Abstract.
There is a need for accurate, high-throughput, functional measures to gauge the efficacy of potential drugs in living cells. As an early marker of drug response in cells, cellular metabolism provides an attractive platform for high-throughput drug testing. Optical techniques can noninvasively monitor NADH and FAD, two autofluorescent metabolic coenzymes. The autofluorescent redox ratio, defined as the autofluorescence intensity of NADH divided by that of FAD, quantifies relative rates of cellular glycolysis and oxidative phosphorylation. However, current microscopy methods for redox ratio quantification are time-intensive and low-throughput, limiting their practicality in drug screening. Alternatively, high-throughput commercial microplate readers quickly measure fluorescence intensities for hundreds of wells. This study found that a commercial microplate reader can differentiate the receptor status of breast cancer cell lines () based on redox ratio measurements without extrinsic contrast agents. Furthermore, microplate reader redox ratio measurements resolve response () and lack of response () in cell lines that are responsive and nonresponsive, respectively, to the breast cancer drug trastuzumab. These studies indicate that the microplate readers can be used to measure the redox ratio in a high-throughput manner and are sensitive enough to detect differences in cellular metabolism that are consistent with microscopy results.
Keywords: microplate reader, redox ratio, breast cancer, fluorescence, drug screening, Herceptin
The development of effective new drugs is limited by costs of equipment, reagents, and personnel, as well as the significant time required to screen and test drug candidates. The average cost to develop a drug from its initial stages to regulatory approval is $1.8 billion, and the process is estimated to take over 13 years.1 Therefore, new technologies are needed to accelerate drug development through accurate, high-throughput, low-cost measures in cells. Breast cancer remains the second leading cause of cancer death among women in the United States.2 Many breast cancer treatments are assigned according to the presence of receptor proteins, particularly estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2). Hormone and targeted therapies intend to mediate the uncontrolled cell proliferation caused by these proteins, but have been met with limited success. Tamoxifen, an ER antagonist, is successful in less than 60% of ER+ patients, and trastuzumab, a HER2 inhibitor, effectively treats only two thirds of HER2+ patients.3,4 Furthermore, breast cancers that lack both of these biomarkers (triple negative) or develop drug resistance remain difficult to treat.5 Therefore, more effective therapies are required to improve breast cancer patient outcomes.
Current in vitro methods for preclinical drug screening rely on protein-based stains for cell viability and growth inhibition as an indicator of drug efficacy.6 These methods require staining procedures that are often costly, time-consuming, toxic to cells, and incapable of monitoring dynamic changes with time, thus limiting the throughput and versatility of drug screens.7
Another gauge of treatment response is cellular metabolism, which is related to chemotherapeutic response, HER2 expression and ER expression.8,9 Cancer cells exhibit the Warburg effect and preferentially undergo aerobic glycolysis.10 When glycolysis is upregulated, nicotinamide adenine dinucleotide (NAD+) is reduced to NADH in the glycolytic pathway more often than flavin adenine dinucleotide (FAD) produced during oxidative phosphorylation, leading to higher cellular levels of NADH than FAD. This relationship is quantified by the optical redox ratio, defined as the intensity of NADH divided by that of FAD. The redox ratio is sensitive to both ER and HER2 overexpression as well as drug response.11–13 NADH and FAD are endogenous fluorophores, and thus enable metabolic measurements without requiring stains or dyes. Therefore, autofluorescence measurements are advantageous for high-throughput drug development.
Confocal and multiphoton microscopy measure NADH and FAD autofluorescence to assess the metabolism on a cell-by-cell basis.13,14 Although they provide high-resolution images of cellular-level metabolism, these low-throughput methods require expensive equipment and sophisticated image analysis procedures. Alternatively, microplate readers are an attractive platform for measuring fluorescence because of their high-throughput capabilities, but no previous literature assesses their application for autofluorescence measurements of metabolism. Microplate readers are already used in research settings to measure absorbance, fluorescence intensity, and luminescence using exogenous labels, and can quickly relay data for microtiter plates of hundreds of wells.
This study tests the sensitivity of a plate reader to differentiate the optical redox ratio of breast cancer cell lines with varying ER and HER2 receptor expression levels, as well as its ability to accurately gauge response to a HER2 inhibitor, trastuzumab. The findings of these experiments demonstrate that microplate readers can accurately identify differences in cellular NADH and FAD autofluorescence intensities that are consistent with published microscopy results.12,13 Therefore, we believe that microplate readers merit further exploration as a valuable tool in drug development and metabolic monitoring.
MDA-MB-231 (triple negative breast cancer), MCF7 (), SKBr3 (), and BT474 () cell lines were cultured in clear Dulbecco’s Modified Eagle Media (DMEM) (Invitrogen, Carlsbad, California) supplemented with 10% fetal bovine serum and 1% penicillin:streptomycin. Cells were plated in a 96-well Costar flat-bottomed transparent plate. Twelve wells were prepared for each group. A consistent volume of of was plated in each well. After 24 h, trastuzumab was administered to the treatment groups at a concentration of , which mimics therapeutic drug dosage in breast cancer patients.15 A HER2 inhibitor used extensively in breast cancer patients, trastuzumab, is a monoclonal antibody capable of arresting cell growth without inducing apoptosis.16 After another 24 h, the autofluorescence from NADH and FAD was measured using a plate reader.
NADH and FAD autofluorescence intensities were measured with a Tecan Infinite M1000 Pro microplate reader (San Jose, California) and Tecan i-Control software. For NADH fluorescence, the cells were excited at 330 to 370 nm and the emission signal was collected at 440 to 480 nm. For FAD fluorescence, the cells were excited at 430 to 470 nm and the emission signal was collected at 515 to 555 nm.12 The instrument was operated under epi-mode with an integration time of , a flash frequency of 400 Hz, and a gain of 80. Measurements of each plate were taken twice within ten minutes to rule out the occurrence of photobleaching, and data were collected on three separate days.
The redox ratio was defined as the NADH fluorescence intensity divided by that of FAD. To account for proliferation rates across cell lines, the redox ratio was normalized to the number of cells per well and quantified using a bright field microscope at magnification for a representative field-of-view. To account for intrinsic system variation across different days, the average redox ratio from each cell line was normalized to the control BT474 measurement on the same day. This normalization is comparable with employing a rhodamine fluorescence reference standard, which is common in fluorescence spectroscopy and quantitative fluorescence imaging.17 Kruskal–Wallis and rank-sum tests were used to determine statistical significance between cell lines and between control and trastuzumab-treated cells, respectively, with indicating significance.
To ensure that the fluorescence signal is primarily emanating from NADH and FAD, a validation experiment was performed using FCCP [carbonyl cyanide-p-(trifluoromethoxy) phenylhydrazone] and the MDA-MB-231 cell line. Since FCCP inhibits oxidative phosphorylation,18 a measurable decrease in cellular redox ratio would ensure that the signal collected by the fluorimeter primarily emanates from NADH and FAD. The MDA-MB-231 cells were chosen for this perturbation because their metabolism is most similar to noncancerous cells used for previous FCCP perturbation experiments,19 but it is acknowledged that different cell lines may respond to FCCP in different ways.18 Twelve wells of MDA-MB-231 cells were treated with DMEM media supplemented with FCCP, and plate reader measurements were taken three times following a 60-s incubation period.19 The significant decrease in redox ratio (, not shown) agrees with previously published microscopy data19 and verifies that the autofluorescence signals detected in these experiments are primarily due to NADH and FAD.
The sensitivity of the plate reader to distinguish between breast cancer receptor status was demonstrated using four human cell lines: MDA-MB-231 (triple negative breast cancer), MCF7 (), SKBr3 (), and BT474 (). MDA-MB-231 cells exhibited the lowest redox ratio. The MCF7, SKBr3, and BT474 cells show increasing redox ratios with a distinct redox ratio for each cell line [, Fig. 1(a)]. These trends match our published confocal and multiphoton microscopy measurements of the optical redox ratio in these cell lines.12,13 As shown previously, the redox ratio values correlate with ER and HER2 expressions with the lowest redox ratio observed in the triple negative breast cancer cell line, MDA-MB-231, and the highest redox ratio in the cell line, BT474. The separate NADH and FAD autofluorescence intensities were also significantly distinct for each cell line [, Figs. 1(b) and 1(c)]. The ability of the plate reader to relate these differences in metabolism is encouraging; it appears to quantify the optical redox ratio as effectively as confocal and multiphoton microscopy, but in a fast, high-volume manner.
Fig. 1.
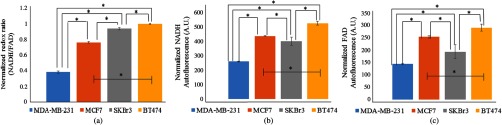
Normalized redox ratios (a), NADH fluorescence intensities (b), and FAD fluorescence intensities (c) of immortalized breast cancer cells, MDA-MB-231 (), MCF7 (), SKBr3 (), and BT474 (). Trends between cell lines are in agreement with published microscopy results.12,13 Autofluorescence intensities are normalized to cell number. *.
In order to test the plate reader’s sensitivity to treatment-induced changes in optical redox ratio, the cell lines were treated with the HER2 inhibitor trastuzumab for 24 h. As expected, the redox ratios of the HER2-overexpressing cell lines, SKBr3 and BT474, decreased with trastuzumab treatment (), while those of the HER2-negative cell lines, MCF7 and MDA-MB-231, did not change [Fig. 2(a)]. These trends match published multiphoton microscopy.13 To reaffirm the significant trends in redox ratios, NADH and FAD intensities were separately quantified for each group. As expected, the NADH and FAD autofluorescence intensities did not differ between the control and treated groups of HER2-negative cells [, Figs. 2(b) and 2(c)]. However, the fluorescence intensities of the SKBr3 and BT474 cells did exhibit significant changes (). Interestingly, the intensities of both NADH and FAD were higher in treated SKBr3 cells than the control, with a greater increase in FAD intensity leading to the decreased redox ratio. Conversely, the fluorescence intensities of both coenzymes in the treated BT474 cells decreased following the treatment; the overall decrease in redox ratio was caused by a greater decrease in NADH intensity than in that of FAD. Importantly, these differing trends in NADH and FAD autofluorescence intensities for each cell line agree with our previously published microscopy data of parallel experiments using BT474 cell monolayers,12,13 and highlight the utility of the redox ratio in measuring drug effect, rather than relying on trends shown by individual fluorophores. These results indicate that the metabolic sensitivity of the plate reader effectively measures changes in cellular metabolic state as an indicator of drug response. The evidence of these changes 24 h after treatment validates the instrument’s ability to quantify the optical redox ratio as accurately as microscopy methods with decreased equipment cost, data acquisition time, and number of samples. Quantification of this metabolic endpoint could be implemented in the early stages of the drug development process as a time- and cost-effective method to identify successful drug candidates.
Fig. 2.
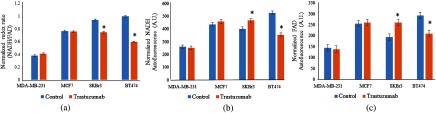
The redox ratios (a), NADH fluorescence (b), and FAD fluorescence (c) of the HER2-overexpressing cell lines (SKBr3 and BT474) changed significantly following trastuzumab treatment (). Conversely, the HER2-negative cell lines (MDA-MB-231 and MCF7) showed no significant changes in redox ratio nor in either of its components (* compared with control) with trastuzumab treatment. These trends match published results using multiphoton microscopy.13
In summary, microplate reader fluorescence measurements are presented as a method for rapid, high-throughput, and accurate assessment of cellular metabolism, breast cancer receptor status, and drug efficacy. The high cost and significant time for new drug development highlights the need for innovative approaches to accelerate drug discovery. We have demonstrated that autofluorescent plate reader measurements, which provide results consistent with published parallel experiments using confocal and multiphoton microscopy,12,13 could potentially meet this need. These methods enable sensitive, fast, and quantitative measurements of endogenous fluorescence in a high-volume platform. Given the high-throughput nature of the plate reader for optical measurements, this instrument merits further exploration in the fields of drug discovery, drug development, and metabolic monitoring.
Acknowledgments
Thank you to Dr. Hak-Joon Sung’s laboratory for use of the microplate reader. Funding includes the DOD BCRP (DOD-BC121998), the NIH/NCI (NIH R00-CA142888, R01-CA185747), and the Mary Kay Foundation (067-14).
References
- 1.Paul S. M., et al. , “How to improve R&D productivity: the pharmaceutical industry’s grand challenge,” Nat. Rev. Drug Discovery 9, 203–214 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Ma J., Jemal A., “Breast cancer statistics,” Chapter 1 in Breast Cancer Metastasis and Drug Resistance, Ahmad A., Ed., pp. 1–18, Springer, New York, (2013). 10.1007/978-1-4614-5647-6 [DOI] [Google Scholar]
- 3.Vogel C. L., et al. , “Efficacy and safety of trastuzumab as a single agent,” J. Clin. Oncol. 20(3), 719–726 (2002). 10.1200/JCO.20.3.719 [DOI] [PubMed] [Google Scholar]
- 4.Chang J., et al. , “Prediction of clinical outcome from primary tamoxifen by expression of biologic markers in breast cancer patients,” Clin. Cancer Res. 6(2), 616–621 (2000). [PubMed] [Google Scholar]
- 5.Onitilo A. A., et al. , “Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival,” Clin. Med. Res. 7, 4–13 (2009). 10.3121/cmr.2008.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd M. R., Padl K. D., “Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen,” Drug Dev. Res. 34, 91–109 (1995). [Google Scholar]
- 7.Suggitt M., Bibby M. C., “50 years of preclinical anticancer drug screening: empirical to target-driven approaches,” Clin. Cancer Res. 11(3), 971–981 (2005). [PubMed] [Google Scholar]
- 8.Zhang D., et al. , “Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer,” Mol. Cell. Proteomics 4, 1686–1996 (2005). 10.1074/mcp.M400221-MCP200 [DOI] [PubMed] [Google Scholar]
- 9.Cheng C. M., et al. , “Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex,” FASEB J. 15, 907–915 (2001). 10.1096/fj.00-0398com [DOI] [PubMed] [Google Scholar]
- 10.Warburg O., “On the origin of cancer cells,” Science 123(80), 309–314 (1956). 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 11.Ostrander J. H., et al. , “Optical redox ratio differentiates breast cancer cell lines based on estrogen receptor status,” Cancer Res. 70, 4759–4766 (2010). 10.1158/0008-5472.CAN-09-2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh A., et al. , “Optical imaging of metabolism in HER2 overexpressing breast cancer cells,” Biomed. Opt. Express 3, 75–85 (2012). 10.1364/BOE.3.000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh A. J., et al. , “Optical metabolic imaging identifies glycolytic levels, subtypes, and early-treatment response in breast cancer,” Cancer Res. 73, 6164–6174 (2013). 10.1158/0008-5472.CAN-13-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skala M. C., et al. , “In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia,” Proc. Natl. Acad. Sci. U. S. A. 104, 19494–19499 (2007). 10.1073/pnas.0708425104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller T. W., et al. , “Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells,” Clin. Cancer Res. 15, 7266–7276 (2009). 10.1158/1078-0432.CCR-09-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junttila T. T., et al. , “Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941,” Cancer Cell 15, 429–440 (2009). 10.1016/j.ccr.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 17.Boens N., et al. , “Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy,” Anal. Chem. 79(5), 2137–2149 (2007). 10.1021/ac062160k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramov A. Y., Scorziello A., Duchen M. R., “Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation,” J. Neurosci. 27, 1129–1138 (2007). 10.1523/JNEUROSCI.4468-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S., Heikal A. A., Webb W. W., “Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein,” Biophys. J. 82(5), 2811–2825 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]


