Abstract
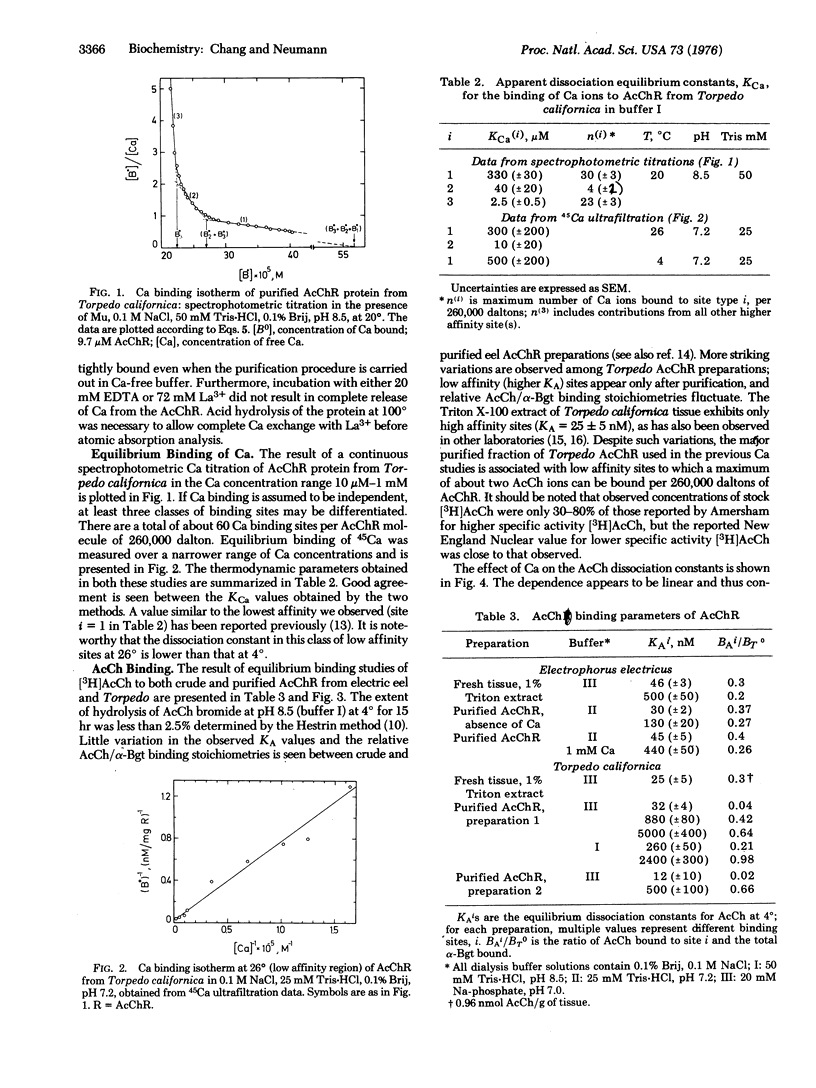
Interaction of Ca and acetylcholine (AcCh) ions with purified acetylcholine receptor (AcChR) from Torpedo californica and Electrophorus electricus has been investigated in view of these ions' role proposed in bioelectricity. Spectrophotometric Ca titration using murexide as an indicator and an ultrafiltration method with 45Ca show that AcChR proteins have a high binding capacity for Ca ions. Per macromolecule of 260,000 daltons, up to 60 Ca ions can be bound with at least three Ca dissociation constants. A linear inhibition of AcCh binding to AcChR by Ca was observed in the 0.1-1 mM Ca range, indicating competition of AcCh and Ca for AcChR. The addition of AcCh to a Ca-AcChR solution at 1.2 mM Ca causes release of four to six bound Ca ions from AcChR when a maximum of two AcCh ions are bound per 260,000 dalton macromolecule. The subsequent addition of alpha-bungarotoxin causes reuptake of up to six Ca ions by AcChR. These results suggest that the neural activator AcCh and the inhibitor alpha-bungarotoxin induce opposing shifts between different conformational states of isolated AcChR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Chang H. W. Purification and characterization of acetylcholine receptor-I from Electrophorus electricus. Proc Natl Acad Sci U S A. 1974 May;71(5):2113–2117. doi: 10.1073/pnas.71.5.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefawi M. E., Eldefawi A. T., Penfield L. A., O'Brien R. D., Van Campen D. Binding of calcium and zinc to the acetylcholine receptor purified from Torpedo California. Life Sci. 1975 Mar 15;16(6):925–935. doi: 10.1016/0024-3205(75)90008-9. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T. Purification and molecular properties of the acetylcholine receptor from Torpedo electroplax. Arch Biochem Biophys. 1973 Nov;159(1):362–373. doi: 10.1016/0003-9861(73)90462-1. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Wilson D. B. Tryptophan and cystein residues of the acetylcholine receptors of Torpedo species. Relationship to binding of cholinergic ligands. Biochemistry. 1975 Sep 23;14(19):4304–4310. doi: 10.1021/bi00690a026. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martinez-Carrion M., Raftery M. A. Use of a fluorescent probe for the study of ligand binding by the isolated cholinergic receptor of Torpedo californica. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1156–1164. doi: 10.1016/s0006-291x(73)80016-6. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Sealock R., Olsen R., Changeux J. P. Purification and properties of the cholinergic receptor protein from Electrophorus electricus electric tissue. Eur J Biochem. 1974 Jun 15;45(2):371–394. doi: 10.1111/j.1432-1033.1974.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Moody T., Schmidt J., Raftery M. A. Binding of acetylcholine and related compounds to purified acetylcholine receptor from Torpedo Californica electroplax. Biochem Biophys Res Commun. 1973 Aug 6;53(3):761–772. doi: 10.1016/0006-291x(73)90158-7. [DOI] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- O'Brien R. D., Gibson R. E. Conversion of high affinity acetylcholine receptor from Torpedo californica electroplax to an altered form. Arch Biochem Biophys. 1975 Aug;169(2):458–463. doi: 10.1016/0003-9861(75)90188-5. [DOI] [PubMed] [Google Scholar]
- O'Brien R. D., Gibson R. E. Two binding sites in acetylcholine receptor from Torpedo marmorata electroplax. Arch Biochem Biophys. 1974 Dec;165(2):681–690. doi: 10.1016/0003-9861(74)90297-5. [DOI] [PubMed] [Google Scholar]
- Paulus H. A rapid and sensitive method for measuring the binding of radioactive ligands to proteins. Anal Biochem. 1969 Oct 15;32(1):91–100. doi: 10.1016/0003-2697(69)90107-9. [DOI] [PubMed] [Google Scholar]
- Penn A. S., Chang H. W., Lovelace R. E., Niemi W., Miranda A. Antibodies to acetylcholine receptors in rabbits: immunochemical and electrophysiological studies. Ann N Y Acad Sci. 1976;274:354–376. doi: 10.1111/j.1749-6632.1976.tb47697.x. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Hess G. P., Eldefrawi A. T., Eldefrawi M. E. Interaction between calcium and ligand-binding sites of the purified acetylcholine receptor studied by use of a fluorescent lanthanide. Biochem Biophys Res Commun. 1976 Jan 12;68(1):56–63. doi: 10.1016/0006-291x(76)90009-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Changeux J. P. Interconversion between different states of affinity for acetylcholine of the cholinergic receptor protein from Torpedo marmorata. Eur J Biochem. 1975 Jul 15;55(3):505–515. doi: 10.1111/j.1432-1033.1975.tb02188.x. [DOI] [PubMed] [Google Scholar]


