Abstract
In Alzheimer’s disease (AD) neurons expressing the enzyme choline acetyltransferase (ChAT) degenerate and a loss of cholinergic activity directly correlates with cognitive decline. Recent studies have suggested that cholesterol plays a role in AD. The aim of the present study was to explore if cholesterol and its oxysterols, 24S-hydroxycholesterol (24S-OH Chol) and 25-hydroxycholesterol (25-OH Chol), affect ChAT-positive neurons in organotypic brain slices of the basal nucleus of Meynert (nBM). We showed that slices expressed approximately 140 ChAT-positive neurons/slice after 2 weeks when incubated with nerve growth factor (NGF). This number markedly decreased when incubated without NGF to approximately 20 neurons/slice. Cholesterol and 24S-OH Chol delayed this decrease in ChAT-positive neurons. In contrast, 25-OH Chol induced a decline in ChAT-positive neurons in 2-week-old slices within 4 days. The effects of cholesterol and its oxysterols were exhibited in a dose- and time-dependent way. Our results show that cholesterol and 24S-OH Chol delays the decrease in ChAT-positive neurons, while 25-OH Chol rapidly decreases ChAT expression, suggesting differential mechanisms on ChAT expression in cholinergic nBM neurons.
Keywords: Cholesterol, Cholinergic neuron, In vitro model, Oxysterols
Introduction
Alzheimer’s disease (AD) is a severe chronic neurodegenerative disease, characterized by beta-amyloid plaques, tau pathology, inflammation and cerebrovascular damage. In addition, neurons, which produce and release the neurotransmitter acetylcholine, degenerate in AD and a lack of acetylcholine directly correlates with cognitive decline [1, 2]. The reasons for AD are unknown, and it is also unclear why the cholinergic neurons degenerate. In addition, it has been demonstrated that cholinergic basal forebrain neurons in the basal nucleus of Meynert (nBM) are the most sensitive neurons to agerelated neurofibrillary degeneration [3]. AD shares some common risk factors with vascular dementia and several vascular risk factors may play a role in the development of AD. One of these risk factors is high cholesterol, and a dysfunction of brain cholesterol metabolism has been extensively studied and linked to AD and amyloid-precursor protein metabolism [4-7]. In fact, hypercholesterolemia modulates β-secretase cleavage in amyloid-precursor protein processing and leads to an enhanced production and aggregation of β-amyloid [4, 6]. Furthermore, cholesterol may have indirect effects via the cholesterol carrier protein apolipoprotein E4, one of the most prevalent genetic risk factor for AD [4, 5, 8]. It has been shown that plasma levels of 24S-hydroxycholesterol (24S-OH Chol), an oxygenated derivate of cholesterol, are elevated in AD patients [6]. Besides, longitudinal studies have suggested a relationship between elevated midlife cholesterol levels and late-life cognitive impairment in AD [9] and there are indications that cholesterol may have positive protective effects in late life [7] .
Cholesterol is an essential component of membranes, but cannot pass the blood-brain barrier [10-12], thus all cholesterol in the brain is thought to result from de novo synthesis. The function of cholesterol comprises the determination of the biophysical properties of membranes and the regulation of membrane-resident proteins [10]. The rate-limiting enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase modulates the cholesterol metabolism [12-15]. Cholesterol is metabolized to different oxysterols, such as 24S-OH Chol or 25-hydroxycholesterol (25-OH Chol) [16-22]. The conversion of cholesterol into its oxysterol 24S-OH Chol is important, because 24S-OH Chol can pass the blood-brain barrier and is able to enter the bloodstream [23, 24]. The oxysterol 25-OH Chol inhibits cholesterol synthesis and has cytotoxic effects on different cell lines, e.g. thymocytes or PC12 cells [25-27] .
The aim of the present study was to explore the effects of cholesterol and its oxysterols 24S-OH Chol and 25-OH Chol on the expression of choline acetyltransferase (ChAT) in cholinergic neurons of the nBM in organotypic brain slices. This enzyme is of importance, because it synthesizes the neurotransmitter acetylcholine from choline and coenzyme A. Our data show that cholesterol and 24S-OH Chol delayed down-regulation of ChAT in axotomized brain slices, while 25-OH Chol induced a rapid decline of ChAT in 2-week-old brain slices.
Material and Methods
Organotypic Brain Slice Cultures
Organotypic brain slice cultures were established as described by us in detail [28-30]. Briefly, the nBM of postnatal day 7–9 Sprague-Dawley rats was dissected under aseptic conditions, 400-μm slices were cut with a tissue chopper (McIlwain, USA), and the slices were placed on a 30-mm diameter Millicell-CM 0.4-μm membrane insert (Millipore, Austria), where they became attached to the membrane. It is important to note that the ipsilateral as well as contralateral brain areas were dissected at the same time, cut on the tissue chopper and all slices pooled in medium. Slices were cultured in six-well plates (Greiner) at 37°C and 5% CO2 with 1.2 ml/well of the following culture medium: 50% MEM/HEPES (Gibco), 25% heat-inactivated horse serum (Gibco/Lifetech, Austria), 25% Hanks’ solution (Gibco), 2 mmol/l NaH-CO3 (Merck, Austria), 6.5 mg/ml glucose (Merck, Germany), 2 mmol/l glutamine (Merck, Germany), pH 7.2. Medium was changed twice a week. In order to test the effects of cholesterol and its oxysterols two assays were performed:
Assay 1: Fresh slices were incubated with or without 10 ng/ml nerve growth factor (NGF) or with 0.02–2 μg/ml cholesterol or 24S-OH Chol or 25-OH Chol for up to 12 weeks.
Assay 2: Slices were incubated for 2 weeks with 10 ng/ml NGF, then for 3 days without (withdrawal) and then with or without 25-OH Chol or with NGF for up to 2 weeks.
At the end of the experiment, slices were fixed for 3 h at 4°C in 4% paraformaldehyde/10 mmol/l phosphate-buffered saline (PBS) and then stored at 4°C in PBS until use. All experiments conformed to Austrian guidelines on the ethical use of animals and all efforts were made to minimize the number of animals used and their suffering. NGF, cholesterol and 25-OH Chol were purchased from Sigma, 24S-OH Chol from Medical Isotopes (USA). The solubility was 2 μg/ml in culture medium and the solutions were agitated until all substances were dissolved.
Immunohistochemistry
Immunohistochemistry was performed as previously described [29]. All incubations were performed free floating for 2 days including 0.1% Triton, such that the antibodies can penetrate from both sides of the slices. Slices were washed for 30 min with 0.1% Triton-PBS at room temperature and pretreated for 20 min with 20% methanol/1% H2O2/PBS. After thorough rinsing, the slices were blocked with 20% horse serum/0.2% bovine serum albumin/Triton-PBS and then incubated for 2 days at 4°C with primary antibodies against ChAT (1:750; Millipore). Then the slices were washed again with PBS and incubated with secondary biotinylated anti-goat antibody for 1 h at room temperature. Following further washing steps with PBS, slices were incubated in an avidin-biotin complex solution (ABC-Elite Vectastain reagent Vector Laboratories) for 1 h. After being washed with 50 mmol/l Tris-buffered saline, the signal was detected by using 0.5 mg/ml 3,3′-diaminobenzidine including 0.003% H2O2 as a substrate in Tris-buffered saline. The slices were mounted on glass slides, air-dried and coverslipped with Entellan (Merck, Germany). Unspecific staining was defined by omitting the primary antibody.
Analysis, Quantification and Statistics
The number of microscopically detectable ChAT-positive neurons was counted in the whole slice visualized under a 20× objective. Quantitative data are presented as mean values ± SEM. Multistatistical analysis was obtained by using one-way ANOVA, followed by a Fisher PLSD post hoc test by comparing controls against respective treatment, where p < 0.05 represents statistical significance.
Results
ChAT-Positive Neurons
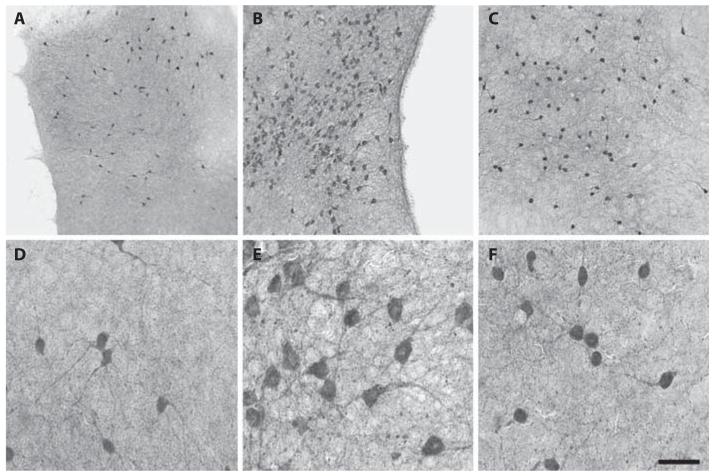
Cholinergic neurons in organotypic brain slices of the nBM were strongly stained by immunohistochemistry for the key enzyme ChAT (fig. 1). When slices were incubated in medium without NGF, only 24 ± 2 ChAT-positive neurons were visible, which were small in size and with few dendritic networks (fig. 1A, D). However, when slices were incubated for 2 weeks with 10 ng/ml NGF the number of ChAT-positive neurons was markedly increased to 140 ± 8 neurons per slice (fig. 1B, E). The number of ChAT-positive neurons decreased with time, displayed 84 ± 6 neurons/slice after 56 days and 54 ± 10 neurons/slice after 84 days when incubated with NGF (fig. 2). Slices incubated without NGF displayed a low number of neurons up to 84 days (fig. 2).
Fig. 1.
Organotypic brain slices of the nBM were cultured with (B, E) or without (A, D) 10 ng/ml NGF or with 2 μg/ml cholesterol (C, F). After 2 weeks slices were fixed and cholinergic neurons were immunohistochemically stained against ChAT (A–F). Higher magnifications of the corresponding slices are shown in D–F. Scale bar = 350 μm (A–C), 70 μm (D–F).
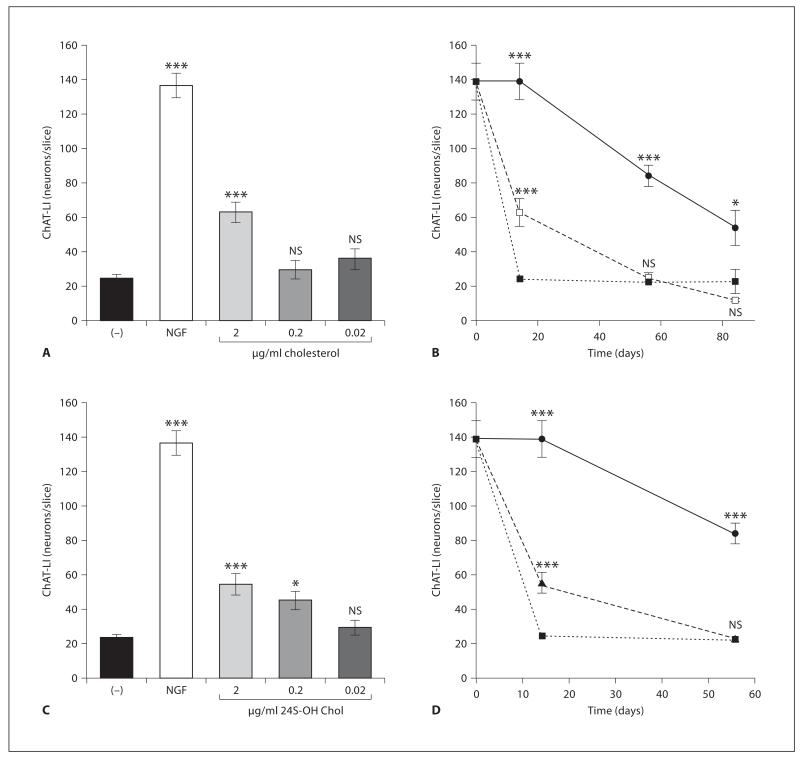
Fig. 2.
Effects of cholesterol and 24S-OH Chol on ChAT in axotomized cholinergic brain slices (assay 1). Brain slices of the nBM were cultured without (−; ■) or with 10 ng/ml NGF (●) or with 0.02, 0.2, or 2 μg/ml cholesterol (□) (A, B) or 24S-OH Chol (▲) (C, D). After 2 weeks the slices were fixed and immunohistochemically stained against ChAT. The number of ChAT-positive neurons was counted in whole slices. Statistical analysis was performed by one-way ANOVA with a Fisher PLSD post hoc test. ***p < 0.001; *p < 0.05; NS = not significant.
Effects of Cholesterol and Oxysterols on ChAT (Assay 1)
When slices were cultured with 2 μg/ml cholesterol for 2 weeks, the number of ChAT-positive neurons was significantly enhanced, compared to slices cultured without NGF (fig. 1C, F, fig. 2A). This effect was dose-dependent and not seen with 0.02 μg/ml and 0.2 μg/ml cholesterol (fig. 2A). The cholesterol effect was time-dependent, displayed a maximal effect after 2 weeks, but not when given for longer time periods (fig. 2B). When slices were incubated for 2 weeks with 2 μg/ml 24S-OH Chol a positive effect on the number of ChAT-positive neurons was found (fig. 2C). Again this effect was dose-dependent and not seen with 0.2 and 0.02 μg/ml 24S-OH Chol (fig. 2C). The effect of 24S-OH Chol exhibited time dependence with a positive effect after 2 weeks but not after 55 days in culture (fig. 2D). No effect of 25-OH Chol was seen (data not shown).
Effects of 25-OH Chol on ChAT (Assay 2)
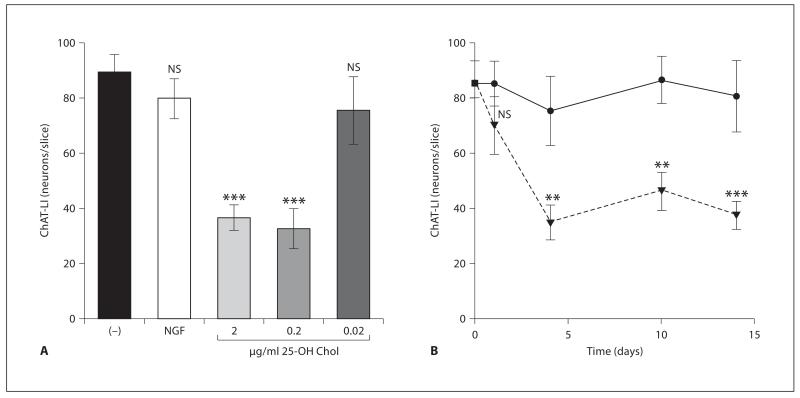
In order to test the effect of 25-OH Chol on cholinergic neurons, brain slices were cultured for 2 weeks with NGF and 3 days without NGF (withdrawal) and then exposed to 25-OH Chol and compared to slices incubated with or without NGF. When 2-week-old brain slices were incubated with 2 or 0.2 μg/ml but not 0.02 μg/ml 25-OH Chol the number of ChAT-positive neurons was significantly reduced (fig. 3A). The effect was already seen after 4 days and was not reversible after 10 and 14 days (fig. 3B). When 2-week-old slices were incubated with cholesterol or 24S-OH Chol, no effects were seen (data not shown).
Fig. 3.
Effects of 25-OH Chol on ChAT expression in 2-week nBM brain slices (assay 2). Slices were incubated for 2 weeks with 10 ng/ml NGF, then for 3 days without NGF and then with (NGF, filled circles) or without (−) NGF, or 0.02, 0.2, 2 μg/ml 25-OH Chol (filled triangles). After 4 days brain slices were fixed and stained for ChAT. The number of ChAT-positive neurons was counted in whole slices. Statistical analysis was performed by one-way ANOVA with a Fisher PLSD post hoc test. ***p < 0.001; **p < 0.01; NS = not significant.
Discussion
Our present study shows for the first time that cholesterol or 24S-OH Chol delays expression of ChAT-positive cholinergic nBM neurons, while 25-OH Chol rapidly decreased ChAT in cholinergic neurons.
The organotypic brain slice model retains many of the features of the intact brain, since it is able to maintain the survival of different cell types, the cytoarchitecture of the tissue, the connections between cells and neuronal properties [31]. In slices, individual cells are in close contact and do not lose density-dependent regulatory mechanisms, three-dimensional architecture as well as tissuespecific transport and diffusion probabilities. Thus, slice cultures provide an easily accessible experimental model for studies of toxic, degenerative and developmental changes in the brain [32]. The organotypic brain slice model has been introduced by Gähwiler and Hefti [33] and Gähwiler et al. [34], modified by Stoppini et al. [35] and is well established in our research group [29, 30, 36, 37]. In the present study, we used single brain slices of the nBM, which are derived from early postnatal day 7–9 rat brains. Cholinergic neurons are labeled by the key enzyme ChAT, which is determined by immunohistochemistry. It is well known that NGF supports the cholinergic phenotype. Although the number of ChAT-positive expression directly correlates with survival of cholinergic neurons, we do not claim that cell death occurs, which needs to be proven by further co-localization studies of ChAT with cell death markers.
Cholesterol has a crucial role in the development and maintenance of neuronal plasticity and function and is involved in the biophysical properties of membranes and biogenesis of synapses [6, 10]. Our data suggest that administration of cholesterol to organotypic brain slices of nearly 1-week-old rat brains delays the down-regulation of ChAT expression. The mechanism by which cholesterol exerts its positive effect on cholinergic neurons is unknown. However, since our nBM slice model is an axotomized in vitro system, it seems likely that cholesterol may support myelination or synaptogenesis of remaining neurons during the first 2 weeks. It is fully clear that cholesterol is not a ‘classic’ neuroprotective factor because it is not as potent as NGF. Alternatively, the positive effect of cholesterol could be mediated by influencing many G-protein-coupled receptors or enzymes in the plasma membrane [38] .
Similarly as cholesterol, brain slices incubated with 24S-OH Chol delayed the down-regulation of ChAT in brain slices. The oxysterol 24S-OH Chol plays an important role in the regulation of cholesterol homeostasis, as it is the major metabolic product, which can pass the blood-brain barrier. Since 24S-OH Chol was as potent as cholesterol to delay down-regulation of ChAT, we suggest that the effect of 24S-OH Chol is linked to cholesterol and AD. The mechanism of 24S-OH Chol is unknown, but it has been shown that 24S-OH Chol is the most potent ligand for the transcription factor LXR, which up-regulates the expression of cholesterol transporters ABCA1 and ABCG1 in the brain [27, 39, 40] and enhances cholesterol transporter proteins, such as apolipoproteins [41]. Thus, the 24S-OH Chol effect might induce cholesterol release from astrocytes [42], which then might directly support myelination or synaptogenesis. In contrast, Kölsch et al. [43] reported a pro-apoptotic effect of 24S-OH Chol on a neuroblastoma cell line and we cannot exclude that 24S-OH Chol may be involved in apoptosis of certain central nervous system cells.
The oxysterol 25-OH Chol, the second oxysterol used in our study, causes toxicity on different cell lines, e.g. in PC12 cells, thymocytes or in oligodendrocyte cell line (158N) [25, 26, 44], and is the most potent suppressor of sterol synthesis [27]. Our present study shows that 25-OH Chol directly and rapidly induced down-regulation of ChAT in nBM cholinergic neurons. The effect of 25-OH Chol could be mediated by the stimulation of inflammation or cellular reactive oxygen species accumulation, e.g. overproduction [22, 45, 46]. Alternatively, 25-OH Chol has been reported to regulate the expression of various inflammatory factors, such as cytokines, chemokines and adhesion molecules e.g. interleukin-8, interleukin-1β or macrophage inflammatory protein-1β [22, 47]. Moreover, 25-OH Chol can cause alterations of the cytoskeletal organization, since it causes progressive disruption of actin filaments or loss of the actin-binding protein vinculin [22]. And additionally, 25-OH Chol has been shown to stimulate apolipoprotein E4 synthesis in human astrocytoma cells [48], which is linked to the pathology of AD.
Taken together we have shown that cholesterol or 24S-OH Chol treatments delay the down-regulation of ChAT in nBM brain slices. In contrast 25-OH Chol rapidly reduced the expression of ChAT. A differential regulation of the cholesterol metabolism may play a role in the regulation of ChAT in nBM cholinergic neurons and possibly in AD.
Acknowledgments
We thank Ursula Kirzenberger-Winkler for her excellent technical help. This study was supported by the Austrian Science Fonds (P19122-B05).
References
- 1.Winkler J, Thal LJ, Gage FH, Fisher LJ. Cholinergic strategies for Alzheimer’s disease. J Mol Med. 1998;76:555–567. doi: 10.1007/s001090050250. [DOI] [PubMed] [Google Scholar]
- 2.Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- 3.Sassin I, Schultz C, Thal DR, Rüb U, Arai K, Braak E, Braak H. Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer’s disease. Neurology. 2001;57:1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- 5.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nature Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 6.Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44:1423–1430. doi: 10.1194/jlr.R300007-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Bohr IJ. Does cholesterol act as a protector of cholinergic projections in Alzheimer’s disease? Lipids Health Dis. 2005;4:4–13. doi: 10.1186/1476-511X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiss AB. Cholesterol and apolipoprotein E in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2005;20:91–96. doi: 10.1177/153331750502000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kivipelto M, Helkala E-L, Hänninen T, Laakso MP, Hallikainen M, Akhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 10.Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie C, Lund EG, Turley SD, Russell DW, Dietschy JM. Quantification of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bojanic DD, Tarr PT, Gale GD, Smith DJ, Bok D, Chen B, Nusinowitz S, Lövgren-Sandblom A, Björkhem I, Edwards PA. Differential expression and function of ABCG1 and ABCG4 during development and ageing. J Lipid Res. 2010;51:169–181. doi: 10.1194/jlr.M900250-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda T, Devi A. Regulation and degradation of HMGCo-A reductase. Appl Microbiol Biotechnol. 2004;66:143–152. doi: 10.1007/s00253-004-1720-5. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 16.Björkhem I, Diczfalusy U. Oxysterols. Friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol. 2002;22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 17.Maxfield FR, Wüstner D. Intracellular cholesterol transport. J Clin Invest. 2001;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Björkhem I, Meaney S, Diczfalusy U. Oxysterols in human circulation: which role do they have. Curr Opin Lipidol. 2002;13:247–253. doi: 10.1097/00041433-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Björkhem I. Do oxysterols control cholesterol homeostasis? J Clin Invest. 2002;110:725–730. doi: 10.1172/JCI16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soccio RE, Breslow JL. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Sottero B, Gamba P, Garguilo S, Leonarduzzi G, Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr Med Chem. 2009;16:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- 23.Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Björkhem I, Meaney S. Brain cholesterol: long secret behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 25.Christ M, Luu B, Mejia JE, Moosbrugger I, Bischoff P. Apoptosis induced by oxysterols in murine lymphoma cells and in normal thymocytes. Immunology. 1993;78:455–460. [PMC free article] [PubMed] [Google Scholar]
- 26.Chang JY, Phelan KD, Liu LZ. Neurotoxicity of 25-OH-cholesterol on NGF-differentiated PC12 cells. Neurochem Res. 1998;23:7–16. doi: 10.1023/a:1022437000893. [DOI] [PubMed] [Google Scholar]
- 27.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 28.Schatz DS, Kaufmann WA, Schuligoi R, Humpel C, Saria A. 3,4-Methylenedioxymetamphetamine (ecstasy) induces c-fos-like protein and mRNA in rat organotypic dorsal striatal slices. Synapse. 2000;36:75–83. doi: 10.1002/(SICI)1098-2396(200004)36:1<75::AID-SYN8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Weis C, Marksteiner J, Humpel C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience. 2001;102:129–138. doi: 10.1016/s0306-4522(00)00452-8. [DOI] [PubMed] [Google Scholar]
- 30.Humpel C, Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices. J Neural Transm. 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- 31.Duff K, Noble W, Gaynor K, Matsuoka Y. Organotypic slice cultures from transgenic mice as disease model systems. J Mol Neurosci. 2002;19:317–320. doi: 10.1385/JMN:19:3:317. [DOI] [PubMed] [Google Scholar]
- 32.Zimmer J, Kristensen BW, Jakobson B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19:7–21. doi: 10.1007/s007260070029. [DOI] [PubMed] [Google Scholar]
- 33.Gähwiler BH, Hefti F. Guidance of acetylcholinesterase-containing fibers by target tissue in co-cultured brain slices. Neuroscience. 1984;40:235–243. doi: 10.1016/0306-4522(84)90088-5. [DOI] [PubMed] [Google Scholar]
- 34.Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 35.Stoppini L, Buch PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 36.Zassler B, Weis C, Humpel C. Tumor necrosis factor-alpha triggers cell death of sensitized potassium chloride-stimulated cholinergic neurons. Mol Brain Res. 2003;113:78–85. doi: 10.1016/s0169-328x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 37.Ullrich C, Humpel C. The pro-apoptotic substance thapsigargin selectively stimulates re-growth of brain capillaries. Curr Neurovasc Res. 2009;6:171–180. doi: 10.2174/156720209788970063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristofiková Z, Kopecký V, Jr, Hofbauerová K, Hovorková P, Ripová D. Complex of amyloid beta peptides with 24-hydroxycholesterol and its effect on hemicholinium-3 sensitive carriers. Neurochem Res. 2008;33:412–421. doi: 10.1007/s11064-007-9443-5. [DOI] [PubMed] [Google Scholar]
- 39.Björkhem I. Are side-chain oxidized oxysterols regulators also in vivo? J Lipid Res. 2009;50:213–218. doi: 10.1194/jlr.R800025-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldán Á , Bojanic DD, Edwards PA. The ABCs of sterol transport. J Lipid Res. 2009;50:80–85. doi: 10.1194/jlr.R800044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol. 2008;59:31–55. [PubMed] [Google Scholar]
- 42.Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC, Jessup W, Hill AF, Garner B. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-β peptide generation. J Biol Chem. 2007;282:2851–2861. doi: 10.1074/jbc.M607831200. [DOI] [PubMed] [Google Scholar]
- 43.Kölsch H, Ludwig M, Lütjohann D, Rao ML. Neurotoxicity of 24-hydroxycholesterol, an important cholesterol elimination product of the brain, may be prevented by vitamin E and estradiol-17β. J Neural Transm. 2001;108:475–488. doi: 10.1007/s007020170068. [DOI] [PubMed] [Google Scholar]
- 44.Trousson A, Bernard S, Petit PX, Liere P, Pianos A, El Hadri K, Lobaccaro J-MA, Ghandour MS, Raymondjean M, Schumacher M, Massaad C. 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase A2 type IIA via LXR beta and PXR. J Neurochem. 2009;109:945–958. doi: 10.1111/j.1471-4159.2009.06009.x. [DOI] [PubMed] [Google Scholar]
- 45.Lemaire-Ewing S, Prunet C, Montange T, Vejux A, Berthier A, Bessède G, Corcos L, Gambert P, Néel D, Lizard G. Comparison of the cytotoxic, prooxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol Toxicol. 2005;21:97–114. doi: 10.1007/s10565-005-0141-2. [DOI] [PubMed] [Google Scholar]
- 46.Joffre C, Leclère L, Buteau B, Martine L, Cabaret S, Malvitte L, Acar N, Lizard G, Bron A, Creuzot-Garcher C, Bretillon L. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr Eye Res. 2007;32:271–280. doi: 10.1080/02713680601187951. [DOI] [PubMed] [Google Scholar]
- 47.Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gueguen Y, Bertrand P, Ferrari L, Batt AM, Siest G. Control of apolipoprotein E secretion by 25-hydroxycholesterol and proinflammatory cytokines in the human astrocytoma cell line CCF-STTG1. Cell Biol Toxicol. 2001;17:191–199. doi: 10.1023/a:1011996515328. [DOI] [PubMed] [Google Scholar]