose (Perkin Elmer NEC042x250UC, 2.5 µl/ml incubation buffer). The 14C glucose uptake was stopped after 30 min by aliquoting 300 µl of the 400 µl adipocyte suspension to Beckman microtubes containing 60 µl of dinonylphtalate (DNP). The adipocyte suspension was centrifuged at 6000 g and frozen at −80°C before the adipocytes were collected and subjected to scintillation counting.
Generation of a stable HEK293 cell line expressing FLAG–SIK2 and large-scale purification of FLAG-SIK2
The generation and stimulation of HEK293 cells with stable inducible expression of wild-type or Ser358Ala FLAG–SIK2 was performed using the FlpIn™ system as described previously (Henriksson et al., 2012). HEK 293 cells expressing wild-type or Ser358Ala FLAG–SIK2 or vector alone were harvested in lysis buffer containing 40 mM HEPES pH 7.4, 1% (w/v) Triton X-100, 1.5 mM sodium orthovanadate, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 120 mM NaCl and complete protease inhibitor (one tablet/50 ml). Lysates (50 mg of total protein) were pre-cleared with protein-G–Sepharose for 1 h, and subsequently incubated with 50 µl of packed anti-FLAG–agarose (Sigma) for 2 h. The beads were washed twice with lysis buffer (defined above) containing 0.5 M NaCl and twice with 10 mM Tris-HCl pH 7.5. Immunoprecipitated proteins were then eluted by incubating for 1 h in 80 µl of 50 mM Tris-HCl pH 7.5, 150 mM NaCl and 200 µg/ml 3×FLAG peptide (Sigma) at room temperature. After concentrating by speed-vac, the eluates were loaded on a polyacrylamide gel, which was subsequently stained with colloidal Coomassie Blue.
Mass fingerprinting
Protein bands were excised as shown in supplementary material Fig. S2 and incubated with 10 mM DTT at 37°C for 20 min, followed by treatment with 50 mM iodoacetamide at room temperature for 20 min in the dark to alkylate Cys residues. After digestion with trypsin for 16 h, the resultant peptides were analysed by mass spectrometric analysis (LC-MS-MS) on a linear ion trap-orbitrap hybrid mass spectrometer (Orbitrap-Classic, Thermo) equipped with a nanoelectrospray ion source (Thermo) and coupled to a Proxeon EASY-nLC system. Peptides were injected onto a Thermo (part number 160321) Acclaim PepMap100 reverse phase C18 3-µm column, 75 µm×15 cm, with a flow rate of 300 nl/min. Peptides were eluted with a linear gradient of 95% solvent A (2% acetonitrile, 0.1% formic acid in H2O) to 35% solvent B (90% acetonitrile, 0.08% formic acid in H2O) at 20 min, followed by a rise to 80% solvent B at 23 min, maintained at 80% solvent B for 5 min, followed by re-equilibration. Data files were analysed using RAW2msm (Matthias Mann, Max-Planck Institute) followed by Mascot searches (www.matrixscience.com) against the SwissProt/Human Database of 25 April 2010.
Statistical methods
Results are presented as means±s.e.m., and analyses were performed using Graph Pad Prism 5. The statistical significance of differences was defined as *P<0.05, **P<0.01, ***P<0.001, and was determined using a paired two-tailed Student's t-test or, where appropriate, one-way ANOVA followed by Holm Sidak's multiple comparison test.
Supplementary Material
Acknowledgments
We thank Eva Ohlson for excellent technical support, Christine Berggreen for assistance in animal experiments, Dr Robert Screaton for the CRTC2 Ser275 phosphospecific antibody and other CRTC2 reagents and Dr Kristopher Clark and Sir Philip Cohen for SIK inhibitors and CRTC3 antibodies. Professor Bente Kiens and Professor Erik Richter are acknowledged for providing SIK2 KO mice.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
E.H. designed and performed most of the experiments, aided in the design of the study and wrote the manuscript draft; O.G. was responsible for the conception and design of the study, performed experiments and edited and submitted the manuscript; J.S., A.G. and S.W. performed experiments and reviewed the paper prior to submission; N.A.M. performed phosphopeptide mapping and reviewed the paper prior to submission; M.F. generated the SIK2 KO mice and reviewed the paper prior to resubmission; A.M.F. bred these mice and aided in the removal of tissue for adipocyte isolation, as well as reviewing the paper prior to resubmission; K.S. and D.G.C. provided reagents, aided in the generation of cell lines and performed the analysis of interacting proteins by mass spectrometry, as well as reviewing the paper prior to resubmission; E.D. and M.E. provided human adipose tissue and reviewed the paper prior to resubmission; K.G.S. performed experiments, provided scientific advice with regard to glucose uptake and GLUT4 analysis, and reviewed the paper prior to submission.
Funding
This work was supported by the Swedish Research Council [grant numbers 2007-5721 and 2012-2869]; STINT Institutional Grant for Younger Researchers [grant number YR2009-7032]; The Novo Nordisk Foundation; The Swedish Diabetes Association; Thuring Foundation; Magnus Bergvall Foundation; The Craaford Foundation; Påhlsson Foundation; The Royal Physiographic Society; and the Blucher Foundation. N.A.M. is supported by Cancer Research UK. D.G.C. is funded by the UK Medical Research Council. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.153932/-/DC1
References
- Altarejos J. Y., Montminy M. (2011). CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 10.1038/nrm3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R., Olson E. N. (2006). Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 75, 19–37 10.1146/annurev.biochem.75.103004.142622 [DOI] [PubMed] [Google Scholar]
- Berdeaux R., Goebel N., Banaszynski L., Takemori H., Wandless T., Shelton G. D., Montminy M. (2007). SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat. Med. 13, 597–603 10.1038/nm1573 [DOI] [PubMed] [Google Scholar]
- Berggreen C., Gormand A., Omar B., Degerman E., Göransson O. (2009). Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am. J. Physiol. 296, E635–E646 10.1152/ajpendo.90596.2008 [DOI] [PubMed] [Google Scholar]
- Berggreen C., Henriksson E., Jones H. A., Morrice N., Göransson O. (2012). cAMP-elevation mediated by β-adrenergic stimulation inhibits salt-inducible kinase (SIK) 3 activity in adipocytes. Cell. Signal. 24, 1863–1871 10.1016/j.cellsig.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Bittinger M. A., McWhinnie E., Meltzer J., Iourgenko V., Latario B., Liu X., Chen C. H., Song C., Garza D., Labow M. (2004). Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr. Biol. 14, 2156–2161 10.1016/j.cub.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Clark K., MacKenzie K. F., Petkevicius K., Kristariyanto Y., Zhang J., Choi H. G., Peggie M., Plater L., Pedrioli P. G., McIver E. et al. (2012). Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc. Natl. Acad. Sci. USA 109, 16986–16991 10.1073/pnas.1215450109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K., Aviles-Hernandez A., Cushman S. W., Malide D. (2001). Insulin-regulated trafficking of dual-labeled glucose transporter 4 in primary rat adipose cells. Biochem. Biophys. Res. Commun. 287, 445–454 10.1006/bbrc.2001.5620 [DOI] [PubMed] [Google Scholar]
- Dentin R., Liu Y., Koo S. H., Hedrick S., Vargas T., Heredia J., Yates J., III and Montminy M. (2007). Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 10.1038/nature06128 [DOI] [PubMed] [Google Scholar]
- Du J., Chen Q., Takemori H., Xu H. (2008). SIK2 can be activated by deprivation of nutrition and it inhibits expression of lipogenic genes in adipocytes. Obesity (Silver Spring) 16, 531–538 10.1038/oby.2007.98 [DOI] [PubMed] [Google Scholar]
- Ferguson B. S., Nam H., Hopkins R. G., Morrison R. F. (2010). Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS ONE 5, e15208 10.1371/journal.pone.0015208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormand A., Berggreen C., Amar L., Henriksson E., Lund I., Albinsson S., Göransson O. (2014). LKB1 signalling attenuates early events of adipogenesis and responds to adipogenic cues. J. Mol. Endocrinol. 53, 117–130 10.1530/JME-13-0296 [DOI] [PubMed] [Google Scholar]
- Hardie D. G., Ross F. A., Hawley S. A. (2012). AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson E., Jones H. A., Patel K., Peggie M., Morrice N., Sakamoto K., Göransson O. (2012). The AMPK-related kinase SIK2 is regulated by cAMP via phosphorylation at Ser358 in adipocytes. Biochem. J. 444, 503–514 10.1042/BJ20111932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M., Lenzner C., Leneuve P., Zaoui R., Hamard G., Vaulont S., Bouc Y. L. (2000). Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res. 28, E92 10.1093/nar/28.21.e92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horike N., Takemori H., Katoh Y., Doi J., Min L., Asano T., Sun X. J., Yamamoto H., Kasayama S., Muraoka M. et al. (2003). Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J. Biol. Chem. 278, 18440–18447 10.1074/jbc.M211770200 [DOI] [PubMed] [Google Scholar]
- Horike N., Kumagai A., Shimono Y., Onishi T., Itoh Y., Sasaki T., Kitagawa K., Hatano O., Takagi H., Susumu T. et al. (2010). Downregulation of SIK2 expression promotes the melanogenic program in mice. Pigment Cell Melanoma Res. 23, 809–819 10.1111/j.1755-148X.2010.00760.x [DOI] [PubMed] [Google Scholar]
- Jansson D., Ng A. C., Fu A., Depatie C., Al Azzabi M., Screaton R. A. (2008). Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl. Acad. Sci. USA 105, 10161–10166 10.1073/pnas.0800796105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh Y., Takemori H., Min L., Muraoka M., Doi J., Horike N., Okamoto M. (2004). Salt-inducible kinase-1 represses cAMP response element-binding protein activity both in the nucleus and in the cytoplasm. Eur. J. Biochem. 271, 4307–4319 10.1111/j.1432-1033.2004.04372.x [DOI] [PubMed] [Google Scholar]
- Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P. et al. (2005). The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 10.1038/nature03967 [DOI] [PubMed] [Google Scholar]
- Küser-Abali G., Ozcan F., Ugurlu A., Uysal A., Fuss S. H., Bugra-Bilge K. (2013). SIK2 is involved in the negative modulation of insulin-dependent muller cell survival and implicated in hyperglycemia-induced cell death. Invest. Ophthalmol. Vis. Sci. 54, 3526–3537 10.1167/iovs.12-10729 [DOI] [PubMed] [Google Scholar]
- Lee C. W., Yang F. C., Chang H. Y., Chou H., Tan B. C., Lee S. C. (2014). Interaction between salt-inducible kinase 2 and protein phosphatase 2A regulates the activity of calcium/calmodulin-dependent protein kinase I and protein phosphatase methylesterase-1. J. Biol. Chem. 289, 21108–21119 10.1074/jbc.M113.540229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G. et al. (2004). LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 10.1038/sj.emboj.7600110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova M. M., Vasquez D. S., Ravnskjaer K., Denechaud P. D., Yu R. T., Alvarez J. G., Downes M., Evans R. M., Montminy M., Shaw R. J. (2011). Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145, 607–621 10.1016/j.cell.2011.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar B., Zmuda-Trzebiatowska E., Manganiello V., Göransson O., Degerman E. (2009). Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell. Signal. 21, 760–766 10.1016/j.cellsig.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K., Foretz M., Marion A., Campbell D. G., Gourlay R., Boudaba N., Tournier E., Titchenell P., Peggie M., Deak M. et al. (2014). The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nat. Commun. 5, 4535 10.1038/ncomms5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L., Saberi M., Zmuda E., Wang Y., Altarejos J., Zhang X., Dentin R., Hedrick S., Bandyopadhyay G., Hai T. et al. (2009). Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 9, 277–286 10.1016/j.cmet.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Takemori H., Yagita Y., Terasaki Y., Uebi T., Horike N., Takagi H., Susumu T., Teraoka H., Kusano K. et al. (2011). SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron 69, 106–119 10.1016/j.neuron.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Screaton R. A., Conkright M. D., Katoh Y., Best J. L., Canettieri G., Jeffries S., Guzman E., Niessen S., Yates J. R., III, Takemori H. et al. (2004). The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 10.1016/j.cell.2004.09.015 [DOI] [PubMed] [Google Scholar]
- Song Y., Altarejos J., Goodarzi M. O., Inoue H., Guo X., Berdeaux R., Kim J. H., Goode J., Igata M., Paz J. C. et al. CHARGE Consortium; GIANT Consortium(2010). CRTC3 links catecholamine signalling to energy balance. Nature 468, 933–939 10.1038/nature09564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori H., Okamoto M. (2008). Regulation of CREB-mediated gene expression by salt inducible kinase. J. Steroid Biochem. Mol. Biol. 108, 287–291 10.1016/j.jsbmb.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Than T. A., Lou H., Ji C., Win S., Kaplowitz N. (2011). Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J. Biol. Chem. 286, 22047–22054 10.1074/jbc.M111.240481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebi T., Tamura M., Horike N., Hashimoto Y. K., Takemori H. (2010). Phosphorylation of the CREB-specific coactivator TORC2 at Ser(307) regulates its intracellular localization in COS-7 cells and in the mouse liver. Am. J. Physiol. 299, E413–E425 10.1152/ajpendo.00525.2009 [DOI] [PubMed] [Google Scholar]
- van der Linden A. M., Nolan K. M., Sengupta P. (2007). KIN-29 SIK regulates chemoreceptor gene expression via an MEF2 transcription factor and a class II HDAC. EMBO J. 26, 358–370 10.1038/sj.emboj.7601479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkinshaw D. R., Weist R., Kim G. W., You L., Xiao L., Nie J., Li C. S., Zhao S., Xu M., Yang X. J. (2013). The tumor suppressor kinase LKB1 activates the downstream kinases SIK2 and SIK3 to stimulate nuclear export of class IIa histone deacetylases. J. Biol. Chem. 288, 9345–9362 10.1074/jbc.M113.456996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Inoue H., Ravnskjaer K., Viste K., Miller N., Liu Y., Hedrick S., Vera L., Montminy M. (2010). Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc. Natl. Acad. Sci. USA 107, 3087–3092 10.1073/pnas.0914897107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Moya N., Niessen S., Hoover H., Mihaylova M. M., Shaw R. J., Yates J. R., III, Fischer W. H., Thomas J. B., Montminy M. (2011). A hormone-dependent module regulating energy balance. Cell 145, 596–606 10.1016/j.cell.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems J., Olson A. L. (2011). Class II histone deacetylases limit GLUT4 gene expression during adipocyte differentiation. J. Biol. Chem. 286, 460–468 10.1074/jbc.M110.157107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems J. C., Griesel B. A., Olson A. L. (2012). Class II histone deacetylases downregulate GLUT4 transcription in response to increased cAMP signaling in cultured adipocytes and fasting mice. Diabetes 61, 1404–1414 10.2337/db11-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Huang X., Feng Y., Handschin C., Feng Y., Gullicksen P. S., Bare O., Labow M., Spiegelman B., Stevenson S. C. (2006). Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc. Natl. Acad. Sci. USA 103, 14379–14384 10.1073/pnas.0606714103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.