Abstract
A variety of cardiovascular and cerebrovascular diseases are associated with alterations in cholesterol levels and metabolism. Moreover, convincing evidence shows that high cholesterol diet can lead to learning and memory impairments. On the other hand, a significant body of research has also demonstrated that learning is improved by elevated dietary cholesterol. Despite these conflicting findings, it is clear that cholesterol plays an important role in these cognitive properties. However, it remains unclear how this blood-brain barrier (BBB)-impenetrable molecule affects the brain and under what circumstances it provides either detrimental or beneficial effects to learning and memory. The aim of this study was to characterize the effects of 5% cholesterol diet on six-month-old inbred Brown Norway rats. More important, we sought to examine the role that cholesterol can play when repeated anesthesia and intravenous infusion disrupts cognitive function. This present study supports previous work showing that enriched cholesterol diet leads to significant alterations in neuroinflammation and BBB disruption. Following repeated anesthesia and intravenous infusion of saline we observe that animals under normal diet conditions exhibit significant deficiencies in spatial learning and cholinergic neuron populations compared to animals under enriched cholesterol diet, which do not show such deficiencies. These findings indicate that cholesterol diet can protect against or counteract anesthesia/infusion-induced cognitive deficits. Ultimately, these results suggest that cholesterol homeostasis serves an important functional role in the brain and that altering this homeostasis can either exert positive or negative effects on cognitive properties.
Keywords: Cholesterol, Anesthesia, Spatial learning, Cholinergic neurons
1. Introduction
Cholesterol is a critical component of the central nervous system involved in the maintenance of normal brain function including synapse formation, receptor function, synaptic plasticity, and signaling. Converging evidence indicates that altered cholesterol levels and/or metabolism are associated with changes in learning and memory (Darwish, Wang, Konat, & Schreurs, 2010; Schreurs, 2010).
Several studies have shown that animals under enriched cholesterol diet exhibit significant cognitive deficits (Darwish et al., 2010; Granholm et al., 2008; Thirumangalakudi et al., 2008) and neuropathological changes (Schreurs, 2010; Sharma, Prasanthi, Schommer, Feist, & Ghribi, 2008; Sparks, Kuo, Roher, Martin, & Lukas, 2000; Xue, Sparks, & Streit, 2007; Zatta, Zambenedetti, Stella, & Licastro, 2002). In a previous study, we demonstrated that Sprague-Dawley rats on a 5% cholesterol diet exhibit prominent cholinergic, learning, and memory deficits, as well as significant losses in blood-brain barrier (BBB) integrity (Ullrich, Pirchl, & Humpel, 2010). Moreover, epidemiological studies have shown that elevated midlife plasma cholesterol levels can increase susceptibility to dementia and that the use of statins, cholesterol-lowering drugs, may provide a protective effect against later development of dementia (Di Paolo & Kim, 2011; Haag, Hofman, Koudstaal, Stricker, & Breteler, 2008; Reiss & Voloshyna, 2012). However, other investigations have shown that enriched cholesterol diet can lead to positive and beneficial effects in learning abilities (Dufour, Liu, Gusev, Alkon, & Atzori, 2006; Micale et al., 2008; Miller & Wehner, 1994; Schreurs, Smith-Bell, Darwish, Stankovic, & Sparks, 2007). Moreover, contradictory results claim that cholesterol reduction through the use of statins does not improve memory, making it difficult to draw conclusions about the relationship between cholesterol levels and cognitive function (Schreurs, 2010). We speculate that these inconsistencies exist due to variations in the amounts of cholesterol given, diet duration, animal age, and animal species/strain.
Convincing evidence demonstrates that cholesterol plays an important role in learning and memory, however, the effects of cholesterol diet and the mechanisms by which cholesterol influences these cognitive functions remain unclear. In this study we sought to examine the effects of high cholesterol diet on spatial learning and brain pathology in Brown Norway rats and what role diet may play when these animals are given multiple rounds of anesthesia and intravenous (i.v.) saline infusion. In future studies, we hope to use these inbred animals for transplant infusion studies and therefore wanted to determine whether this treatment (and associated stress) would lead to any changes in cognition, the cholinergic system, or neuroinflammation. Previous studies have been inconsistent in providing a clear understanding of the effects of anesthesia. Thus, we thought it would be important to establish these effects in our animal model.
Here, we evaluated spatial learning using an 8-arm maze and cholinergic system alterations by staining cholinergic neurons for choline acetyltransferase and by measuring cortical NGF levels, a growth factor associated with the survival and function of this neuronal population. Neuroinflammation was investigated by staining for microglial marker OX-42 and measuring cortical levels of pro-inflammatory markers MCP-1, MIP-2, TNF-α, and IL-1β. Interestingly, our data suggests that cholesterol diet can protect against the cognitive deficits induced by repeated anesthesia/infusion treatment.
2. Materials and methods
2.1. Animals and diet
Six-month-old male Brown Norway rats were housed at the Innsbruck Medical University animal facility providing 12 h/12 h light-dark cycles and open access to food and water. Animals received either a normal diet (controls, n = 17) or cholesterol diet (n = 16), supplemented with 5% cholesterol, for five months. The diet consisted of: 450 g/kg cornstarch, 140 g/kg casein, 155 g/kg maltodextrin, 100 g/kg sucrose, 40 g/kg soybean oil, 50 g/kg fiber, 35 g/kg mineral mix, 1.8 g/kg l-Cystine, 1.4 g/kg choline chloride, 0.0008 g/kg butylhydroxytoluol, 10 g/kg vitamin mix (without folic acid), 1 g/kg chocolate aroma, and 0.002 g/kg folic acid with an additional 50 g/kg cholesterol for animals on the cholesterol diet (Ssniff special diet GmbH; Soest Germany). All animal experiments were approved by the Austrian Ministry of Science and Research (BMWF-66.011/0044-II/3b/2011 and BMWF-66.011/0059-II/3b/2011) and conformed to the Austrian guidelines on animal welfare and experimentation. All possible steps were taken to reduce suffering and the number of animals used.
2.2. Repeated anesthesia and intravenous infusion
After three months, animals on the normal diet (n = 10) and the cholesterol diet (n = 10) were given weekly i.v. injections containing 100 μl of heparinized saline for two months via the dorsal penile vein. Prior to injection, each rat was anaesthetized by an intraperitoneal injection of thiopental (400 μl/100 g; 12.5 mg/ml). For the rest of this manuscript, we refer to repeated anesthesia and i.v. infusion of saline as anesthesia/infusion. Animals receiving a five-month normal diet (n = 7) or cholesterol diet (n = 6) undisrupted by anesthesia/infusion treatments served as control animals. These animals were occasionally handled, but were never given anesthesia or i.v. infusions.
2.3. Evaluation of spatial learning and memory using an 8-arm radial maze
Following a five-month diet course, spatial memory was assessed in animals using a partially-baited 8-arm radial maze (Pan-Lab, Spain) as previously described by us (Pirchl, Ullrich, & Humpel, 2010; Ullrich et al., 2010). The maze consisted of a central starting arena from which eight identical Plexiglas arms radiated. Food goal cups were placed at the ends of each arm where food pellets could be left as a reward (bait). To facilitate spatial navigation, small high contrast visual cues were placed above the entrance to four arms. Prior to behavioral testing, all animals were placed on a restricted diet to increase motivation (2 g food pellets/animal/day for three days) and subsequently acclimatized to the maze and experimental set-up. Spatial learning and memory performance was tested during five consecutive daily sessions (S) consisting of five trials per day. The first two days also included one training session prior to experiment trials. Four arms were baited with food pellets (chocolate cereal). Once all baits were found or the 10 min time limit was exceeded, the trial was ended. To exclude olfactory interference, crumbs from the baits were placed under each food cup and following each trial the maze apparatus was cleaned with 70% ethanol. The behavioral test equipment was automatically controlled and monitored by a computer equipped with Mazesoft 8.1.9 Software. Quantification of cognitive performance (spatial learning and memory) was done as previously described (Pirchl, Ullrich, Sperner-Unterweger, & Humpel, 2012; Pirchl et al., 2010) by calculating the percentage of correct visits (choices) as a measure for enhanced learning. The percentage of correct choices was calculated accordingly: (baits/visits) (baits 100/4). The calculated values distinguish between the performance of animals within trials, where all baits were found (4 baits max.), from trials where only 1, 2, or 3 baits were found. Total errors were calculated by adding the number of reference memory errors (RME), working memory errors (WME), and reference-working memory errors together and dividing by the number by trials per session. Following behavioral testing and prior to tissue collection, animals were placed back on their regular diets for three days.
2.4. Tissue collection
Animals were anesthetized by subcutaneous sodium thiopental (12.5 mg/ml, 1 ml) injection. Brains were removed and the frontal cortices were dissected from the left hemisphere. One section of cortex was frozen at −80 °C (for ELISA evaluation). The right brain hemisphere was post-fixed overnight in 4% paraformaldehyde (PFA) and then stored in 20% sucrose/sodium azide solution.
2.5. Preparation of cortical extracts
Frozen cortical tissue for ELISA evaluation was dissolved in 100 μl ice-cold PBS containing protease inhibitor cocktail (P-8340, Sigma), homogenized using an ultrasonic device (Hielscher Ultrasonic Processor, Germany) and then centrifuged at 16,000g for 10 min at 4 °C. The supernatant was collected and samples were stored at −80 °C until further use. Total protein was determined by Bradford protein assay.
2.6. NGF elisa
Cortical nerve growth factor (NGF) levels were measured in cortex extracts using an indirect sandwich enzyme-linked immunosorbent assay (ELISA; Promega) as previously described (Böttger, Ullrich, & Humpel, 2010; Zassler & Humpel, 2006). Briefly, 96-well ELISA plates were coated with a monoclonal anti-NGF antibody diluted in carbonate coating buffer (pH 9.7) and incubated overnight at 4 °C. Plates were then blocked using 1× blocking buffer (200 μl/well) for 1 h at 20 °C. Following incubation, NGF standards (0–100 pg/well) or tissue extracts were added to plates and incubated for 6 h at 20 °C. After washes, plates were incubated with a monoclonal rat anti-NGF antibody overnight at 4 °C. After a second round of washes, the plate was incubated with horseradish peroxidase-conjugated anti-rat antibody (1:4000) for 2 h at 20 °C. Plates were again washed and incubated with enzyme substrate (TMB One solution, Promega) for 15 min at 20 °C. The enzyme reaction was stopped by adding 1 N HCl and the absorbance was measured at 450 nm by a microplate ELISA reader (Zenyth 3100 ELISA reader or LambdaE, MWG). Sample values were calculated from a standard curve in the linear range. The assay detection limit was 10 pg/ml NGF.
2.7. Inflammatory marker ELISA
The detection of inflammatory proteins (monocyte chemotactic protein-1, MCP-1; macrophage inflammatory protein-2, MIP-2; tumor necrosis factor-α, TNF-α; interleukin-1β, IL-1β) was performed using the Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Vienna) according to the manufacture’s recommendations (Bio-Rad) and as previously described by us (Hohsfield & Humpel, 2010). Briefly, cell extracts (diluted 1:2 in diluent) or calibrated standards were added to coated wells of the provided plate and incubated for 3 h. After washing, the biotinylated antibodies were added and following 30 min incubation the wells were washed again and incubated with streptavidin-horseradish peroxidase conjugate. After the final washing step the SuperSignal Chemiluminescent Substrate was added. All incubation steps were carried out on a shaker at 20 °C. The luminescent signal was detected using a compatible CCD imaging and analysis system and the absorbance was measured at 450 nm. The concentration of each sample was quantified by comparing the spot intensities with the corresponding standard curves calculated from the standard sample results using the SearchLight Array Analyst Software. Integrated density values were proportional to the concentrations of bound proteins. Standard curves, raw data and final pg/ml concentrations for each analyte and each sample were reviewed in the array software and exported to Microsoft Excel Software for further statistical analysis. Sample values were calculated from a standard curve in the linear range.
2.8. Immunohistochemistry
Immunohistochemistry was performed as previously described under free-floating conditions (Pirchl et al., 2012; Ullrich et al., 2010). Following PFA fixation and sucrose immersion, the right brain hemisphere was placed on a cork, frozen in a CO2 stream and subsequently cut into 40-μm cryostat (Leica CM 1950) sections. The brain sections were then washed with PBS and incubated in PBS/0.1% Triton (T-PBS) for 30 min at 20 °C while shaking. To quench endogenous peroxidase, sections were treated with PBS/1%H2O2/5% methanol. After incubation, the sections were then blocked in T-PBS/20% horse serum (GIBCO Invitrogen)/0.2% BSA (SERVA) for 30 min at 20 °C shaking. Following blocking, brain sections were incubated with primary antibodies against OX-42 (CD11b, 1:500, Millipore) or choline acetyltransferase (ChAT, 1:750, AB144P, Millipore) in T-PBS/0.2% BSA overnight at 20 °C. The sections were then washed and incubated with biotinylated secondary antibody (1:200, Vector Laboratories) in T-PBS/0.2% BSA for 1 h at 20 °C shaking. To evaluate BBB permeability, some sections were also incubated with biotinylated rabbit anti-rat IgG (1:400, Vector) as previously described (Pirchl et al., 2012; Ullrich et al., 2010). Following secondary antibody incubation, sections were rinsed with PBS and incubated in avidin-biotin complex solution (Elite ABC kit, Vector Laboratories) for 1 h at 20 °C shaking. Finally, the sections were washed with 50 mM Tris-buffered saline (TBS) and then incubated in 0.5 mg/ml 3,3′-diaminobenzidine (DAB, Sigma)/TBS/0.003% H2O2 at 20 °C in the dark until a signal was detected. Once DAB staining was visible, the reaction was stopped by adding TBS to the sections. The brain sections were rinsed with TBS, mounted onto glass slides, cover-slipped with Entellan (Merck, Darmstadt, Germany), and then evaluated under the microscope. Images were captured with an Olympus BX61 (ProgRes C14 camera) microscope equipped with Openlab 5.5.0 imaging software. Quantification of ChAT-positive neurons in the nucleus basalis of Meynert (NBM) was done by a blind observer and evaluated in four sections chosen at −0.8, −1.5, −2.2, and −3.0 mm from bregma according to a rat brain atlas (Paxinos & Watson, 1986). OX-42 staining was evaluated in three to five random sections of cortex per brain and measured for optical density using NIH ImageJ software. To count IgG-positive spots, three to five brain sections per animal were evaluated in the cortex by a blind observer as previously done (Ullrich et al., 2010).
2.9. Statistical analyses
All data are reported as mean ± SEM (n = independent experiments or individual animals). Statistical analysis was performed using a three-way repeated measures analysis of variance (ANOVA) for cognitive performance and ChAT staining. A two-way ANOVA was carried out for all other measures. If a significant interaction was found between diet and anesthesia/infusion, a Fisher LSD post-hoc comparison was made to determine the effect of anesthesia/infusion or vice versa. All statistical tests were performed at a p < 0.05 level of significance.
3. Results
3.1. The effects of diet and repeated anesthesia/infusion on animal weight
In order to characterize the effects of enriched cholesterol diet, adult male Brown Norway rats were fed a normal diet or a special diet supplemented with 5% cholesterol for five months. After three months of normal or cholesterol diet, animals were treated with weekly i.v. infusions of sterile saline via the dorsal penile vein for two months. Intravenous infusion was performed using the penile dorsal vein, where introduction of material into circulation could be easily assessed, compared to tail vein infusions, where tail thickness and pigmentation lead to increased difficulties. Prior to infusion, animals were anaesthetized by an intraperitoneal injection of thiopental.
A two-way ANOVA was used to test whether diet and/or infusion have a significant impact on animal weight. The results of this analysis show that diet has no significant effect on weight. Surprisingly, weights remained unchanged between animals under normal (373 ± 6 g, n = 7) and cholesterol (371 ± 6 g, n = 6) diet indicating that a five-month diet of 5% cholesterol diet does not result in a significant accumulation of fatty tissue in inbred Brown Norway rats. In normal-fed animals receiving infusion, weights were slightly lower (358 ± 9 g, n = 10) compared to those on the same diet receiving no infusions (373 ± 6 g, n = 9), however, we found no significant main effect of infusion on weight alone. Instead, we observed a significant interaction between diet and infusion, F(1,28) = 4.485, p = .043. Subsequent Fisher LSD post-hoc comparisons demonstrated that the weights of normal-fed infused animals were significantly lower compared to cholesterol-fed infused, p = .004, indicating that animals on a normal diet are more susceptible to weight loss during infusion compared to those on a cholesterol diet. No other significant differences were observed between the animal groups.
3.2. The effects of diet and anesthesia/infusion on cognitive performance
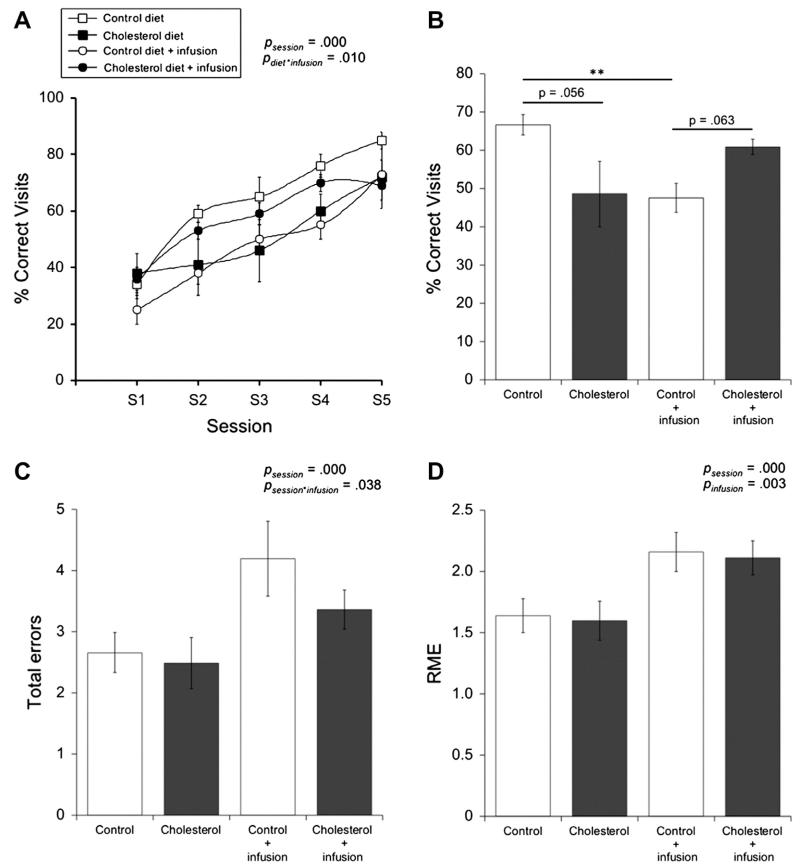
Following diet and infusion, animals were evaluated for spatial learning and memory performance in an 8-arm maze measured as the percentage of correct visits made to baited arms. A three-way repeated measures ANOVA was used to test whether diet and/or infusion can lead to significant differences in correct visits over time (Fig. 1A). Here, we found that time (session) has a significant effect on this performance alone, F(1,2.844) = 57.723, p = .000, however, no significant interaction exists between time and diet and/or infusion. Further analysis demonstrated that diet and infusion alone have no main effect on correct visits, but that there is a significant interaction between these two treatments, F(1,28) = 7.684, p = .010 (Fig. 1A). Fisher LSD post-hoc comparisons indicate that normal-fed animals receiving infusions exhibited significantly reduced correct visits compared to normal-fed animals without infusion treatment, p = .009. It appears that infused normal-fed animals exhibit significant disruptions in spatial learning compared to their non-infused normal-fed counterparts. Interestingly, cholesterol-fed animals exhibited no significant changes in their spatial learning performance following anesthesia /infusion. By this same analysis we also observed under control conditions (no infusion) cholesterol diet slightly, but not significantly, worsens cognitive function, p = .056, whereas it slightly, but not significantly, improves cognitive function when animals receive infusion treatment, p = .063 (Fig. 1A and B).
Fig. 1.
The effects of diet and anesthesia/infusion on cognitive performance. Brown Norway rats were fed either a normal (control; open square, n = 7) or cholesterol diet (filled square, n = 6) for 5 months. For the last remaining 2 months, some normal-fed (open circle, n = 10) and cholesterol-fed (filled circle, n = 9) animals were given repeated anesthesia and i.v. sterile saline infusions. Spatial learning was evaluated in these animals using an 8-arm radial maze, where cognitive performance was quantified as the percentage of correct visits made to baited arms. Performance was measured over 5 sessions (S) and each session value was calculated from the average of 5 trials (A). Performance was also quantified by averaging the number of correct visits made across sessions 2-4 (B). The number of total memory errors (i.e. reference memory errors (RME), working memory errors, and reference-working memory errors) and RME are also given (C and D). Statistical analysis was performed using a three-way repeated measures ANOVA. The p-values represent either a significant effect of diet or infusion or a significant interaction between diet and infusion. Values = mean ± SEM. Asterisks indicate a significant difference, as seen by Fisher LSD post-hoc comparison, between two groups (**p < 0.01).
In order to determine whether diet and/or infusion leads to significant differences in total errors (working memory, reference memory, reference working memory) made over time, we again employed a three-way repeated measures ANOVA. This analysis confirmed that time (session) does have a significant effect on total errors alone, F(4,112) = 12.208, p = .000 and that there is a significant interaction between session and infusion, F(4,112) = 2.633, p = .038. Following Fisher LSD post-hoc comparisons, we observed that animals receiving infusion perform worse or make more errors compared to animals without infusion during session 4, p = .044. More specifically, it appears that normal-fed animals receiving infusions make approximately 1.5 more total errors on average compared to normal-fed animals without infusions (Fig. 1C).
Significant effects and interactions in reference memory errors (RME) between time and diet and/or infusion was evaluated using a three-way repeated measures ANOVA. Time (session) did have in a significant effect on RME made by animals, F(3.909,109.460) = 18.243, p = .000. We also observed a slight, but not significant, interaction between time and infusion, F(3.909,109.460) = 2.038, p = .096 and time, diet and infusion, F(3.909,109.460) = 2.175, p = .078. Comparing the mean number of RME made during sessions 2–4, we observed that normal-fed animals receiving infusion make approximately 0.5 more RME on average compared to normal-fed animals without infusion. This is also true for cholesterol animals, in which cholesterol animals receiving infusions make approximately 0.5 more RME on average compared to cholesterol animals without infusion (Fig. 1D).
Through similar statistical analysis we also observed that working memory errors (WME) made by the animals remained unchanged over time. There is a significant interaction between time and infusion, F(3.537,99.047) = 1.387, p = .022, however, upon further post-hoc testing, we observed no significant differences between animals receiving infusion and animals without infusion (data not shown).
3.3. The effects of diet and anesthesia/infusion on the cholinergic system
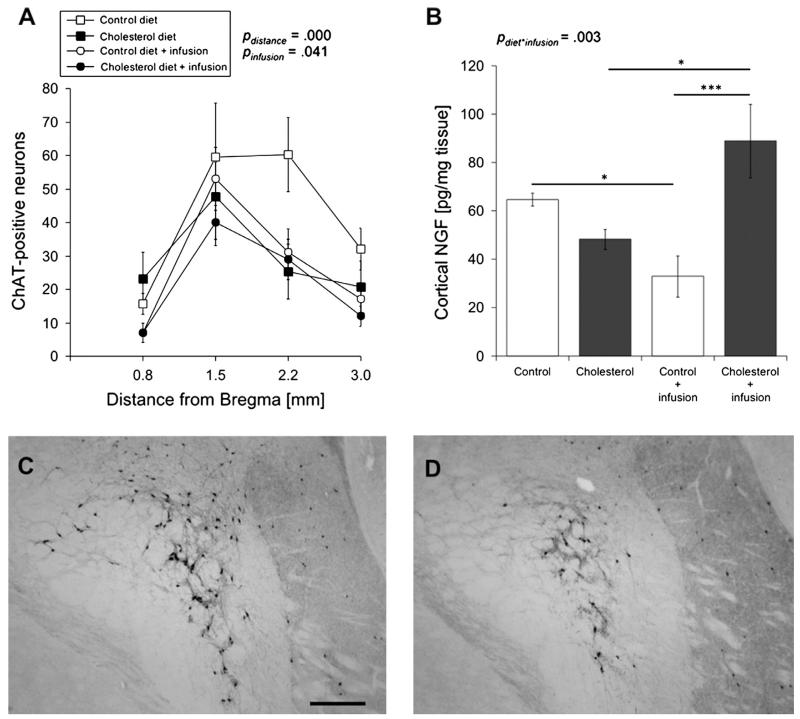
The cholinergic system was evaluated by counting the number of ChAT-positive cholinergic neurons in the NBM, located between 0.8 and 3.0 mm from Bregma, as well as measuring cortical levels of NGF, a growth factor involved in cholinergic neuron survival and function. By three-way repeated measures ANOVA analysis we observed that the distance from Bregma, F(2.601,46.811) = 17.183, p = .000, and infusion treatment, F(1,28) = 4.864, p = .041, have a significant effect on the number of ChAT-positive neurons in the NBM (Fig. 2A). Fisher LSD posthoc comparison demonstrated that normal-fed infused animals display significantly reduced ChAT-positive neurons compared to non-infused animals, p = .046. This, however, was not true in cholesterol-fed animals, suggesting that cholesterol diet makes animals less susceptible to infusion-dependent neuronal loss. In addition, no significant differences were found in ChAT-positive neuronal staining between normal and cholesterol-fed infusion groups. Although the number of ChAT-positive neurons was reduced by approximately 50% in cholesterol-fed animals (Fig. 2D) compared to control-fed animals (Fig. 2C) at 2.2 mm from Bregma, diet did not have a significant effect on cholinergic neuron survival.
Fig. 2.
The effects of diet and anesthesia/infusion on the cholinergic system. Brown Norway rats were fed either a normal (control; open square, n = 7) or cholesterol diet (filled square, n = 6) for 5 months. For the last remaining 2 months, some normal-fed (open circle, n = 10) and cholesterol-fed (filled circle, n = 9) animals were given repeated anesthesia and i.v. sterile saline infusions. The number of choline acetyltransferase (ChAT)-positive neurons was evaluated in the nucleus basalis of Meynert, located between 0.8 and 3.0 mm from Bregma (A), as an indicator of cholinergic neurodegeneration. Cortical tissue was also analyzed for nerve growth factor (NGF), a growth factor associated with cholinergic neuron survival and function, content (B). Values = mean ± SEM ChAT-positive neurons or pg NGF/mg tissue. Statistical analysis was performed using a three-way repeated measures ANOVA (A) or a two-way ANOVA (B). The p-values represent either a significant effect of diet or infusion or a significant interaction between diet and infusion. Asterisks indicate a significant difference, as seen by Fisher LSD post-hoc comparison, between two groups (*p < 0.05, ***p < 0.001). C and D are images that represent ChAT staining in normal-fed (C) and cholesterol-fed animals. Scale bar in C = 80 μm.
Furthermore, we found a significant interaction, F(1,27) = 10.810, p = .003, between diet and infusion in comparing NGF cortical levels using two-way ANOVA analysis. Fisher LSD post-hoc comparisons indicated that normal-fed infused animals express significantly reduced levels of cortical NGF compared to their non-infused counterparts, p = .038. However, cholesterol-fed animals exhibit significantly elevated cortical NGF levels, p = .020, indicating that anesthesia/infusion might lead to cholinergic system dysfunction under normal diet. Furthermore, cholesterol-fed animals receiving infusion treatment also exhibited significantly elevated levels of cortical NGF compared to normal-fed animals receiving anesthesia/infusion, p = .000 (Fig. 2B).
3.4. The effects of diet and anesthesia/infusion on cortical inflammatory markers
A two-way ANOVA was used to test whether diet and/or infusion have a significant impact on inflammatory and cortical properties. Diet proved to have a significant effect on MIP-2, F(1,28) = 8.4, p = .007, and TNF-α, F(1,28) = 5.2, p = .029, in which cholesterol-fed animals expressed decreased levels of this cytokine compared to normal-fed animals. However, there appeared to be no significant difference in MCP-1 or IL-1β levels in cholesterol or normal-fed animals (Fig. 3A and B). Diet also had a significant effect on the microglial marker OX-42 staining, F(1,28) = 5.3, p = .028. Interestingly, non-infused cholesterol-fed animals displayed higher cortical staining patterns for OX-42, indicative of cell activation, compared to non-infused normal fed-animals (Fig. 3B and C). Moreover, diet also had a significant effect on anti-rat IgG immunoreactivity, F(1,28) = 8.0, p = .008, in which IgG staining, indicative of BBB compromise, appeared elevated in cholesterol compared to normal-fed animals (Fig. 3B and D). These data suggest that enriched cholesterol diet can lead to slight alterations in spatial learning impairment and cholinergic neurons. However, the most prominent effects of cholesterol diet in Brown Norway rats involve elevated microglial and IgG cortical staining indicative of microglia activation, a marker for neuroinflammation, and BBB disruption.
Fig. 3.
The effects of diet and anesthesia/infusion on cortical inflammatory markers. Brown Norway rats were fed either a normal (control; n = 7) or cholesterol diet (n = 6) for 5 months. For the last remaining 2 months, some normal-fed (n = 10) and cholesterol-fed (n = 9) animals were given repeated anesthesia and i.v. sterile saline infusions. Cortical tissue was analyzed for pro-inflammatory markers (A; monocyte chemotactic protein-1, MCP-1; macrophage inflammatory protein-2, MIP-2; tumor necrosis factor-α, TNF-α; interleukin-1β, IL-1β), OX-42, a marker for microglia, staining (B and C), and anti-rat IgG, a marker for blood-brain barrier leakage, staining (B and D). Values = mean ± SEM pg/mg tissue (inflammatory markers), optical density (OX-42) or IgG-positive spots/cortex. Statistical analysis was performed using a two-way ANOVA. The p-values represent a significant effect of either diet or infusion. C and D are images that represent OX-42 (C) and IgG (D) staining observed in normal-fed (upper image) and cholesterol-fed animals (lower image). Scale bar in C = 80 μm.
Infusion had an overall significant effect on proinflammatory marker levels of MCP-1 F(1,28) = 94.4, p = .000, MIP-2 F(1,28) = 13.2, p = .001, and TNF-α F(1,28) = 16.4, p = .000. In cholesterol- and normal-fed animals, it appears that cortical MCP-1 levels were elevated in animals receiving anesthesia /infusion compared to non-infused animals. Proinflammatory markers MIP-2, p < 0.01, and TNF-α, p < 0.01, were significantly reduced by repeated infusion (Fig. 3A) in both diet groups. We observed no significant effect of infusion treatment on microglial OX-42 staining (Fig. 3B), anti-rat IgG staining (Fig. 3B) or IL-1β levels (Fig. 3A).
4. Discussion
Our present study indicates that 5% cholesterol diet in Brown Norway rats can lead to significant elevations in OX-42 microglia marker staining and anti-rat IgG staining, indicative of microglia activation and BBB disruption, respectively. In addition, we show that repeated anesthesia/infusion can lead to significant spatial learning deficits, reduced ChAT-positive neurons, and altered levels of cortical NGF, MCP-1, MIP-2, and TNF-α in animals receiving normal diet. Most important, our data suggest that cholesterol diet can protect against spatial learning and cortical NGF deficits induced by anesthesia/infusion.
4.1. The effects of cholesterol diet on Brown Norway rats
Altered cholesterol levels and metabolism, induced by high cholesterol diet, can lead to changes in learning and memory. However, it remains unclear how cholesterol specifically affects these cognitive properties. On one hand, animal studies have shown that high cholesterol diet can result in working memory deficits in the water maze (Darwish et al., 2010; Granholm et al., 2008; Thirumangalakudi et al., 2008), whereas other studies have reported improved cognitive performance in learning water maze tasks (Dufour et al., 2006; Micale et al., 2008; reviewed in Schreurs, 2010). There are relatively few studies on memory retention following cholesterol diet, however, a recent investigation indicates that dietary cholesterol may retard long-term memory (Schreurs et al., 2012). In addition to changes in learning and memory, studies have also shown that cholesterol can impact brain pathology. Some of these effects include BBB breakdown, microglia activation, apoptosis, elevated inflammatory marker expression, myelin sheath and axon damage, β-amyloid accumulation, tau phosphorylation, and cerebrovascular changes (Berkman et al., 2009; Hooijmans et al., 2007; Schreurs et al., 2012; Sharma et al., 2008; Sparks et al., 2000; Xue et al., 2007; Zatta et al., 2002). Our previous investigations have shown that diet-induced hypercholesterolemia, or high cholesterol, in male Sprague Dawley rats results in impaired learning and memory, reduced survival of cholinergic neurons in the NBM, reduced cortical acetylcholine, elevated inflammatory factors, elevated cortical β-amyloid, tau, and phospho-tau 181 and increased presence of small cortical BBB bleedings (Ullrich et al., 2010).
The current study was performed in male rats to avoid estrogen’s influence on cholesterol homeostasis. Brown-Norway inbred rats were chosen to avoid the risk of unwarranted host vs. graft immune reaction, and subsequent rejection, in future planned transplant experiments. Similar to our findings in Sprague Dawley rats (Ullrich et al., 2010), albeit less pronounced, these animals exhibited some spatial learning impairments and slightly reduced cholinergic neurons in the NBM. However, the significant changes we observed in these animals were elevated microglia immunoreactivity in the cortex and increased deposition of BBB bleedings. These findings are supported by previous studies that show high cholesterol diet leads to learning task deficits, BBB compromise and microglia activation (Sparks et al., 2000; Xue et al., 2007; Zatta et al., 2002). In Brown-Norway rats, however, we did not observe a significant difference in weight change between cholesterol-fed and normal-fed animals. We also observed that diet had a significant effect on inflammatory markers and cortical NGF levels. Our data suggest that cholesterol diet resulted in a reduction of these cortical proteins. In contrast, other reports and our previous study in Sprague Dawley rats have demonstrated that significantly elevated levels of inflammatory markers and growth factors are correlated to high cholesterol diet (Rahman, Van Dam, Schultzberg, & Crisby, 2005; Ullrich et al., 2010; Wang et al., 2011). Despite lower expression of inflammatory factors, Brown-Norway rats did display elevated microglia staining indicative of cell activation and the possible onset of neuroinflammation. One possible explanation for these contradictory findings could be that the area excised and measured from the frontal cortex was too small to serve as an adequate representation of the entire microenvironment of the cortex in these animals. It could also be possible that the measurement of these proteins was not optimal for recognizing subtle changes in expression. Although the Brown-Norway and Sprague Dawley rats received the same treatment conditions, it could also be possible that differences in strain variability contributed to these inconsistent findings. Since the Brown-Norway rats did not develop as severe learning and cholinergic deficiencies, another explanation could be that the effects of cholesterol were slower progressing than those in the Sprague Dawley rats and other reported animal models. It should also be pointed out that interpretation of cognitive performance in high cholesterol-fed animals is not without some consideration. A recent study has shown that fasting impacts behavior and neuroimmunity in low fat diet mice, however, not in high fat diet mice (Lavin et al., 2011). These findings indicate that dietary restriction in high fat diet rodents is a potential confounding element in cognitive tests and neuroinflammatory status. In our study it is important to consider that cholesterol-fed rats may be more resistant to the motivational effects of dietary restriction compared to normal-fed rats reflecting resistance to these effects rather than to cognitive impairment. This may also explain why cholesterol-fed rats experience higher microglial activation compared to normal-fed animals.
4.2. Repeated anesthesia/infusion impairs cognition in Brown Norway rats
Thiopental is a thiobarbituarate that can cross the BBB and act, partly, on the GABAA receptor in the central nervous system to produce anesthesia. Previous investigations have shown that anesthesia can cause profound cognitive impairment in learning and memory by affecting brain function at multiple levels, including cell membranes, receptors, ion channels, neurotransmitters, and cerebral blood flow (Culley, Baxter, Yukhananov, & Crosby, 2003; Wang & Orser, 2011).
Following two months of anesthesia /infusion, we observed significant deficiencies in spatial learning, cholinergic neuron survival, and pro-inflammatory markers alterations in infused compared to non-infused normal-fed animals. MCP-1, a chemokine responsible for the recruitment of cells to sites of inflammation, was highly elevated in the cortex of infused animals. One explanation for this is that anesthesia /infusion can lead to enhanced inflammation, however, since other inflammatory markers (i.e. MIP-2 and TNF-α) were reduced in the cortex and OX-42 microglia staining was unchanged, we do not believe that this treatment leads to gross neuroinflammation. These findings indicate that anesthesia /infusion can have marked effects on cognition and related biomarkers in normal-fed rats.
Previous studies indicate that anesthesia alone might impair spatial learning in aged rats (Culley et al., 2003; Gong et al., 2012). However, the results are conflicting. One study reports that learning is improved in rats two weeks following anesthesia, as is spatial memory in mice following repeated exposure to anesthesia (Butterfield, Graf, Ries, & MacLeod, 2004; Crosby, Culley, Baxter, Yukhananov, & Crosby, 2005; Komatsu et al., 1998; Valentim, Alves, Olsson, & Antunes, 2008). In contrast, others report that repeated or single anesthesia confers no immediate or prolonged cognitive impairment in mice (Butterfield et al., 2004; Valentim et al., 2008). Although the mechanism of thiopental-induced cognitive impairment remains unknown, we believe that the behavioral, cellular, and biochemical deficits in these animals are a result of repeated anesthesia exposure. It could be possible that thiopental promotes enhanced GABA release resulting in inhibitory synaptic transmission (Hirota, Sasaki, & Yamazaki, 2012).
However, we cannot exclude that stress, from repeated anesthesia /infusion may have also played a role in the altered phenotype of these animals. It should also be noted that we cannot distinguish whether repeated anesthesia alone or repeated i.v. saline infusion alone resulted in these changes. To characterize these differences would be out of the scope of this study. Our main purpose was to establish an effective method for intravenous delivery to six-month-old Brown Norway rats as well as test the learning and cognitive properties that are affected by this method.
4.3. Cholesterol diet prevents anesthesia and infusion-induced cognitive deficits
In this present study we observed a protective effect of high cholesterol diet against cognitive deficits induced by anesthesia / infusion. This was indicated when, unlike normal-fed animals, cholesterol fed animals were able to maintain their spatial learning abilities following anesthesia/infusion treatment. Since we observed no significant differences in RME or WME made by infused cholesterol-fed and normal-fed animals, we speculate that cholesterol may play a role in reference working memory. Cortical NGF levels in cholesterol-fed infused animals were also significantly enhanced compared to normal-fed infused animals. In agreement with other studies reporting prolonged recovery in lean animals compared to those with high body fat, these data demonstrate the importance of fat reservoirs for the redistribution of thiopental from central circulation (Dugdale, 2010). Previous investigations have also suggested that anesthetics can bind to membrane proteins, such as ion channels, and possibly alter their function (Rehberg, Urban, & Duch, 1995). However, little evidence exists evaluating the relationship between cholesterol and anesthesia. In addition, one study indicates that cholesterol can modulate anesthetic suppression of ion channel currents (Rehberg et al., 1995). It could be possible that high cholesterol diet helps maintain cholesterol homeostasis needed on the membrane for appropriate ion channel initiated signal transduction, which is disrupted by repeated exposure to anesthesia (Levitan, Fang, Rosenhouse-Dantsker, & Romanenko, 2010). Another theory postulates that anesthetic molecules can destroy phase separation in the lipid bilayer effectively fluidizing the entire bilayer and disrupting Na+ channel conformational change needed for ion passage. It states that cholesterol may have the opposite effect of anesthetics by stabilizing phase separation making the lipid bilayer more rigid and allowing proper Na+ channel structure and function (Bastiaanse, Höld, & Van der Laarse, 1997). However, it is important to remember that dietary cholesterol cannot cross the BBB. Thus, more studies are needed to better understand the relationship between altered cholesterol levels and metabolism and disruptions in central nervous system function.
In conclusion, our data indicate that anesthesia/infusion can lead to spatial learning impairment and cholinergic system dysfunction. Moreover, these findings suggest that cholesterol diet can provide some protection against these anesthesia/infusion-induced deficits.
Acknowledgments
This study has been supported by the Austrian Science Funds (P24541-B24). We would like to thank Ursula Kirzenberger-Winkler for her excellent technical assistance. We thank Mag. Dr. Michael Pirchl for his help and technical advice on using the radial 8-arm maze. We also thank Prof. Dr. Georg Kemmler for his professional advice on statistical analyses and interpretation.
References
- Bastiaanse EM, Höld KM, Van der Laarse A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovascular Research. 1997;33:272–283. doi: 10.1016/s0008-6363(96)00193-9. [DOI] [PubMed] [Google Scholar]
- Berkman Z, Tanriover G, Acar G, Sati L, Altug T, Demir R. Changes in the brain cortex of rabbits on a cholesterol-rich diet following supplementation with a herbal extract of Tribulus terrestris. Histology and Histopathology. 2009;24:683–692. doi: 10.14670/HH-24.683. [DOI] [PubMed] [Google Scholar]
- Böttger D, Ullrich C, Humpel C. Monocytes deliver bioactive nerve growth factor through a brain capillary endothelial cell monolayer in vitro and counteract degeneration of cholinergic neurons. Brain Research. 2010;1312:108–119. doi: 10.1016/j.brainres.2009.11.062. [DOI] [PubMed] [Google Scholar]
- Butterfield NN, Graf P, Ries CR, MacLeod BA. The effect of repeated isoflurane anesthesia on spatial and psychomotor performance in young and aged mice. Anesthesia & Analgesia. 2004;98:1305–1311. doi: 10.1213/01.ane.0000108484.91089.13. [DOI] [PubMed] [Google Scholar]
- Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesthesia & Analgesia. 2005;101:1389–1392. doi: 10.1213/01.ANE.0000180835.72669.AD. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesthesia & Analgesia. 2003;96:1004–1009. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- Darwish DS, Wang D, Konat GW, Schreurs BG. Dietary cholesterol impairs memory and memory increases brain cholesterol and sulfatide levels. Behavioral Neuroscience. 2010;124:115–123. doi: 10.1037/a0018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nature Reviews Neuroscience. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour F, Liu QY, Gusev P, Alkon D, Atzori M. Cholesterol-enriched diet affects spatial learning and synaptic function in hippocampal synapses. Brain Research. 2006;1103:88–98. doi: 10.1016/j.brainres.2006.05.086. [DOI] [PubMed] [Google Scholar]
- Gong M, Chen G, Zhang XM, Xu LH, Wang HM, Yan M. Parecoxib mitigates spatial memory impairment induced by sevoflurane anesthesia in aged rats. Acta Anaesthesiologica Scandinavica. 2012;56:601–607. doi: 10.1111/j.1399-6576.2012.02665.x. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. Journal of Alzheimer’s Disease. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam study. Journal of Neurology, Neurosurgery, & Psychiatry. 2008;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- Hirota K, Sasaki R, Yamazaki M. Pre-synaptic function explains age-dependent actions of general anesthetics in the rat hippocampal synaptic transmission. Toxicology In Vitro. 2012;26:872–877. doi: 10.1016/j.tiv.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Hohsfield LA, Humpel C. Homocysteine enhances transmigration of rat monocytes through a brain capillary endothelial cell monolayer via ICAM-1. Current Neurovascular Research. 2010;7:192–200. doi: 10.2174/156720210792231787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiology of Disease. 2007;28:16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Nogaya J, Kuratani N, Ueki M, Yokono S, Ogli K. Repetitive post-training exposure to enflurane modifies spatial memory in mice. Anesthesiology. 1998;89:1184–1190. doi: 10.1097/00000542-199811000-00019. [DOI] [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, et al. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity. 2011;19:1586–1594. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Cholesterol and ion channels. Subcellular Biochemistry. 2010;51:509–549. doi: 10.1007/978-90-481-8622-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale V, Scapagnini G, Colombrita C, Mazzola C, Alkon DL, Drago F. Behavioral effects of dietary cholesterol in rats tested in experimental models of mild stress and cognitive tasks. European Neuropsychopharmacology. 2008;18:462–471. doi: 10.1016/j.euroneuro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Miller S, Wehner JM. Cholesterol treatment facilitates spatial learning performance in DBA/2lbg mice. Pharmacology Biochemistry and Behavior. 1994;49:257–261. doi: 10.1016/0091-3057(94)90487-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed Academic Press; Sydney, Australia: 1986. [Google Scholar]
- Pirchl M, Ullrich C, Humpel C. Differential effects of short- and longterm hyperhomocysteinaemia on cholinergic neurons, spatial memory and microbleedings in vivo in rats. European Journal of Neuroscience. 2010;32:1516–1527. doi: 10.1111/j.1460-9568.2010.07434.x. [DOI] [PubMed] [Google Scholar]
- Pirchl M, Ullrich C, Sperner-Unterweger B, Humpel C. Homocysteine has anti-inflammatory properties in a hypercholesterolemic rat model in vivo. Molecular and Cellular Neuroscience. 2012;49:456–463. doi: 10.1016/j.mcn.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SM, Van Dam AM, Schultzberg M, Crisby M. High cholesterol diet results in increased expression of interleukin-6 and caspase-1 in the brain of apolipoprotein E knockout and wild type mice. Journal of Neuroimmunology. 2005;169:59–67. doi: 10.1016/j.jneuroim.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Rehberg B, Urban BW, Duch DS. The membrane lipid cholesterol modulates anesthetic actions on a human brain ion channel. Anesthesiology. 1995;82:749–758. doi: 10.1097/00000542-199503000-00017. [DOI] [PubMed] [Google Scholar]
- Reiss AB, Voloshyna I. Regulation of cerebral cholesterol metabolism in Alzheimer disease. Journal of Investigative Medicine. 2012;60:576–582. doi: 10.231/JIM.0b013e318246d973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Darwish DS, Stankovic G, Sparks DL. High dietary cholesterol facilitates classical conditioning of the rabbit’s nictitating membrane response. Nutritional Neuroscience. 2007;10:31–43. doi: 10.1080/10284150701232034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG. The effects of cholesterol on learning and memory. Neuroscience & Biobehavioral Reviews. 2010;34:1366–1379. doi: 10.1016/j.neubiorev.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Wang D, Smith-Bell CA, Burhans LB, Bell R, Gonzalez-Joekes J. Dietary cholesterol concentration and duration degrade long-term memory of classical conditioning of the rabbit’s nictitating membrane response. International Journal of Alzheimer’s disease. 2012;2012:732634. doi: 10.1155/2012/732634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Prasanthi RPJ, Schommer E, Feist G, Ghribi O. Hypercholesterolemia-induced Abeta accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiology of Disease. 2008;32:426–432. doi: 10.1016/j.nbd.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Kuo YM, Roher A, Martin T, Lukas RJ. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation. Preliminary observations. Annals of the New York Academy of Sciences. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. Journal of Neurochemistry. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Molecular & Cellular Neuroscience. 2010;45:408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentim AM, Alves HC, Olsson IA, Antunes LM. The effects of depth of isoflurane anesthesia on the performance of mice in a simple spatial learning task. Journal of the American Association for Laboratory Animal Science. 2008;47:16–19. [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Orser BA. Inhibition of learning and memory by general anesthetics. Canadian Journal of Anesthesia. 2011;58:167–177. doi: 10.1007/s12630-010-9428-8. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Miki T, Ding Y, Wang SJ, Gao YH, Wang XL, et al. A high cholesterol diet given to apolipoprotein E-knockout mice has a differential effect on the various neurotrophin systems in the hippocampus. Metabolic Brain Disease. 2011;26:185–194. doi: 10.1007/s11011-011-9252-z. [DOI] [PubMed] [Google Scholar]
- Xue QS, Sparks DL, Streit WJ. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. Journal of Neuroinflammation. 2007;4:20. doi: 10.1186/1742-2094-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zassler B, Humpel C. Transplantation of NGF secreting primary monocytes counteracts NMDA-induced cell death of rat cholinergic neurons in vivo. Experimental Neurology. 2006;198:391–400. doi: 10.1016/j.expneurol.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Zatta P, Zambenedetti P, Stella MP, Licastro F. Astrocytosis, microgliosis, metallothionein-I-II and amyloid expression in high cholesterolfed rabbits. Journal of Alzheimer’s Disease. 2002;4:1–9. doi: 10.3233/jad-2002-4101. [DOI] [PubMed] [Google Scholar]