Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative illness affecting the elderly and is characterized by beta-amyloid (Aβ) deposition in the brain (plaques) and in microvessels (Aβ-angiopathy). The reasons for Aβ deposition are not clear, but an impaired clearance of Aβ at the blood-brain barrier may be implicated and oxidative stress possibly plays a major role in this process. Platelets are of particular interest, because they contain high levels of the amyloid precursor protein (APP) and in AD an abnormal expression of platelets APP fragments was found. The aim of the present study was to investigate (1) if oxidative stress induced by hydrogen peroxide (H2O2) affects APP expression in rat and human platelets and (2) to compare the APP changes with platelets of AD patients. In rat platelets, all three fragments of APP (130-110-106 kilo Dalton, kDa) were found. H2O2 (10 mM, 20 minutes) significantly reduced all three fragments in rat platelets, did not affect CD62P-staining and slightly increased the size of actin as seen in the Western blot. The effect was not seen at 1 mM H2O2 and was counteracted by glutathione. Immunohistochemistry for CD62P, CD61, APP and Annexin-V was used to verify the changes at the cellular level. In platelets of young volunteers (age = 33 ± 4 years), 10 mM H2O2 markedly reduced the smaller APP 110 and 106 kDa fragments after 20 minutes. Our data show that platelets of AD patients (age = 80 ± 1 years) had a significant reduced 130 kDa fragment compared to controls (age = 70 ± 2 years). In summary, oxidative stress may account for a dysfunctional processing of APP in rat and human control platelets and possibly in AD patients.
Keywords: Platelets, APP processing, hydrogen peroxide, oxidative stress, Alzheimer’s disease
Introduction
Platelets contain the highest amyloid precursor protein (APP) levels of all peripheral tissues and APP is secreted by platelets spontaneously, as well as during their activation, aggregation or degranulation [1, 2]. Platelets express mainly APP770 and APP751 which contain a Kunitz-type serine protease inhibitor domain (KPI) and are also known as APP-KPI + forms, while the APP695 form lacks this domain (APP-KPI− form) and is mainly found in neuronal tissue [3, 4]. APP 770 is the predominant form in platelets, but the role of platelets APP is still unclear. There are strong indications that soluble APP (sAPP) fragments may play a role in regulating thrombosis and hemostasis [5]. While the major population of APP is stored in alpha-granules as proteolyzed fragments, including sAPPα, approx. 10% of platelets APP is associated as a full length protein in the membrane [1, 5–9]. Activation of platelets with thrombin increases the surface expression of APP [7] and full length APP770 is cleaved by a calcium-dependent cysteine protease during platelet activation [10]. Platelets possess all the enzymatic machinery necessary for APP processing to beta-amyloid (Aβ) peptides [2, 11]. Platelets contain α- (ADAM10) and β-secretases (BACE1), but the α-secretase is more dominant and sAPPα levels are much higher than Aβ levels [2]. Interestingly, platelets are the source of 90% of peripheral β-amyloid in the blood and secrete mainly the Aβ(1-40) peptide [1, 11].
In plasma of Alzheimer’s disease (AD) patients a decrease of a larger 130 kilo Dalton (kDa) APP fragment, a decrease of α-secretases and an increase of β-secretases have been reported [12, 13]. Such changes may be caused by alterations in processing of the mRNA [14], altered posttranslational modifications [15–17] or might be the result of proteolytic cleavage by distinct secretases. Thus, these findings suggest an important role of platelets in AD and make them useful as a possible biomarker to diagnose AD [8, 18, 19].
There is evidence that oxidative stress is implicated in the pathomechanism of AD and neurons are particularly vulnerable [20, 21]. Age is a key risk factor for AD and it is likely that free radicals damage neurons over years. Hydrogen peroxide (H2O2) is one such reactive radical and commonly used to induce oxidative stress in vivo and in vitro [22–24]. There is evidence that H2O2 directly affects APP processing in human neuroblastoma cells where it induces apoptosis [25]. The aim of the present study was to use rat platelets as a simple and easy accessible model to study the effect of H2O2 on APP expression. Furthermore we compared the effects of oxidative stress in young healthy controls and AD patients. We showed that oxidative stress differentially affects APP fragments in all three used models. We hypothesize that oxidative stress may cause an aberrant APP processing and Aβ secretion in platelets, which may play a role in Aβ-angiopathy.
Methods
Collection of blood
For the animal experiments adult Sprague Dawley rats were used. The rats were anaesthetized with a high dose of thiopental (Sandoz) and blood was directly drawn from the heart by using a 21 gauge butterfly blood collection system (BD Valu- Set, BD). The blood was collected in ethylenediaminetetraacetate (EDTA) tubes (S-monovettes, Sarsted) and gently mixed. All experiments on animal tests were approved by the Austrian ethical committee. For human platelets blood was taken from young healthy volunteers from the lab (age = 33 ± 4 years, n = 6), collected in EDTA tubes and immediately processed. For old healthy controls (age = 70 ± 2 years, mini mental state examination (MMSE) = 27 ± 1, n = 15) and AD patients (age = 80 ± 1 years, MMSE = 18.0 ± 1.1, n = 25) the blood was used from a previous study [26] and processed within 3 hours.
Platelets isolation from rat and humans
Immediately after blood collection, anticoagulated rat or human blood was centrifuged at 250 × g for 15 minutes to obtain platelet rich plasma (PRP). All centrifugation steps were performed at room temperature. PGI2 (Prostaglandin, 500 nM, Sigma) was added to prevent platelet activation during processing. Platelets were separated from PRP by centrifugation at 2300 × g for 10 minutes and washed in calcium-free Tyrode buffer (136 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgSo4, 5 mM glucose, pH 6.5). After further centrifugation at 2300 × g for 10 minutes, platelets were finally resuspended in 1 ml Tyrode buffer (adjusted to pH 7.4) and further processed. To induce oxidative stress, platelets were exposed to freshly prepared H2O2 (30%, Merck) at a concentration of 1 or 10 mM for 20 minutes. In order to test protective effects, the antioxidant glutathione (1 mM, Sigma) was added 30 minutes prior and during H2O2 treatment. To study secretion of platelet markers, 1 × 108 platelets were incubated for 10 minutes (serotonin), 20 minutes (sAPPα and sAPPβ), 3 hours (matrix-metalloproteinase-2; MMP-2) or 24 hours (Aβ40+42) in Tyrode buffer (pH 7.4) at 37° C, then centrifuged for 10 minutes at 2300 × g and the supernatant was analyzed.
Immunohistochemistry
Immunohistochemistry was performed as described recently [26]. Platelets were pipetted onto glass slides and postfixed in 4% paraformaldehyde for 30 minutes at 4° C. Cells were washed 30 minutes with 0.1 % Triton/phosphate-buffered saline (T-PBS), pretreated with 5% methanol/1% H2O2/PBS. Then the cells were washed three times for 5 minutes with phosphate buffered saline, blocked with 20% horse serum/0.2% bovine serum albumin/T-PBS and further incubated with the primary antibody mouse anti-APP (Millipore; 1:2000), mouse anti-cluster of differentiation 41 (CD41, integrin alpha 2b) (Serotec, 1:2000), rabbit anti-cluster of differentiation 62P (CD62P, P-selectin) (Abcam, 1:1000) or mouse anti-cluster of differentiation 61 (CD61, integrin beta-3) (Serotec, 1:200) in 0.2% bovine seum albumin/T-PBS at room temperature overnight. Cells were washed and incubated with secondary biotinylated anti-mouse or anti-rabbit immunoglobulin G (IgG, 1:200 BA-2001 and BA-1000, Vector Laboratories) antibody for 1 hour at room temperature. After rinsing three times in PBS, slides were incubated in avidin-biotin complex solution (ABC; Elite Standard PK 6100, Vector Laboratories) for 1 hour, and then washed three times in 50 mM Tris-buffered saline (TBS). The signal was detected using 0.5 mg/ml 3,3′ diaminobenzidine (DAB) in TBS with 0.0003% H2O2 as substrate. Reaction was stopped in TBS and slides were allowed to dry and were cover slipped.
Annexin-V-Alexa 568 staining
Treated platelets were centrifuged at 2300 × g for 5 minutes, dissolved in 500 μl Annexin-V-labeling solution (Roche, 20 μl/ml Annexin, 10 mM 2-(4-2-(4-(2-hydroxyethyl)-1-piperazinyl)-ethansulfonacid (HEPES), pH adjusted to 7.4, 140 mM NaCl, 5 mM CaCl), then incubated 15 minutes at room temperature, pipetted onto glassslides and covered with Vectashield (H-1000, Vector Laboratories).
Western blot
To analyze the expression of APP fragments and platelet markers, Western blot analysis was performed as described recently [27]. Briefly, the platelet pellet was dissolved in 100 μl ice-cold PBS with a protease inhibitor cocktail (P-8340, Sigma), homogenized by using an ultrasonic device (Branson sonifier 250, Danburry), centrifuged at 16 000 × g for 10 minutes at 4° C and the pellet was discarded. Then platelet extracts (approximately 30–50 μg) were loaded onto 4–12% Bis-Tris polyacrylamide gel (Invitrogen) and electrophoresed for 45 minutes at 200 V. Samples were electrotransferred to nylon PVDF Immobilon-PSQ membranes (Millipore) for 90 minutes at 30 V with 20% methanol blotting buffer (Invitrogen). For detection, the Western Breeze Chemiluminescent System (Invitrogen) was used. Blots were blocked for 30 minutes with blocking buffer, then incubated overnight at 4°C with the primary antibody mouse anti-APP (1:2000, Millipore), rabbit anti-CD62P (1:1000, Abcam), mouse anti-CD61 (1:200, Serotec), or rabbit anti-actin antibody (1:1000, Sigma-Aldrich). Blots were washed and incubated with alkaline phosphatase-conjugated anti-mouse or anti-rabbit antibodies for 30 minutes at room temperature. After being washed, blots were incubated in CDP-Star chemiluminescent substrate solution (Invitrogen) and the signal was visualized with a cooled CCD camera (SearchLight, Thermoscience). The used monoclonal antibody 22C11/APP recognizes three APP fragments at 106, 110 and 130 kDa. Due to methodological difficulties to differentiate between the 106 and 110 kDa band both bands were merged for the calculations.
ELISA for rat Aβ(1-40+42), MMP-2, sAPPα and sAPPβ
For the secretion studies rat or human platelets were incubated with 1 or 10 mM H2O2 for 10 minutes, 20 minutes (human), 3 hours, or 24 hours, centrifuged at 2300 × g for 10 minutes to remove platelets and the supernatant was analyzed. ELISA measurements were performed as described by the manufacturers (Wako 292-64501 Aβ(1-42); Wako 294-64701 Aβ(1-40)). MMP-2 ELISA was performed as described by a SearchLight Array (Searchlight, Aushon Biosystems) [28]. Soluble APPα and APPβ measurements were performed by ELISA as described by the manufacturers (IBL, 27734 sAPPα and Covance, 38960 sAPPβ).
Analysis of serotonin
Serotonin was measured by high-performance liquid chromatography (HPLC) and electrochemical detection, as described by us [26]. Rat platelets were incubated with 1 or 10 mM or without (controls) H2O2 for 10 minutes, centrifuged and the supernatant (20 μl) was injected onto the HPLC. The samples were separated on a reversed phase C18 Nucleosil column (Bartelt) at a flow rate of 1 ml/minute using the following mobile phase: 0.05 M trichloric acid, 0.26 mM EDTA, 1.36 mM NaCl, 1.81 mM heptane sulfonic acid and 15% acetonitril in HPLC water. Detection was performed with an electrochemical detector (Antec-Leyden) at +0.55 V at 30° C. All unknown samples were correlated to external standards of serotonin (Sigma) according to the peak heights.
Quantification and statistical analysis
Measurement of optical density in Western blots was performed by computer-assisted densitometry (Photoshop). The Western blot signal has been found to be linear up to 60 μg protein loaded (R2 = 0.934). The number of platelets was counted under the microscope (Olympus, BX61) at a 40 × magnification. Statistical analysis was performed by one way analysis of variance (ANOVA) with a subsequent Fisher LSD posthoc test and by students-t test; A p-value <0.05 was considered as statistically significant.
Results
Rat control platelets characterization
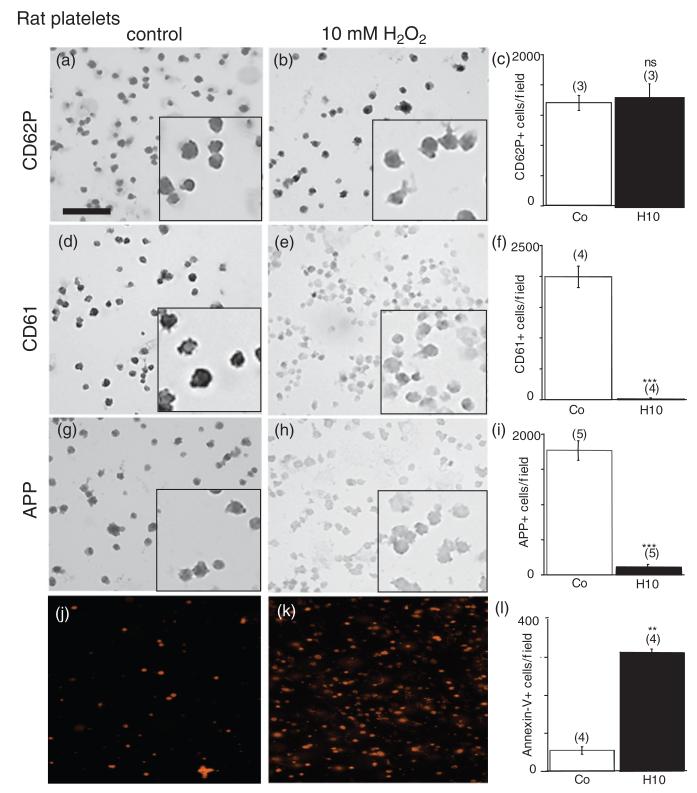
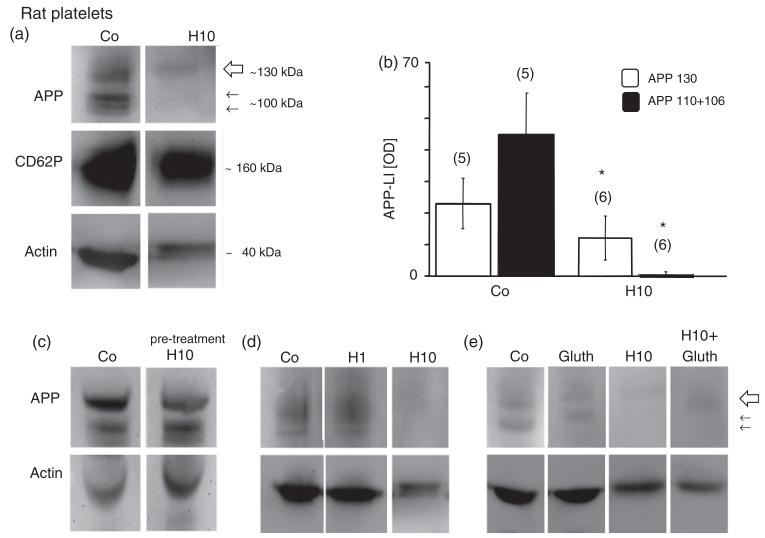
Immunohistochemistry of control platelets revealed a high number of discoid CD62P+ (Figure 1(a)) and CD61+ cells (Figure 1(d)). Control platelets were also positive for APP-like immunohistochemistry (Figure 1(g)). Annexin-V staining displayed a very low number of positive cells (360 ± 67 positive cells/mm2, n = 4, Figure 1(j)) in controls. Western blot analysis of control platelets showed three APP fragments at 130, 110, and 106 kDa (Figure 2(a)) and a strong band of CD62P at approximately 160 kDa (Figure 2(a)). Actin was used as a control, which was detectable at approximately 40 kDa (Figure 2(a)).
Figure 1.
Immunohistochemical staining of rat platelets. Rat platelets were treated with 10mM H2O2 for 20 minutes (b, e, h, k) or without (a, d, g, j) and spotted onto glasslides. Immunohistochemistry against CD62P (a, b) or CD61 (d, e) or amyloid precursor protein (APP) (g, h) or Annexin-V-staining (j, k) was performed. H2O2 markedly reduced the number of CD61 (e, f) and APP-positive platelets (h, i), but not of CD62P (b, c) and enhanced Annexin-V-positive cells (k, l). Platelets were counted under the microscope at 40 × magnification in a 336 × 448 μm field. Values are given as mean ± SEM. Statistical analysis was performed by Students t-test. Values in parenthesis give the number of experiments. *** p < 0.001;**p < 0.01; ns, not significant; Scale bar in (a) = 24 μm (large image) and 9 μm (small inserts), and 40 μm (j, k).
Figure 2.
Western blot analysis revealed three bands of amyloid precursor protein (APP) fragments at 130 (large arrow) and 110, 106 (small arrows) kilo Dalton (kD) in rat platelets (a). CD62P was used as a well established marker for control platelets and revealed a size of approximately 160 kDa (a). Actin was used for quantitative correlation and revealed a size of approximately 40 kDa (a) in control rat platelets. Incubation of rat platelets with 10mM H2O2 (H10) dramatically decreased the APP immunoreactivity of all 3 bands after 20 minutes (a), but did not affect CD62P (a). The size of actin was slightly increased after H2O2 treatment (a). H2O2 markedly reduced the APP 130, 110, and 106 kDa fragments, as shown in quantitative analysis (b). Pre-treatment of blots with 10mM H2O2 did not affect immunogenicity of the monoclonal 22C11/APP antibody (c). The effect of H2O2 was dose-dependent (d) and seen at 10mM H2O2 (H10), but not at 1mM H2O2 (H1). Glutathione (Gluth) partly counteracted the H2O2-induced (H10) decline of APP expression (e). Each lane represents an individual subject. Values are given as mean ± SEM optical density (OD). Statistical analysis was performed by students t-test. Values in parenthesis give the number of subjects; *p < 0.05.
Rat platelets and H2O2
Treatment of rat platelets with 10 mM H202 did not change the number of CD62P+ cells (Figure 1(b) and (c)), but significantly reduced the number of CD61+ platelets (Figure 1(e) and (f)). Incubation of platelets with 10 mM H2O2 significantly reduced the number of APP-positive cells (Figure 1(h) and (i)). H2O2 (10 mM, 20 minutes) markedly increased the number of Annexin-V positive cells (Figure 1(k) and (l)). Treatment of rat platelets with 10 mM H2O2 for 20 minutes resulted in a significant deletion of the 130, 110 and 106 kDa APP fragments (Figure 2(a) and (b)), but not CD62P, as shown in Western blot analysis. To preclude that H2O2 impairs the immunogeneity of the monoclonal antibody 22C11/APP the transblot was incubated with 10 mM H2O2 for 20 minutes before detection was performed (Figure 2(c)). The effect of H202 was dose-dependent with a marked effect at 10 mM H202, while 1 mM H2O2 did not affect APP-fragments (Figure 2(d)). In order to test protective effects, rat platelets were incubated with glutathione with or without H2O2. Glutathione (1 mM) partly counteracted the H2O2-induced decrease of APP fragments (Figure 2(e)). Incubation of platelets with glutathione alone did not alter APP fragments (Figure 2(e)).
Effect of H2O2 on secretion of serotonin, MMP-2, Aβ(1-40) and Aβ(1-42)
Serotonin secretion from rat platelets was detectable in controls and H2O2 did not significantly affect serotonin secretion after 10 minutes (Table I). MMP-2 secretion was not enhanced by H2O2 after 3 hours (Table I). Secreted Aβ(1-40) levels were clearly detectable in controls, but not changed after treatment with 1 or 10 mM H2O2 (Table I). Aβ(1-42) levels were very low in controls and slightly decreased after 1 and 10 mM H2O2 treatment.
Table I.
Secretion of serotonin, matrix-metalloproteinase-2 (MMP-2), beta-amyloid (Aβ).
| Controls | 1 mM H2O2 | 10 mM H2O2 | |
|---|---|---|---|
| Serotonin | 27 ± 3 (8) - | 45.5 ± 29 (6) ns | 30.5 ± 13 (3) ns |
| MMP-2 | 115 ± 24 (5) - | 207 ± 99 (3) ns | 131 ± 19 (3) ns |
| Aβ(1-40) | 17.1 ± 0.6 (6) - | 13.6 ± 2.4 (4) ns | 19.9 ± 2.4 (6) ns |
| Aβ(1-42) | 3.2 ± 1 (5) - | 1.2 ± 0.4 (4) * | 1.0 ± 0.4 (6) * |
Rat platelets (1×108) were incubated without (controls) or with 1 or 10mM hydrogen peroxide (H2O2) for 10 minutes (serotonin), 3 hours (MMP-2), or 24 hours (Aβ), then centrifuged and supernatant was evaluated by HPLC-EC (serotonin) or ELISA (MMP-2, Aβ). Values are given as mean ± SEM ng/mlx10 minutes (serotonin), pg/mlx3 hours (MMP-2), or pM/24 hours (Aβ). Statistical analysis was performed by one way analysis of variance with a subsequent Fisher LSD posthoc test. Values in parenthesis give the number of experiments.
p < 0.05;
ns, not significant.
Characterization of human platelets
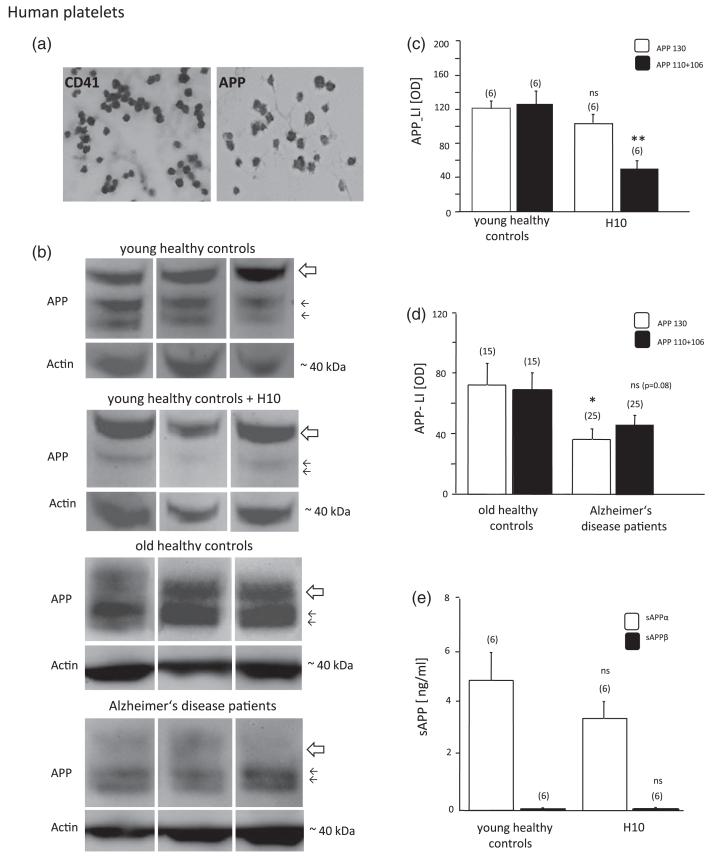
Immunohistochemistry of control platelets revealed discoid CD41+ (Figure 3(a)) and APP+ cells (Figure 3(a)). Western blot analysis detected 130, 110 and 106 kDa fragments of APP in young (age = 33 ± 4 years, n = 6) healthy control platelets (Figure 3(b)) and in old healthy controls (age = 70 ± 2 years, n = 15; Figure 3(b)). Human platelets contained approximately 20–50 times more sAPPα than sAPPβ (Figure 3(e)).
Figure 3.
Immunohistochemical staining of human platelets for CD41 and APP was performed (a). Western blot analysis of amyloid precursor protein (APP) in human platelets extracts revealed three fragments of APP at 130 (large arrow) and 110, 106 (small arrows) kilo Dalton (kDa) in young healthy controls (age = 33 ± 4 years; b) and old healthy controls (70 ± 2 years; b). Actin (40 kDa) was used for quantitative correlation. Each lane represents an individual subject. In platelets of Alzheimer disease (AD) patients the larger 130 kDa APP fragment was significantly decreased (b), while the smaller 110 and 106 fragments were only slightly reduced (p = 0.08), as shown by quantitative analysis (d). Treatment of platelets of young volunteers with 10mM H2O2 (H10) significantly reduced the smaller 110 and 106 kDa APP fragments (b, c), but did not affect the large 130 kDa form. Soluble APPα (sAPPα) levels were higher than soluble APPβ (sAPPβ) levels, however not changed after H2O2 treatment (e). Values are given as mean ± SEM optical density (OD; c, d) or ng/ml (e). Statistical analysis was performed by students t-test. Values in parenthesis give the number of subjects, *p < 0.05; ns, not significant.
Effect of H2O2 on APP expression in human platelets
Treatment of human control platelets from young healthy volunteers with 10 mM H2O2 significantly decreased the lower 106 and 110 kDa APP fragments, but did not affect the 130 kDa APP fragment (Figure 3(b) and (c)). H2O2 treatment did not affect secretion of sAPPα and sAPPβ after 20 minutes (Figure 3(e)).
APP expression in AD patients
In AD patients (Figure 3(b), age = 80 ± 1 years, n = 25) the APP 130 kDa fragment was significantly decreased (Figure 3(b) and (d)), but the 106 and 110 kDa APP fragments were only slightly decreased (Figure 3(b) and (d); p = 0.08, n = 25).
Discussion
Our present study shows (1) that rat platelets contain all three APP fragments, (2) that H2O2 induces a decline of APP in rat platelets, and (3) that H2O2 affects APP expression in human control platelets.
Rat platelets
In order to compare isolated rat platelets with human platelets we characterized rat platelets with the well established markers CD61 and CD62P. CD61 is also known as the integrin β3 unit and forms together with CD41 the gpIIIa/IIb complex, which is found on rat platelets and megacaryocytes mediating cell adhesion. CD61 is expressed on resting platelets and serves as a subunit of the receptor for fibrinogen [29]. Binding of fibrinogen to this receptor is required for platelet aggregation and endothelial adherence. CD62P is stored in alpha-granules of platelets, but is not expressed on the surface. This calcium-dependent protein migrates during platelet activation to the plasma membrane and mediates platelets interaction with endothelial cells or leukocytes and CD62P serves as a marker for activated platelets. In our Western blot experiments we found high CD62P levels in platelet extracts after sonication, which most likely reflects the total granular content. Thus CD62P levels may not be fully appropriate as an activation marker by using Western blot but is highly useful in immunohistochemistry where membrane bound CD62P can be seen. In rat platelets APP fragments of 130, 110 + 106 kDa were detectable using the same antibody as in human platelets, but the APP expression levels were markedly lower. When comparing the APP ratios from young, old and rat control platelets no significant difference was observed, although a tendency for a decrease in the control rat platelets ratio was seen (data not shown). We also noted that the APP content markedly varied during different preparations and partly displayed a high heterogeneity, which may be explained by cleavage of the full length APP into smaller fragments without transmembrane and cytoplasmic domains during the preparation [1]. Indeed, we observed enhanced expression of a smaller APP form (probably sAPP as shown in the ELISA) in supernatants of control platelets. Also we cannot exclude that a part of the isolated platelets undergo rapid cell death, as we also found low actin levels in the supernatant (data not shown). Our data showed that rat platelets provide a fast, simple and easy model to study processing of APP. Moreover, rat platelets are useful to investigate effects of oxidative stress. This has the advantage to use a well controlled cell system, while in humans unknown cofactors, such as drug intake or age-dependent alterations cannot be excluded. Indeed, in agreement recently Yu et al. [30] explored the proteome of human and rat platelets suggesting the usefulness of rat platelets as a reliable alternative platelet model.
Human platelets
Human platelets were isolated from young healthy volunteers, older healthy controls, and AD patients. The isolation procedure and characterization (CD41) has been recently publicated by us in detail [26]. In order to test if human platelets react to oxidative stress we isolated platelets from younger volunteers from the lab and found 130, 110 + 106 APP fragments with an expression level which was not different to elderly controls. Oxidative stress mediated by 10 mM H2O2 (20 minutes) significantly reduced the APP 110 + 106 kDa fragments but not the APP 130 kDa fragment. This was interestingly to note because it seems that especially the smaller fragments are more sensitive to oxidative stress. In this study we used the same controls and AD patients as in a previous study [26]. We recently reported that the ratio of APP 130/110-106 was slightly reduced in AD patients [26] and show that the APP 130 kDa cleavage product was significantly reduced and the APP 110 + 106 kDa fragments were only slightly reduced. This is in full line with several previous reports showing altered APP metabolism in AD patients [8, 12, 13, 18, 19, 26].
H2O2 mediated oxidative stress in platelets
Oxidative stress may play a role in AD neuropathology [20, 31]. Hydrogen peroxide is commonly used to induce oxidative stress in vivo as well as in vitro [22–24]. Under physiological conditions the mean hydrogen peroxide level is <0.7 μM and does not induce oxidative stress. In blood, neutrophils produce sufficient amounts of H2O2 leading to high local endogenous peroxide levels in vivo [32, 33]. Furthermore, hyperoxic conditions after stroke or heart attack may give rise to high H2O2 concentrations in the brain [34] and generation of H2O2 may also be induced by different drugs, such as sulfonamide [35]. In general, exogenously applied H2O2 to cells normally results in approximately 7 to 10-fold less intracellular levels [36] and thus, in our experiments oxidative stress was induced by 10 mM H2O2. The lack of comparability between the in vivo H2O2 concentration and the dose of H2O2 that was used in vitro is a limitation of this study. However, there are some good reasons for using H2O2 levels in the mM range. (1) Hydrogen peroxide is a very unstable reactive molecule and rapidly reacts with other molecules. (2) We used a relative high density of platelets and it is known that platelets contain H2O2-degrading enzymes [32, 37], which may limit the effects of H2O2 treatment at low concentrations. (3) The number of morphologically CD62P+ platelets was not affected by this H2O2 treatment. It cannot be excluded that CD62P is already activated during the isolation or culturing conditions. (4) We could not see any effects on APP expression with 1 mM H2O2 and the most prominent effect on APP was only observed at 10 mM H2O2. As mentioned before, immunodetection of CD62P was not influenced by H2O2 treatment, as seen in Western blot analysis or in immunohistochemistry, clearly showing that H2O2 did not cause morphological discrimination of the platelets. CD61 immunodetection was affected and the sensitivity of the gpIIb/IIIa complex (CD 61) to H2O2 may also be indicated by the finding that platelets aggregation is modified by hydrogen peroxide [38, 39]. In addition, our experiments showed a slight increase in molecular weight of actin, indicating actin polymerization during H2O2 treatment. This alteration in molecular weight may advise to an early apoptotic process in response to H2O2, which is well established [26]. Interestingly, we found low levels of actin also in the supernatant of control and H2O2-treated rat platelets (data not shown), which possibly points to rapid cell death of a few platelets during the isolation procedure. In addition, we showed enhanced Annexin-V-staining in rat platelets after 20 minutes of 10 mM H2O2 treatment, but not in control platelets. It is well known that the translocation of phosphatidylserine from the inner part of the plasma membrane to the outer layer binding site is seen during platelet activation. Taken together, our findings may indicate activation in rat and human platelets in vitro due to oxidative stress.
Serotonin and MMP-2 in platelets during oxidative stress
To proof functionality of our platelets preparation during oxidative stress, we measured the serotonin and MMP-2 secretion. Platelets contain high levels of serotonin, which directly reflect the blood serotonin levels. Platelets do not synthesize serotonin and this biogenic amine is taken up from the plasma, stored in dense granules and secreted upon platelets activation. Serotonin secretion was detectable in controls, but not changed by 1 and 10 mM H2O2 treatment, which again indicated a moderate peroxide effect. However, at 100 mM H2O2 (data not shown) a significant serotonin secretion has been seen, being in agreement with others [32]. Interestingly, serotonin is also implicated in APP processing [40, 41] and may thus contribute to any APP metabolic processes during isolation. MMPs are zink-dependent endopeptidases, which are capable of degrading extracellular matrix proteins [42]. MMP-2 is expressed and secretion by platelets and is associated in blood-brain barrier impairment and axonal regeneration [43, 44]. It is interesting to note that MMP-2 is capable to degrade Aβ-peptides [45, 46], possibly indicating a role in Aβ-clearance. In AD a reduction in MMP-2 levels in the cerebrospinal fluid [47] and decreased levels in platelets have been found [26], pointing to an important role of MMP-2 in AD pathology. In the present study the secretion of MMP-2 was not altered after treatment with 1 and 10 mM H2O2, suggesting that MMP-2 is not directly involved in the H2O2-induced APP processing.
APP and Aβ in platelets during oxidative stress
Aβ-peptides are derived from amyloid precursor protein after processing by different secretases mainly leading to Aβ(1-40) and Aβ(1-42) peptides, which accumulate in the brain (plaques) and vessels (angiopathy) of AD patients. Platelets contain high levels of APP [1, 2, 11] and share some metabolic similarities with neurons, as they store and release neurotransmitters, such as e.g. serotonin. The processing of APP is altered in patients suffering from AD [4] and a reduced ratio between upper (130 kDa) and lower (106-110 kDa) APP fragments has been reported in AD compared to controls [12, 13], suggesting that APP ratios in platelets could be a biomarker for AD [10, 20]. The different sizes of APP detected by Western blot analysis do not necessarily reflect different APP fragments [15] but rather APP cleavage products of a different size. Reasons for differences in molecular weight may also be due to alternative splicing or posttranslational modifications, such as glycosylation, phosphorylation and sulfatation [15–17]. In the present study, using Western blot analysis, we could detect three immunoreactive bands at 106, 110, and 130 kDa size in rat and human platelets. The monoclonal antibody 22C11 detects mature and immature APP, which may differ in the degree of glycosylation, as well as secreted APP fragments, at the N-terminal cysteine containing region [48]. Since the 106, 110 kDa fragments were hardly to distinguish, both bands were combined for measurements in the study. H2O2 markedly decreased the APP 106 110 kDa bands and less the 130 kDa band indicating cleavage and processing of full length APP and different metabolic APP-fragments. Interestingly, after H2O2 treatment of neuroblastoma cells a 5.5 kDa peptide was detected, possibly containing the Aβ-sequence [25]. Indeed, we were able to detect a smaller APP product at approximately 80 kDa in platelet supernatants, maybe indicating a secreted form of APP and indicating platelet activation and APP processing during platelets isolation. sAPPα is generated by the α-secretase and may have neuroprotective effects [49] sAPPα levels are much higher than Aβ levels due to the fact that the non-amyloidogenic pathway is predominant in platelets [2]. This is in line with our study where higher sAPPα than sAPPβ levels were measured in human platelets. Thus, higher levels of sAPPα point to a predominant non-amyloidocenic pathway with α-secretase cleavage. Interestingly, treatment with H2O2 did not affect the secretion of sAPPα and sAPPβ in platelets.
Indeed, we observed a higher variability in APP fragments during different platelet isolations and the H2O2 treatment did not exactly mimic the AD platelets phenotype. In fact, AD has a multifaceted pathogenesis and oxidative stress may only partly account for the changes seen in this disease. One limitation of measuring APP ratios is the strong variability of APP isoforms. Several studies suggested the usefulness of APP as a biomarker for AD [8, 18, 19], but these studies also reported that a high number of subjects was necessary for verification due to a high variance or instability during isolation of APP isoforms. This could be a reason for the observation that the changes seen in young treated platelets after oxidative stress did not exactly mimic the AD platelet profile. Furthermore, APP isoform expression in platelets may be influenced by many other factors, such as age, gender, and obesity. It is important to note that we used a calcium-free buffer to prevent calcium-dependent APP processing [8]. To eliminate the possibility that H2O2 may directly destroy the APP molecule we pretreated the Western blot membrane with 10 mM H2O2 just before detection of APP. Our data clearly show that all 3 APP bands were detectable pointing to a direct effect of H2O2 on APP processing and not just an artificial modification of the APP-binding site. In neuroblastoma cells H2O2-induced cleavage of APP into Aβ(1-42) was observed, accompanied by a downregulation of the α-secretase, and up-regulation of β- and γ-secretase enzymes [50]. Others reported diminished levels of full length APP and a modification of APP-processing mediated by H2O2 in human neuroblastoma cells [25]. The predominant isoform of Aβ in platelets is the 40 amino acids long peptide Aβ(1-40) and in our study, Aβ(1-40) was clearly detectable in controls and after treatment with H2O2. It has been shown that Aβ(1-40) accumulates in apoptotic platelets [51], indicating declined Aβ(1-40) secretion. The longer Aβ(1-42) form is the peptide which more likely aggregates and contributes to plaque formation. It has been reported that H2O2 can increase Aβ production in human neuroblastoma cells [25], but Aβ(1-42) can also mediate H2O2-induced cytotoxicity [52]. Our data show slightly decreased Aβ(1-42) secretion after treatment with H2O2, indicating an accumulation of Aβ(1-42) in platelets, which could affect the Aβ clearance at the blood-brain barrier and thus, contribute to plaque development in a long term.
Counteracting H2O2-mediated APP processing
In order to investigate if anti-oxidants counteract the aberrant APP processing upon H2O2 treatment, we tested the well known anti-oxidant glutathione. Glutathione prevents damage caused by reactive oxygen species and is generated in many cells [53]. A ratio between reduced and oxidized form of glutathione may indicate the intensity of oxidative stress [54]. It is well established, that H2O2-induced cytotoxicity in hippocampal brain slice cultures is attenuated by pretreatment with the anti-oxidant glutathione [55]. In our present study, glutathione partly counteracted the altered APP processing in H2O2-treated rat platelets indicating that indeed anti-oxidative molecules may be useful in AD therapy.
Conclusion
In conclusion our data show altered APP processing in rat platelets exposed to oxidative stress, which was partly prevented by glutathione, similar to the aberrant APP processing in AD. Oxidative stress similarly declined APP processing in young healthy volunteers and a decline of APP fragments has been seen in platelets of AD patients. Thus, chronic mild longlasting oxidative stress may influence the peripheral APP processing in platelets. In combination with a dysfunctional Aβ clearance these processes may play an important role in the development of AD and β-amyloid angiopathy.
Acknowledgements
This study was supported by the Austrian Science Funds (L429-B05). We thank Ursula Kirzenberger-Winkler for her excellent technical help. We thank Prof. Josef Marksteiner (Landeskrankenhaus Hall/Tirol) for continuous support on blood collection.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
References
- 1.Bush Al, Martis RN, Rumble B, Moir R, Fuller S, Milward E, Currie J, Ames D, Weidemann A, Fischer P, Multhaup G, Beyreuther K, Masters CL. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J Biol Chem. 1990;265:15977–15983. [PubMed] [Google Scholar]
- 2.Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL. Secretion of Alzheimer’s disease Aß-amyloid peptide by activation of human platelets. Lab Invest. 1998;78:461–469. [PubMed] [Google Scholar]
- 3.Di Luca M, Colciaghi F, Pastorino L, Borroni B, Padovani A, Cattabeni F. Platelets as a peripheral district where to study pathogenetic mechanisms of alzheimer disease: The case of amyloid precursor protein. Eur J Pharmacol. 2000;405:277–283. doi: 10.1016/s0014-2999(00)00559-8. [DOI] [PubMed] [Google Scholar]
- 4.Li QX, Fuller SJ, Beyreuther K, Masters CL. The amyloid precursor protein of Alzheimer disease in human brain and blood. J Leukoc Biol. 1999;66:567–574. doi: 10.1002/jlb.66.4.567. [DOI] [PubMed] [Google Scholar]
- 5.Evin G, Zhu A, Holsinger RM, Masters CL, Li QX. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein in brain and platelets. J Neurosci Res. 2003;74:386–392. doi: 10.1002/jnr.10745. [DOI] [PubMed] [Google Scholar]
- 6.Canobbio I, Catricalà S, Balduini C, Torti M. Calmodulin regulates the non-amyloidogenic metabolism of amyloid precursor protein in platelets. Biochim Biophys Acta. 2011;13:500–506. doi: 10.1016/j.bbamcr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Li QX, Berndt MC, Bush AI, Rumble B, Mackenzie I, Friedhuber A, Beyreuther K, Masters CL. Membrane-associated forms of the beta A4 amyloid protein precursor of Alzheimer’s disease in human platelet and brain: Surface expression on the activated human platelet. Blood. 1994;84:133–142. [PubMed] [Google Scholar]
- 8.Tang K, Hynan LS, Baskin F, Rosenberg RN. Platelet amyloid precursor protein processing: A bio-marker for Alzheimer’s disease. J Neurol Sci. 2006;240:53–58. doi: 10.1016/j.jns.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skovronsky DM, Lee VM, Praticò D. Amyloid precursor protein and amyloid beta peptide in human platelets. Role of cyclooxygenase and protein kinase C J Biol Chem. 2001;20:17036–17043. doi: 10.1074/jbc.M006285200. [DOI] [PubMed] [Google Scholar]
- 10.Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer’s disease beta A4 amyloid precursor protein in human platelets. J Biol Chem. 1995;270:14140–14147. doi: 10.1074/jbc.270.23.14140. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Inestrosa NC, Ross GS, Fernandez HL. Platelets are the principal source of amyloid beta-peptide in human blood. Biochem Biophys Res Commun. 1995;213:96–103. doi: 10.1006/bbrc.1995.2103. [DOI] [PubMed] [Google Scholar]
- 12.Borroni B, Perani D, Broli M, Colciaghi F, Garibotto V, Paghera B, Agosti C, Giubbini R, Di Luca M, Padovani A. Pre-clinical diagnosis of Alzheimer disease combining platelet amyloid precursor protein ratio and rCBF spect analysis. J Neurol. 2005;252:1359–1362. doi: 10.1007/s00415-005-0867-z. [DOI] [PubMed] [Google Scholar]
- 13.Borroni B, Agosti C, Marcello E, Di Luca M, Padovani A. Blood cell markers in Alzheimer disease: Amyloid precursor protein form ratio in platelets. Exp Gerontol. 2010;45:53–56. doi: 10.1016/j.exger.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Vignini A, Sartini D, Morganti S, Nanetti L, Luzzi S, Provinciali L, Mazzanti L, Emanuelli M. Platelet amyloid precursor protein isoform expression in Alzheimer’s disease: Evidence for peripheral marker. Int J Immunopathol Pharmacol. 2011;24:529–534. doi: 10.1177/039463201102400229. [DOI] [PubMed] [Google Scholar]
- 15.Blasko I, Marx F, Grubeck-Loebenstein B, Marksteiner J. Abnormalities of amyloid beta precursor protein expression in platelets of patients with Alzheimer disease: Do we understand them well enough? Arch Neurol. 1999;56:889–891. doi: 10.1001/archneur.56.7.889. [DOI] [PubMed] [Google Scholar]
- 16.Weidemann A, König G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 17.Pahlsson P, Spitalnik SL. The role of glycosylation in synthesis and secretion of beta-amyloid precursor protein by Chinese hamster ovary cells. Arch Biochem Biophys. 1996;331:177–186. doi: 10.1006/abbi.1996.0296. [DOI] [PubMed] [Google Scholar]
- 18.Padovani A, Pastorino L, Borroni B, Colciaghi F, Rozzini L, Monastero R, Perez J, Pettenati C, Mussi M, Parrinello G, et al. Amyloid precursor protein in platelets: A peripheral marker for the diagnosis of sporadic AD. Neurology. 2001;57:2243–2248. doi: 10.1212/wnl.57.12.2243. [DOI] [PubMed] [Google Scholar]
- 19.Borroni B, Agosti C, Marcello E, Di Luca M, Padovani A. Blood cell markers in Alzheimer Disease: Amyloid Precursor Protein form ratio in platelets. Exp Gerontol. 2010;45:53–56. doi: 10.1016/j.exger.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: New insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaykhalishahi H, Yazdanparast R, Ha HH, Chang YT. Inhibition of H2O2-induced neuroblastoma cell cytotoxicity by triazine derivative, AA3E2. Eur J Pharmacol. 2009;622:1–6. doi: 10.1016/j.ejphar.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P. Melatonin protects against hydrogen peroxide-iduced cell death signaling in SH-SY5Y cultured cells: Involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res. 2006;41:116–123. doi: 10.1111/j.1600-079X.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 24.Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000;39:6951–6959. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Zhao B, Yew DT, Kusiak JW, Roth GS. Processing of Alzheimer’s amyloid precursor protein during H2O2-induced apoptosis in human neuronal cells. Biochem Biophys Res Commun. 1997;235:245–848. doi: 10.1006/bbrc.1997.6698. [DOI] [PubMed] [Google Scholar]
- 26.Hochstrasser T, Ehrlich D, Marksteiner J, Sperner-Unterweger B, Humpel C. Matrix metalloproteinase-2 and epidermal growth factor are decreased in plateletsof Alzheimer patients. Curr Alz Res. 2011 doi: 10.2174/156720512803251156. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. 2010;45:408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirchl M, Ullrich C, Humpel C. Differential effects of short- and long-term hyperhomocysteinaemia on cholinergic neurons, spatial memory and microbleedings in vivo in rats. E J Neurosci. 2010;32:1516–1527. doi: 10.1111/j.1460-9568.2010.07434.x. [DOI] [PubMed] [Google Scholar]
- 29.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb-IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 30.Yu Y, Leng T, Yun D, Liu N, Yao J, Dai Y, Yang P, Chen X. Global analysis of the rat and human platelet proteome-the molecular blueprint for illustrating multi-functional platelets and cross-species function evolution. Proteomics. 2010;10:2444–2457. doi: 10.1002/pmic.200900271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark RA, Klebanoff SJ. Myeloperoxidase-mediated platelet release reaction. J Clin Invest. 1979;63:177–183. doi: 10.1172/JCI109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henson PM, Johnston RB. Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physio. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 35.Jacob HS, Ingbar SH, Jandl JH. Oxidative hemolysis and erythrocyte metabolism in hereditary acatalasia. J Clin Invest. 1965;44:1187–1199. doi: 10.1172/JCI105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone JR, Yang S. Hydrogen peroxide: A signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 37.Levine PH, Weinger RS, Simon J, Scoon KL, Krinsky NI. Leukocyte-platelet interaction. Release of hydrogen peroxide by granulocytes as a modulator of platelet reactions. J Clin Invest. 1976;57:955–963. doi: 10.1172/JCI108372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodvein R, Lindon JN, Levine PH. Physiology and ultrastructure of the blood platelet following exposure to hydrogen peroxide. Br J Haematol. 1976;33:19–26. doi: 10.1111/j.1365-2141.1976.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 39.Canoso RT, Rodvien R, Scoon K, Levine PH. Hydrogen peroxide and platelet function. Blood. 1974;43:645–656. [PubMed] [Google Scholar]
- 40.Lezoualc’h F, Robert SJ. The serotonin 5-HT4 receptor and the amyloid precursor protein processing. Exp Gerontol. 2003;38:159–166. doi: 10.1016/s0531-5565(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 41.Nitsch RM, Wurtman RJ, Growdon JH. Regulation of proteolytic processing of the amyloid beta-protein precursor by first messengers. A novel potential approach for the treatment of Alzheimer’s disease. Arzneimittelforschung. 1995;45:435–438. [PubMed] [Google Scholar]
- 42.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metallo-proteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 43.Santos-Martínez MJ, Medina C, Jurasz P, Radomski MW. Role of metalloproteinases in platelet function. Thromb Res. 2008;121:535–542. doi: 10.1016/j.thromres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg GA. Matrixmetalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 45.Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem Biophys Res Commun. 1994;205:1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- 46.Liao MC, Van Nostrand WE. Degradation of soluble and fibrillar amyloid β-protein by matrix metalloproteinase (MT1-MMP) in-vitro. Biochemistry. 2010;49:1127–1136. doi: 10.1021/bi901994d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mlekusch R, Humpel C. Matrix metalloproteinases-2 and -3 are reduced in cerebrospinal fluid with low beta-amyloid1-42 levels. Neurosci Lett. 2009;466:135–138. doi: 10.1016/j.neulet.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem. 2009;29(284):15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilbich C, Mönning U, Grund C, Masters CL, Beyreuther K. Amyloid-like properties of peptides flanking the epitope of amyloid precursor protein-specific monoclonal antibody 22C11. J Biol Chem. 1993;268:26571–26577. [PubMed] [Google Scholar]
- 50.Quiroz-Baez R, Rojas E, Arias C. Oxidative stress promotes JNK-dependent amyloiogenic processing of normally expressed human APP by differential modification of alpha-, beta- and gamma-secretase expression. Neurochem Int. 2009;55:662–670. doi: 10.1016/j.neuint.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Casoli T, Di Stefano G, Balietti M, Solazzi M, Giorgetti B, Fattoretti P. Peripheral inflammatory biomarkers of Alzheimer’s disease: The role of platelets. Biogerontology. 2010;11:627–633. doi: 10.1007/s10522-010-9281-8. [DOI] [PubMed] [Google Scholar]
- 52.Sorg O. Oxidative stress: A theoretical model or biological reality? C R Biol. 2004;327:649–662. doi: 10.1016/j.crvi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 54.Asensi M, Sastre J, Pallardo FV, Lloret A, Lehner M, Garcia-de-la Asuncion J, Viña J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999;299:267–276. doi: 10.1016/s0076-6879(99)99026-2. [DOI] [PubMed] [Google Scholar]
- 55.Feeney CJ, Frantseva MV, Carlen PL, Pennefather PS, Shulyakova N, Sniffer C, Mills L. Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res. 2008;1198:1–15. doi: 10.1016/j.brainres.2007.12.049. [DOI] [PubMed] [Google Scholar]