Abstract
We recently identified the DPY19L2 gene as the main genetic cause of human globozoospermia (70%) and described that Dpy19l2 knockout (KO) mice faithfully reproduce the human phenotype of globozoospermia making it an excellent model to characterize the molecular physiopathology of globozoospermia. Recent case studies on non-genetically characterized men with globozoospermia showed that phospholipase C, zeta (PLCζ), the sperm factor thought to induce the Ca2+ oscillations at fertilization, was absent from their sperm, explaining the poor fertilization potential of these spermatozoa. Since 30% of globozoospermic men remain genetically uncharacterized, the absence of PLCζ in DPY19L2 globozoospermic men remains to be formally established. Moreover, the precise localization of PLCζ and the reasons underlying its loss during spermatogenesis in globozoospermic patients are still not understood. Herein, we show that PLCζ is absent, or its presence highly reduced, in human and mouse sperm with DPY19L2-associated globozoospermia. As a consequence, fertilization with sperm from Dpy19l2 KO mice failed to initiate Ca2+ oscillations and injected oocytes remained arrested at the metaphase II stage, although a few human oocytes injected with DPY19L2-defective sperm showed formation of 2-pronuclei embryos. We report for the first time the subcellular localization of PLCζ in control human sperm, which is along the inner acrosomal membrane and in the perinuclear theca, in the area corresponding to the equatorial region. Because these cellular components are absent in globozoospermic sperm, the loss of PLCζ in globozoospermic sperm is thus consistent and reinforces the role of PLCζ as an oocyte activation factor necessary for oocyte activation. In our companion article, we showed that chromatin compaction during spermiogenesis in Dpy19l2 KO mouse is defective and leads to sperm DNA damage. Together, these defects explain the poor fertilization potential of DPY19L2-globozoospermic sperm and the compromised developmental potential of embryos obtained using sperm from patients with a deletion of the DPY19L2 gene.
Keywords: male infertility, globozoospermia, DPY19L2, phospholipase C zeta, acrosome, ICSI
Introduction
Approximately 15% of couples seek assistance from reproductive clinics for subfertility or infertility. The causes of infertility are numerous (societal, environmental, chromosomal or genetic) and this heterogeneity has slowed down the comprehension of the etiology of infertility. Identification of the cause of a patient's infertility is always long and costly and is thus often neglected and replaced by a palliative treatment based on assisted reproductive technologies (ART), which do not repair or improve the deficient physiological processes but merely bypass the defects. Nevertheless, despite progresses in ART, including the development of ICSI, genetic causes of infertility associated with defects such as macrozoospermia, globozoospermia or azoospermia remain difficult to overcome and many couples fail to achieve a pregnancy. Therefore, a better understanding of the molecular defects underlying these diseases will help to elucidate unknown mechanism(s) of spermatogenesis, while offering the perspective of more rational therapeutic approaches.
In the last few years, we have focused our attention on one phenotype of male infertility, globozoospermia (OMIM #613958, http://omim.org/entry/613958), which is characterized by the presence in the ejaculate of a large majority of round spermatozoa devoid of acrosome (Dam et al., 2007a). Some patients present a complete phenotype, type I, with 100% abnormal spermatozoa, whereas others have a mosaic of normal and globozoospermic sperm. Despite the different phenotypes, globozoospermia seems to have a genetic origin, since familial cases have been described. A familial mutation of SPATA16 has been associated with globozoospermia (Dam et al., 2007b), although a single mutation in SPATA16 thus far was subsequently identified among a large cohort of patients with this phenotype (Karaca et al., 2014), suggesting that globozoospermia is genetically heterogeneous. Recently, research on a cohort of Tunisian patients with type I globozoospermia revealed that 15 of the 20 analyzed patients displayed complete deletion of the DPY19L2 gene (Harbuz et al., 2011); this finding was confirmed by two other studies including one in a population of Chinese subjects (Koscinski et al., 2011; Zhu et al., 2013). Subsequently, point mutations in the DPY19L2 gene were found in some of the patients that did not show DPY19L2 gene deletion (Coutton et al., 2012; Elinati et al., 2012; Zhu et al., 2013). Together, these results implicate the DPY19L2 gene as the major cause of type I globozoospermia.
Studies into the mechanism of the deletion of the DPY19L2 gene showed that it was produced by non-allelic homologous recombination (NAHR) between two repeated sequences located on each side of the gene. Sequence analysis demonstrated that at the center of the breakpoint, there was a 13-nucleotide recognition motif for the PRDM9 protein, confirming the link between the presence of this motif and the occurrence of NAHRs (Coutton et al., 2013). The frequency of the heterozygous deletion in the general population is estimated to be 1 in 220 individuals predicting a prevalence of globozoospermia of around ∼1/200,000 males, a value that is compatible with the rarity of the phenotype. Although these findings established the association of the DPY19L2 gene with globozoospermia, the function of the gene product was unknown. Therefore, more fundamental studies about its possible function were carried out using a genetic knockout (KO) mouse model with a deleted Dpy19l2 gene. In those studies, we found that DPY19L2 is located in the inner nuclear membrane and is necessary for anchoring the acrosome to the nuclear envelope, because in its absence, the inner nuclear membrane becomes separated from the outer nuclear membrane, leading to the detachment of the acrosome associated with the outer nuclear membrane (Pierre et al., 2012).
Before the development of ART, and more particularly ICSI, the treatment of globozoospermia was not possible and affected men remained sterile. In 1994, Lundin et al. using ICSI reported the first successful pregnancy and offspring from a man with globozoospermia (Lundin et al., 1994); other teams later reported similar outcomes. Despite this initial success, other studies using ICSI reported an absence of fertilization when using globozoospermic sperm (Liu et al., 1995; Battaglia et al., 1997). The low efficiency of ICSI with globozoospermic sperm, characterized by low rates of egg activation and fertilization, raised the possibility that these sperm were deficient in the factor responsible for egg activation. After many years of study, such a factor was proposed to be a testis-specific phospholipase C, zeta (PLCζ), because it is appropriately expressed in sperm and injection of the corresponding complementary RNAs (cRNA) into oocytes induced Ca2+ oscillations (Saunders et al., 2002). The role of PLCζ was further confirmed in numerous publications (Amdani et al., 2013). PLCζ is located in the head of mammalian sperm in areas surrounding the equatorial region, which is where the fusion between gametes is proposed to begin (Yoon and Fissore, 2007; Grasa et al., 2008). Consistent with a possible role in globozoospermia, recent studies on men presenting this condition showed that protein expression of PLCζ was greatly reduced or absent from these patients (Yoon et al., 2008; Heytens et al., 2009). Further, later studies using motile sperm organelle morphology evaluation (MSOME) showed that few globozoospermic sperm can retain fragments of the acrosome, known as acrosomal buds, and in these cases some PLCζ is detected on the sperm head and/or midpiece, which suggests heterogeneity in expression of this protein in globozoospermic patients (Taylor et al., 2010; Kashir et al., 2012). Given that the DPY19L2 gene was only described in 2011, the association between globozoospermia, DPY19L2-deletion and PLCζ expression remains to be demonstrated.
Contrary to humans where only two genes have been associated with globozoospermia, the analyses of KO mice allowed identifying nine genes whose absence leads to defective acrosome biogenesis and the globozoospermia-like sperm phenotype (Pierre et al., 2012). A better characterization of the molecular underpinnings responsible for the infertility of globozoospermic patients could be carried out in mouse models (Heytens et al., 2010). Toward that end, one of these models, the Dpy19l2 KO mouse, displays a reproductive phenotype remarkably similar to the human disease, as males are completely infertile and 100% of their sperm are globozoospermic (Pierre et al., 2012). Therefore, this model represents an opportunity to assess the role of PLCζ in DPY19L2-dependent globozoospermia.
Using both human and mouse sperm from individuals/animals with DPY19L2-dependent globozoospermia, we show that PLCζ is absent/reduced in sperm of both species and this absence seems to underlie the characteristic defect in oocyte activation associated with fertilization by ICSI when using these sperm. Using optical and electronic microscopy, we identified for the first time with a high degree of precision the subcellular localization of PLCζ in human sperm in the perinuclear theca. This distribution is consistent with its activity as an oocyte activation factor and with its loss during spermiogenesis in DPY19L2-defective sperm, which display disruption of the nuclear envelope, resulting in detachment of the acrosome and loss of the acrosome and surrounding material including the perinuclear region and PLCζ.
Materials and Methods
Biological samples
Human sperm were obtained from patients consulting at the fertility department of Grenoble and Marseille Hospitals (France) or Clinique des Jasmins (Tunis, Tunisia), following approval by the ethical committee and informed consent from the patients. All patients gave an informed consent for the conservation of the remaining sperm in the Germetheque biobank and their use in studies on human fertility in accordance with the Helsinki Declaration of 1975 on human experimentation. The Germetheque Scientific Committee approved the present study design. Globozoospermic patients underwent genetic analysis as previously described (Harbuz et al., 2011) and homozygously DPY19L2-deleted patients selected. Some of these patients were included in Harbuz et al. (2011). This previous study focused on the genotypes of a cohort of globozoospermic patients and included none of the results presented here, including those involving ICSI. All animal procedures were performed according to the French and to the IACUC UMASS guidelines on the use of living animals in scientific investigations with the approval of the respective local ethical review committees (Grenoble-Institut des Neurosciences—ethical committee, agreement number 004). Dpy19l2 KO mice were obtained from Mutant Mouse Regional Resource Center (MMRRC), University of California, Davis, CA, USA. Human sperm were collected by masturbation and washed twice in phosphate-buffered saline (PBS) after semen liquefaction.
Collection of gametes for ICSI
Sperm from caudae epididymides of different mouse strains (Dpy19l2 KO and wild-type (WT) B6D2F1) were allowed to swim for 10 min at 37°C in 1 ml of Nuclear Isolation Medium (NIM) containing (in mM) KCl 125, NaCl 2.6, Na2HPO4 7.8, KH2PO4 1.4 and EDTA 3 (pH 7.0). Sperm were then washed twice by centrifugation at 500 g with NIM, then resuspended in 100 µl NIM + 12% poly(vinylpyrrolidone) (PVP), average Mr≈360,000 (Sigma-Aldrich, France) medium. The sperm head was separated from the tail by the application of several piezo pulses (PiezoXpert®, Eppendorf) or by sonication (2 × 15 s).
Oocyte preparation
B6D2F1 female mice, 7–11 weeks old, were superovulated by IP injection of 7.5 IU pregnant mare's serum gonadotrophin (PMSG; Intervet) followed by 7.5 IU of hCG (Intervet) 48 h later. Oocytes were collected from oviducts about 14 h after hCG injection. Cumulus cells were removed with 0.1% bovine testicular hyaluronidase (300 USP U/mg; ICN Biochemicals, Costa Mesa, CA, USA) in M2 medium (Sigma-Aldrich, France) for 5–10 min. Oocytes were rinsed thoroughly and kept in M2 medium at 15°C for at least 15 min until required for ICSI.
Media
M2 medium (Sigma) and CZB.HEPES (CZB.H, in mM) (HEPES 20, NaCl 81.6, KCl 4.8, MgSO4 1.2, CaCl2 1.7, KH2PO4 1.2, EDTA.Na2 0.1, Na-lactate 31, NaHCO3 5, Na-pyruvate 0.3, polyvinyl alcohol 0.1 mg/ml, phenol red 10 mg/ml (0.5% (w/v) in DPBS (Dulbecco's phosphate-buffered saline)), pH 7.4) was used for gamete handling and ICSI in air. Potassium simplex optimized medium (KSOM) (100×, Life technologies) supplemented with 1% essential amino acids (EAA) (100×, Life technologies) was used for embryo culture (KSOM/EAA).
ICSI procedures
ICSI was performed according to the method described by Yoshida and Perry (2007). For microinjection, sperm were stored in 50 µl of NIM, 12% PVP medium and moved directly to the injection chamber. Sperm were introduced into the ooplasm by using micromanipulators (Micromanipulator InjectMan®, Eppendorf) mounted on an inverted Nikon TMD microscope. The sperm suspension was replaced every 30 min during the ICSI experiment. Oocytes that survived the ICSI procedure were incubated in KSOM/EAA medium at 37°C under an atmosphere of 5% CO2. Pronuclei (PN) formation was checked at 6 h after ICSI, and outcomes were scored up to the blastocyst stage.
[Ca2+]i monitoring
[Ca2+]i monitoring was carried out as described by our laboratory (Yoon et al., 2008). Briefly, mouse eggs were loaded with fura-2-AM (Molecular Probes; Invitrogen) prior to performing ICSI. Eggs were then transferred to a monitoring dish (Mat-Tek Corp., Ashland, MA, USA) containing 50 μl drops of TL-HEPES medium under mineral oil. Eggs were monitored simultaneously under a 20× objective on an inverted microscope (Nikon) outfitted for fluorescence measurements and with a temperature-controlled stage. Excitation wavelengths of 340 and 380 nm were alternated using a filter wheel (Ludl Electronic Products Ltd., Hawthorne, NY, USA). Fluorescence ratios were taken every 20 s. After passing through a 510-nm barrier filter, the emitted light was collected by a Cool-SNAPES digital camera (Roper Scientific, Tucson, AZ). SimplePCI imaging software was used to run all the hardware and capture images (Hamamatsu, Sewickley, PA, USA). [Ca2+]i values are reported as the ratio of 340:380 nm fluorescence in the whole egg.
Western blotting
Sperm from caudae epididymides of different mouse strains (Dpy19l2 KO and WT B6D2F1) were allowed to swim for 10 min at 37°C in 1 ml of NIM. Sperm were then washed in 1 ml of PBS, resuspended in Laemmli sample buffer without β-mercaptoethanol, and boiled for 5 min. After centrifugation, 5% β-mercaptoethanol was added to the supernatants, and the mixture was boiled again for 5 min. Protein extracts equivalent to 1–2 × 106 sperm were loaded per lane into a 4–20% sodium dodecyl sulfate polyacrylamide gel, and resolved proteins were transferred onto polyvinylidene difluoride membranes (PVDF, Millipore) using a Mini Trans-Blot Cell (Bio-Rad). The membranes were blocked in 6% nonfat dry milk in PBS–0.1% Tween and incubated overnight at 4°C with anti-PLCζ antibodies (1 : 500); this was followed by 1 h of incubation with a horseradish peroxidase labeled secondary antibodies. Immunoreactivity was detected using chemiluminescence detection kit reagents and a Chimidoc™ Station (Bio-Rad). Western blotting procedures were repeated at least three times per sample.
Immunofluorescence
Sperm were fixed in PBS/4% paraformaldehyde for 1 min at room temperature. After washing in 1 ml PBS, the sperm suspension was spotted onto 0.1% poly l-lysine precoated slides (Thermo Scientific). After attachment, sperm were permeabilized with 0.1% (v/v) Triton X-100–DPBS (Triton X-100; Sigma-Aldrich) for 5 min at room temperature. Slides were then blocked in 5% corresponding normal serum–DPBS (normal goat or donkey serum; GIBCO, Invitrogen) and incubated overnight at 4°C with primary antibodies. Washes were performed with 0.1% (v/v) Tween 20–DPBS, followed by 1 h incubation at room temperature with Alexa Fluor 555-labeled goat anti-rabbit or Dylight 488-labeled goat anti-rabbit (1:400) secondary antibodies. Samples were counterstained with 5 μg/ml Hoechst 33342 and mounted with DAKO mounting media (Life Technology).
Fluorescence images were captured with confocal microscopy (Zeiss LSM 710) outfitted with a 63× oil immersion objective for mice sperm and 100× oil immersion objective for human sperm and analyzed with ZEN lite software (Zeiss). Whole images were reconstructed and projected from Z-stack images using ZEN software.
Immunogold labeling
Purified spermatids were fixed for 2 h in 0.1 M phosphate buffer, pH 7.3, containing 2% paraformaldehyde and 0.2% glutaraldehyde. Cells were then washed twice in 0.1 M phosphate buffer and once in phosphate buffer 0.1 M, pH 7.2, containing glycine 50 mM before being centrifuged and embedded in 10% gelatin diluted in the same buffer at 37°C. After solidification on ice, this cell pellet was incubated for 4 h in 2.3 M sucrose and frozen in liquid nitrogen. Cryosections of these samples were made at −120°C using an ultra-cry-microtome (Lexica-Reichert) and retrieved with a 1:1 solution of 2.3 M sucrose and 2% methylcellulose according to Liou et al. (1996). For labeling, cryosections were first incubated with primary human PLCζ (hPLCζ) antibody and revealed with protein A-gold conjugated (CMC, Utrecht, the Netherlands).
Primary antibodies
Anti-human acrosin antibodies were obtained from Sigma-Aldrich; two anti-PLCζ antibodies were used: for mouse sperm, antibody was raised against a 19-mer peptide sequence (GYRRVPLFSKSGANLEPSS) of the mouse PLCζ (anti-mPLCζ) (Kurokawa et al., 2005); the anti-mPLCζ was from whole serum, therefore concentration is not known. For human sperm, antibody was raised against a 15-mer-peptide sequence (305KETHERKGSDKRGDN319) of the human PLCζ1 protein (anti-hPLCζ) (Yoon et al., 2008). The anti-hPLCζ was affinity purified and stored at a concentration of 1 μg/μl. The dilution used was of 1:1000 for western blot and 1:100 for immunofluorescence.
Statistical analyses
Statistical analyses were performed with SigmaPlot (Systat Software Inc.). Student's T-test and paired t-test were used to compare the fertility rate. If necessary, data are presented as mean ± SD. Statistical tests with a two-tailed P value ≤0.05 were considered as statistically significant.
Results
DPY19L2-deficient sperm display poor oocyte activation capacity
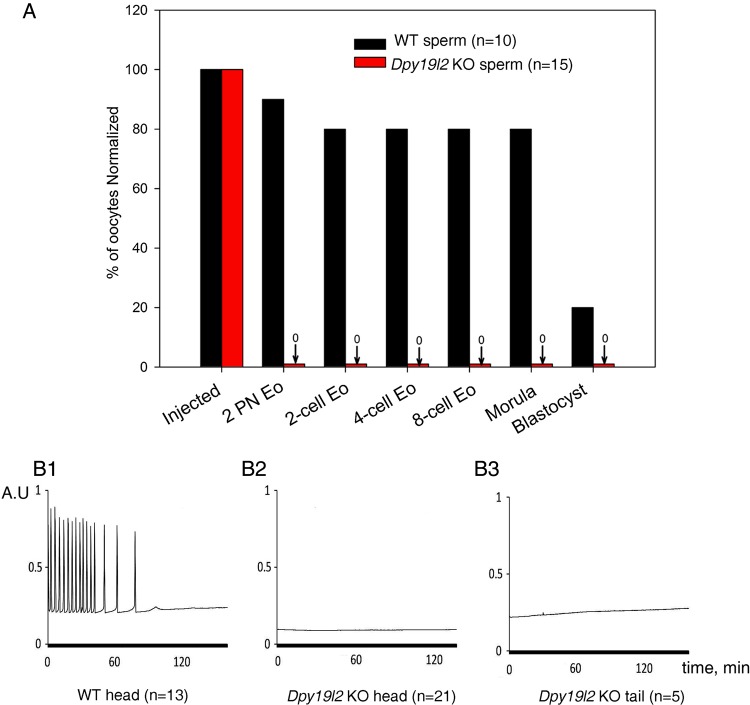
To investigate the ability of Dpy19l2 KO mice sperm to induce oocyte activation, we injected control and Dpy19l2 KO sperm heads and tails into WT oocytes, and embryo development was followed up to the blastocyst stage. Injection of Dpy19l2 KO sperm failed to induce activation, as evaluated by the presence of formation of 2-PN or division to the 2-cell stage, demonstrating failure to induce oocyte activation. In contrast, ∼80% of the oocytes injected with WT sperm underwent activation and reached the morula stage (Fig. 1A). The absence of oocyte activation following injection of Dpy19l2 KO sperm was confirmed by recording Ca2+ oscillations after ICSI. As expected, injection of WT sperm induced Ca2+ oscillations (13/13), whereas injection of Dpy19l2 KO sperm heads (0/21) or flagella (0/5) failed to induce Ca2+ responses (Fig. 1B1–3).
Figure 1.
Absence of oocyte activation in Dpy19l2 knockout (KO) globozoospermic mouse sperm was correlated with defective calcium signaling. (A) Absence of 2-pronuclei and subsequent absence of development demonstrate that oocyte activation was defective in ICSI experiments performed with Dpy19l2 KO nuclei (red columns), contrary to those performed with wild-type (WT) sperm nuclei (black columns). Sperm were from three different KO and two WT males. There was a significant difference between WT and KO sperm for all stages (P < 0.001). (B1–B3) Contrary to ICSIs performed with WT sperm (13/13, D1), those performed with globozoospermic sperm head (0/21, D2) or flagellum (0/5, D3) do not elicit calcium signaling. Sperm were from three different KO and two WT males.
The ICSI outcomes of globozoospermic patients are variable, with some medical teams reporting high pregnancy rates and others reporting low fertilization rates, early abortion and finally an absence of delivery. Nevertheless, in the absence of genetic characterization of the cause of globozoospermia in those reports, it is difficult to draw conclusion about the oocyte activation capacity of globozoospermic sperm. In this report, we followed nine couples corresponding to 13 stimulation cycles with males presenting globozoospermia due to a full deletion of the DPY19L2 gene. Unlike results in the mouse, a low degree of fertilization was observed in these patients, as in 7 of 13 cycles, 2-PN embryos were obtained, although the rate of oocyte activation remained very low, with a mean rate of 7.25% ± 9.76 2-PN formation (Table I).
Table I.
Outcomes following hormonal stimulation and ICSI in nine couples where the men presented with type I globozoospermia due to the full deletion of the DPY19L2 gene.
| Patient number | M1 | G1-1 | G1-2 | G1-3 | T1.1 | T1.2 | T3 | T4 | T5.1 | T5.2 | T7 | T8 | T9 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, male (years) | 30 | 37 | 38 | 38 | 31 | 31 | 50 | 40 | 36 | 38 | 30 | 42 | 38 | ||
| Age, female (years) | 28 | 29 | 30 | 30 | 27 | 36 | 37 | 36 | 24 | 26 | 26 | 36 | 28 | 30.23 | 4.28 |
| Nber MII oocytes | 14 | 10 | 4 | 5 | 10 | 6 | 8 | 4 | 29 | 19 | 10 | 11 | 9 | ||
| Nber 2-PN embryos | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 | 1 | 1 | 1 | ||
| % of 2-PN embryos/MII* | 14.3 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 34.5 | 5.3 | 10 | 9 | 11.1 | 7.25 | 9.76 |
MII, metaphase II oocytes retrieved; PN, pronuclei.
*Oocyte activation rate upon ICSI in 13 different stimulation cycles.
PLCζ is absent in Dpy19l2 KO mouse sperm
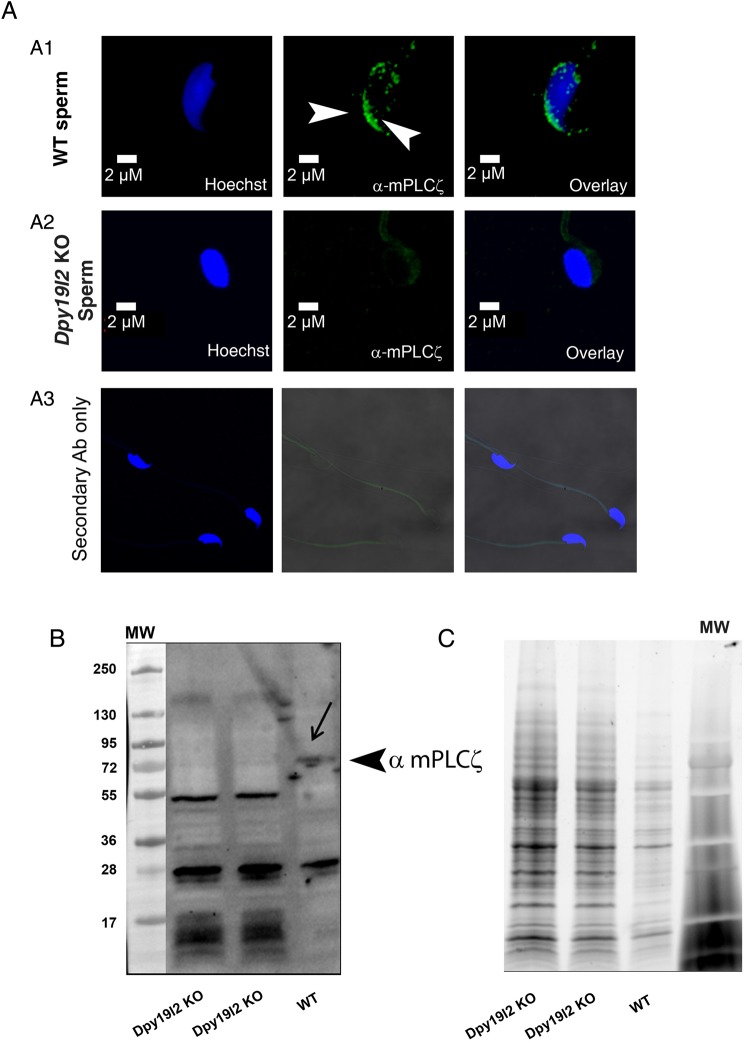
The absence of oocyte activation in ICSI experiments performed with Dpy19l2 KO sperm and the low rate of fertilization with human sperm suggested that these sperm lacked the sperm factor thought to be associated with oocyte activation, i.e. PLCζ. To confirm this possibility, we used immunohistochemistry and western blotting to detect expression of PLCζ in both WT and Dpy19l2 KO sperm. As previously shown, PLCζ is located in the post-acrosomal area of the mouse sperm heads (arrowheads, Fig. 2A1), although additional, less specific staining has also been described in the acrosome region (Yoon and Fissore, 2007; Grasa et al., 2008; Young et al., 2009). In contrast, Dpy19l2 KO sperm failed to show any immunoreactivity toward PLCζ, strongly suggesting that these sperm are devoid of PLCζ (Fig. 2A2). The absence of PLCζ in Dpy19l2 KO sperm was confirmed by western blotting, whereas the anti-mPLCζ antibody recognized a band just above 72 kDa in WT sperm, this band was absent in Dpy19l2 KO sperm lysates (Fig. 2B, representative WB, n = 4); equal loading of WT and Dpy19l2 sperm extracts was demonstrated using TGX stain free™ precast gels (Fig. 2C).
Figure 2.
PLCζ is absent in Dpy19l2 knockout (KO) globozoospermic mouse sperm. (A) Sperm were stained with Hoechst (blue) and antibodies against mouse phospholipase C ζ (mPLCζ, green staining). PLCζ was located in the acrosome crescent and in the post-acrosomal area in wild-type (WT) sperm (A1). No PLCζ staining was observed on globozoospermic sperm (A2). No staining was observed with secondary antibody only (A3). (B) In WT sperm extracts, antibodies targeting mPLCζ immunodecorated a band of ∼74 kDa, which is the expected molecular weight of mouse PLCζ. Any band around 74 kDa and corresponding to PLCζ was detected in sperm extracts from Dpy19l2 KO globozoospermic sperm. (C) Similar loading of the different sperm extracts was controlled with TGX stain free™ precast gels.
Reduced and abnormal localization of PLCζ in DPY19L2-deficient human sperm
To examine the expression and localization of PLCζ in DPY19L2-deleted human sperm, we first performed immunofluorescence in control sperm using an anti-human PLCζ (anti-hPLCζ) antibody that we have previously characterized (Yoon et al., 2008; Lee et al., 2014). In agreement with those results, we found that PLCζ is mostly located around the equatorial/post-acrosomal area of human sperm, where it shows a strong and compact staining in the form of a band, the acrosome staining being considerably weaker (Fig. 3A and Supplementary Fig. S1AC). To more accurately determine the location of PLCζ, images obtained with the confocal microscope on the z-axis were examined. In the first section where a signal was detected, here named 0 µm (Supplementary Fig. S1D), PLCζ staining appears as a post-acrosomal band, whereas in more central sections the shape of the staining appears as peripheral dots (Supplementary Fig. S1E and S1F). Finally, as expected, moving the focus to higher confocal sections allowed observing a staining similar to that observed in the 0 µm section (Supplementary Fig. S1G and S1H). These results strongly suggest that PLCζ is not located inside the nucleus, but rather in the vicinity of the nuclear envelope. Lastly, we also performed western blotting studies, which confirmed the specificity of the antibody, as it prominently recognized in three out of four human sperm extracts a band of ∼72 kDa, which is the expected molecular weight of hPLCζ (Supplementary Fig. S1I).
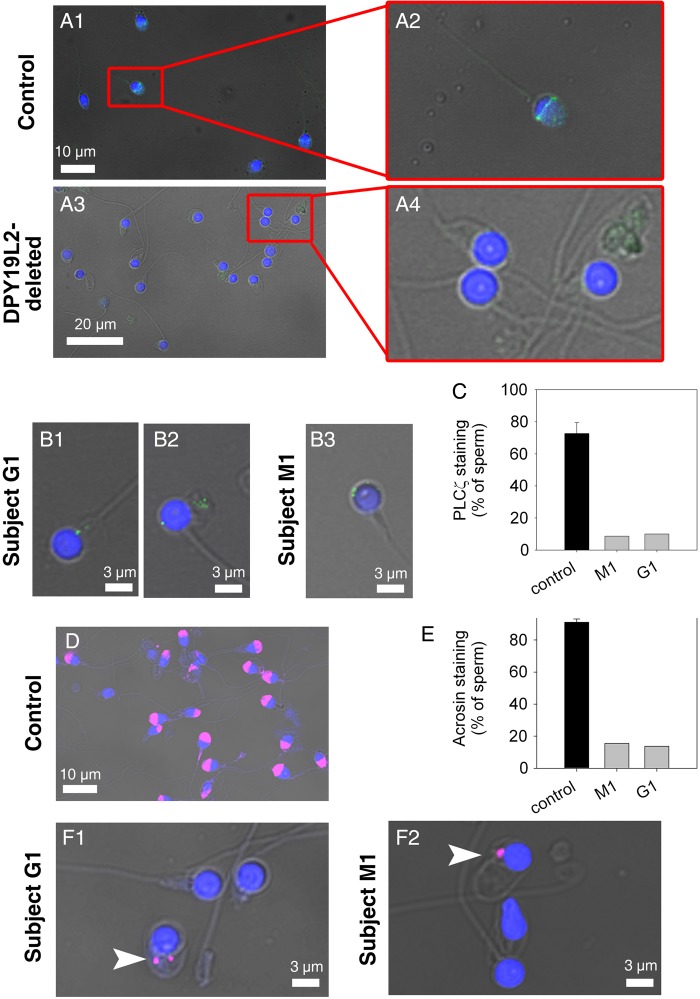
Figure 3.
PLCζ is absent in most DPY19L2-dependent globozoospermic human sperm. (A1) Sperm from a man with normal fertility. Sperm were stained with anti-hPLCζ antibodies (green staining) and counterstained with Hoechst (blue). The equatorial and the acrosomal areas are marked. (A2) Enlargement of the red square in (A1). Total image length 14 µm. (A3) Sperm from DPY19L2-deleted patients. Sperm were stained with anti-hPLCζ antibodies (green staining) and counterstained with Hoechst (blue). No staining was detected in most sperm. (A4) Enlargement of the red square in (A3). Total image length 22 µm. (B1–B3) Dot shape PLCζ staining in globozoospermic sperm in two different subjects G1 and M1. (C) Only a small fraction of DPY19L2-dependent globozoospermic sperm present a punctiform staining in M1 and G1 subjects, in contrast to sperm from men presenting with normal fertility (n = 5). There was a significant difference between sperm from control and DPY19L2-deleted men (P < 0.001). (D) Sperm from a man with normal fertility were stained with anti-acrosin antibodies (magenta staining) and counterstained with Hoechst (blue) in order to show the acrosomal vesicle. (E) Contrary to control sperm from three different men with normal fertility, only around 15% of DPY19L2-dependent globozoospermic sperm from subjects M1 and G1 were stained with anti-acrosin antibody, identifying acrosomal buds (F1–F2). There was a significant difference between sperm from control and DPY19L2-deleted men (P < 0.001).
The presence of PLCζ in DPY19L2-deleted patients was studied in two subjects with globozoospermia and part of the cohort presented in Table I. We observed that in the large majority of sperm, the anti-PLCζ antibody failed to display any reactivity (Fig. 3A3 and A4), which is consistent with the lack of oocyte activation following injection of these sperm. Remarkably, staining with the anti-PLCζ antibody showed a punctiform staining in ∼10% of the sperm (Fig. 3B1–B3 and 3C; more than 150 sperm were analyzed per subject) and although minor, this staining is likely to represent specific staining of PLCζ, because when the primary antibody was omitted, it was no longer observed (Supplementary Fig. S2). It is worth noting that using an anti-acrosin antibody, we observed similar punctiform staining in ∼15% of the sperm (Fig. 3E, F1 and F2; more than 150 sperm were analyzed per subject), which suggested the presence of acrosomal buds in a minority of DPY19L2-deleted sperm. While co-staining with hPLCζ and acrosin antibodies was not possible, as both antibodies were raised in rabbits, the results suggest that in those globozoospermic sperm that retain remnants of acrosomal content, they might also contain abnormally distributed PLCζ. Taken together, immunofluorescence and western blotting studies using mouse and human sperm clearly demonstrate that PLCζ is absent, or its presence highly reduced, in Dpy19l2-dependent globozoospermic sperm.
PLCζ localizes to the inner acrosomal membrane/perinuclear theca in the post-acrosomal region of human sperm
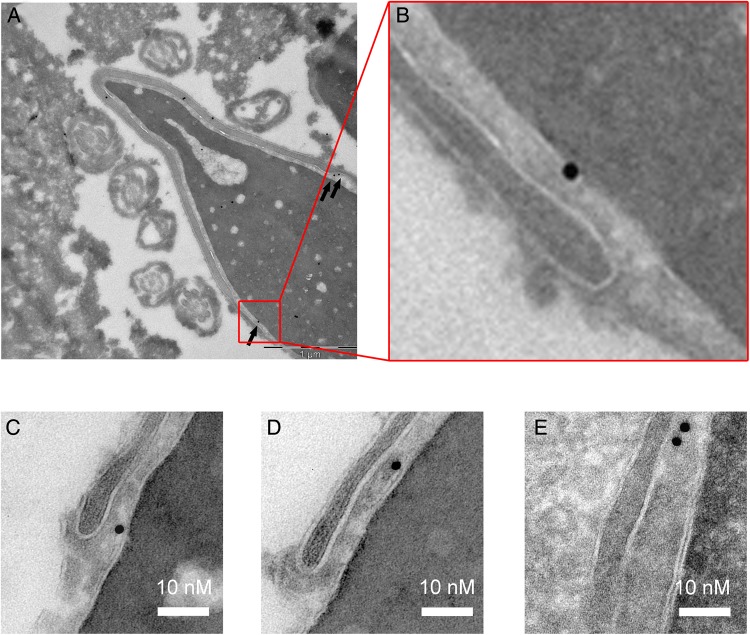
To determine the localization of PLCζ at the ultrastructural level, we used electronic microscopy (EM) and the anti-hPLCζ antibody subsequently revealed with protein A-gold conjugated (Fig. 4A). A total of 195 gold particles were studied and most of the gold particles were located around (104/195) or on (91/195) the inner acrosomal membrane (IAM) (Fig. 4B). Given that the observed position of the gold particles does not allow us to directly determine the precise localization of the antigenic site, i.e. PLCζ, as due to the lengths of the two IgGs used in the IgG-gold complexes (15 nm × 2 (IgG × 2) = 30 nm), the gold particles actually fall in a circle of ∼60 nm diameter around the antigenic site, we estimated localization of PLCζ by measuring the shortest distance from the edge of the gold particle to the line corresponding to the IAM; the distances were counted positively when heading toward the nucleus and negatively when heading toward the acrosome. Since the distribution of the gold particles in the circle is random, the median of the sample should be around 0 for a location on the IAM. A box plot corresponding to the numerical values of the distances of the 104 gold particles located around the IAM is shown in Fig. 4C and reveals a median of −6.7 nm with the 10th/90th percentile values indicating that 80% of the gold particles fall inside the expected circle. This result strongly suggests that in mature sperm PLCζ is located on the perinuclear theca side bound to the IAM. In addition, numerous gold particles and gold clusters were found at the end of the base of the acrosomal vesicle (Fig. 5A–E), which is consistent with immunofluorescence images in Fig. 3 and Supplementary Fig. S1, showing that PLCζ staining is mostly located in the post-acrosomal area. It is worth noting that a few gold particles were found inside the nucleus (Figs. 4A and 5A), although the specificity of this location was considered weak, as similar density of gold particles was found outside of the cell (Fig. 5A). Finally, in control experiments which were incubated only with secondary gold conjugates, few gold particles were observed and at a very low density (Supplementary Fig. S3).
Figure 4.
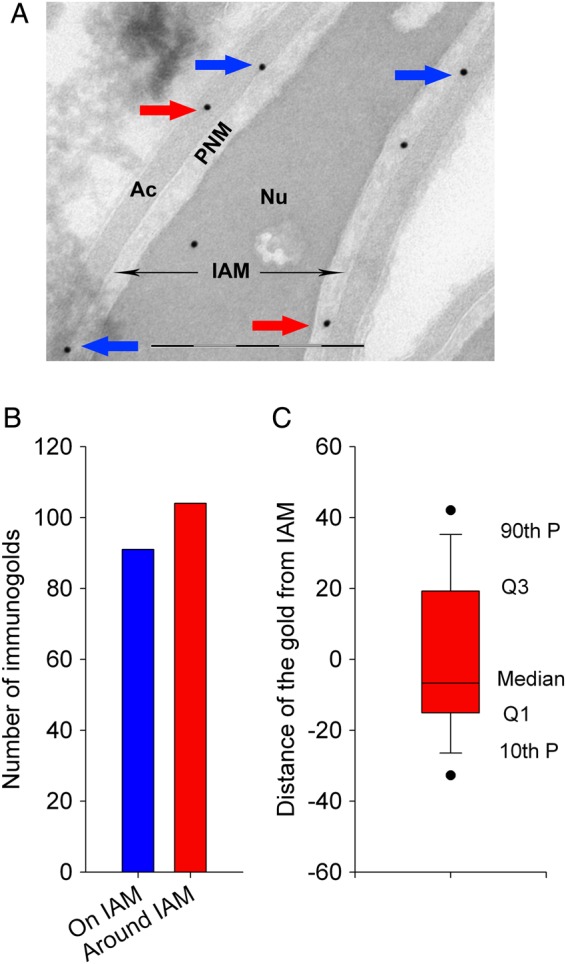
Immunogold localization of PLCζ in ejaculated human sperm. (A) Gold particles targeting PLCζ antigenic sites were observed along the IAM (blue arrows). About half of the gold particles were also observed at short distances associated with the acrosomal content or the perinuclear theca (red arrows). Scale bar 500 nm. (B) Quantification of the localization of 104 gold particles. Blue column corresponds to gold particles located along the IAM and red column to gold particles located in the acrosome or in the nuclear theca. (C) Box plot showing the repartition of the numerical values of the distances measured from particles located either in the acrosome (counted as positive) or in the nuclear theca (counted as negative). Nu, nucleus; Ac, acrosome; IAM, inner acrosomal membrane; PNM, perinuclear theca.
Figure 5.
Presence of numerous gold particles at the base of the acrosome vesicle is consistent with immunohistochemistry results showing post-acrosomal localization of PLCζ in human sperm. (A) Gold particles targeting PLCζ antigenic sites were often observed at the base of the acrosome (black arrows) in a control human sperm. (B) Enlargement of the red box drawn in (A). (C–E) Examples of the presence of gold particles in the equatorial segment in different human sperm.
Discussion
Globozoospermia is a rare, well-known and complex disease that causes male infertility and is associated with the loss of organelles and proteins during spermatogenesis. Nevertheless, the pathogenesis of the disease is not well-known and this was due in part to the failure to find the molecular defect(s) responsible for it. Indeed, several genes have been shown to be associated with globozoospermia-like infertility in the mouse, including Csnk2a2 (casein kinase IIa), Gopc (Golgi-associated PDZ and coiled-coil motif containing protein), Hrb (HIV-1 Rev binding protein), Pick1 (protein interacting with C kinase 1), Hsp90b1 (gp96; glucose-related protein 94 [Grp94]), Vps54 (vacuolar protein sorting-associated protein 54) and sperm acrosome associated 1 (Spaca1) (Xu et al., 1999; Kang-Decker et al., 2001; Yao et al., 2002; Xiao et al., 2009; Audouard and Christians, 2011; Paiardi et al., 2011; Fujihara et al., 2012). Importantly, with the exception of a mutation in PICK1 found in a Chinese family (Liu et al., 2010), the aforementioned genes do appear mutated in men with globozoospermia. In contrast, our recent studies identified the Dpy19l2 gene as the main genetic cause of human globozoospermia (Harbuz et al., 2011; Coutton et al., 2012, 2013) and showed that the loss of this gene phenocopies globozoospermia in mice (Pierre et al., 2012) demonstrating that defects associated with the DPY19L2 gene are the main cause of the disease in these species. Herein, we were able to compare human and mouse DPY19L2 globozoospermia. We observed that mouse spermatozoa display complete absence of PLCζ whereas ∼10% of human sperm showed traces of PLCζ associated with small remnants of the acrosome. These observations are concordant with the findings of complete fertilization failure following ICSI in mice whereas fertilization was occasionally achieved in men. Furthermore, we identified the precise localization of PLCζ in human sperm, which is attached to the IAM in the sperm head, and which helps to explain the mechanism leading to its disappearance during spermatogenesis of globozoospermic men.
The location of PLCζ is consistent with its role as the sperm factor of mammalian sperm
Numerous studies have shown that PLCζ is the likeliest candidate to be the sperm factor, and the strongest supporting data are provided by the ability of the recombinant protein or PLCζ cRNA injection to initiate sperm-like oscillations (reviewed in Amdani et al. (2013). In addition, immunoprecipitation studies have shown that removal of PLCζ from sperm extracts depletes the ability of these extracts to initiate Ca2+ oscillations or hydrolyze phosphatidylinositol 4,5-bisphosphate (PiP2) (Kurokawa et al., 2007). Herein, we present evidence that the location of PLCζ in mouse and human sperm is consistent with its role as the sperm factor. First, using EM, we showed that in human sperm the gold particles that evidence the recognition of PLCζ by our specific antibody are located in the perinuclear theca facing the acrosome and are likely associated with the IAM and the perinuclear theca facing the acrosome. This location is consistent with previous results obtained by Fujimoto et al showing by mass spectrometry that all oocyte-activating fractions of perinuclear theca contain PLCζ (Fujimoto et al., 2004). Further, because both IAM and perinuclear theca remain after the acrosome reaction, the PLCζ associated with it would be available to be delivered after sperm–oocyte fusion. In addition, we found greater reactivity in the equatorial zone of the sperm, which is the area that first undergoes fusion with the oocyte, consistent with a role in triggering Ca2+ release following fusion of the gametes. Interestingly the equatorial zone remains relatively unaltered during the acrosome reaction in humans (Zanetti and Mayorga, 2009), which would preserve this PLCζ pool. Lastly, we previously showed that in Dpy19l2-dependent globozoospermia, the separation of the nuclear envelope leads to the detachment of the whole acrosome, associated with the perinuclear theca and the outer nuclear envelope, with these structures being then discarded during elimination of the residual body during the compaction stage (Escalier, 1990; Pierre et al., 2012). It is therefore likely possible that PLCζ which is bound to the IAM is discarded from the cell during elimination of the residual body. Altogether, these data demonstrate for the first time a location of PLCζ consistent with its role in human sperm.
While the evidence for PLCζ is accumulating, a recent study has shown that another sperm protein may be capable of initiating oscillations in mouse and human eggs (Aarabi et al., 2014). In that study, it was shown that injection of a recombinant form of the sperm protein post-acrosomal WW binding protein (PAWP) triggered Ca2+ oscillations in mouse eggs, and oscillations were also induced in human eggs by injection of a cRNA encoding human PAWP. Further, a blocking peptide based on a WW binding motif present in PAWP inhibited oscillations initiated by injection of PAWP or by fertilization (Aarabi et al., 2014). Although these results are intriguing and represent the first step toward the validation that PAWP may participate in the early stages of mammalian fertilization, important questions remain unanswered, including a very recent report that raises questions about PAWP's ability to initiate Ca2+ oscillations, as injection of mouse PAWP recombinant protein or cRNA failed to initiate oscillations in mouse eggs (Nomikos et al., 2014). Besides those results, other questions deserve consideration. For example, although oscillations are present in all mammals during fertilization, different species display distinct patterns of oscillations (Igusa and Miyazaki, 1986; Taylor et al., 1993; Halet et al., 2004; Malcuit et al., 2006). These patterns are faithfully replicated by injection of species-specific PLCζ cRNAs (Saunders et al., 2002; Ito et al., 2008; Cooney et al., 2010; Sato et al., 2013), although thus far the oscillations induced by PAWP do not appear to show this degree of subtlety (Aarabi et al., 2014). Another important unknown about PAWP, and the suppression of oscillations by the inhibitor peptide, are the site of action. In other words, it was not shown whether or not the blocking peptide is actually binding PAWP or some other target or both. In the same vein, it is unknown whether or not PAWP is necessary to induce oscillations during fertilization. Toward that end, it would be useful to determine if anti-PAWP-specific antibodies could be used to deplete the [Ca2+]i-oscillation inducing activity present in mammalian sperm extracts. If this turns out to be the case, as shown to be the case for PLCζ (Saunders et al., 2002; Kurokawa et al., 2007), it will represent a compelling result, which will require elucidation of the mechanism by which it triggers oscillations in mammals.
Oocyte activation by human globozoospermic sperm
Unlike ICSI with Dpy19l2 KO mouse sperm, ICSI with globozoospermic human sperm induced oocyte activation in 53% of cycles (7/13, see Table I Nber 2-PN embryos), although only 7.25% ± 9.76 of the MII oocytes formed 2-PN embryos (total MII oocytes: 139 obtained in 13 different cycles). The females involved in this cohort were young (mean age 30.23 ± 4.28) and presented no evidence of infertility, which highlights the low activation rates in these couples compared with couples with a tuboperitoneal female factor and normal spermatogram, whose activation rates after ICIS are ∼70% (Bukulmez et al., 2000). These low fertilization rates are consistent with previous publications examining non-genotyped globozoospermic patients (Liu et al., 1995; Battaglia et al., 1997). It is notable that despite near complete absence of PLCζ in the sperm of our patients, some oocytes became activated. Although the oolema deformation and the subsequent rupture of the membrane caused by the ICSI procedure cause Ca2+ influx, it is generally not sufficient to activate human oocytes (Tesarik et al., 1994; Sato et al., 1999). Nevertheless, it is possible that in some cases, this Ca2+ influx together with the low traces of PLCζ associated with acrosomal buds might be capable of supporting oocyte activation. The importance of acrosomal buds in oocyte activation was initially suggested in a SPATA16/DPY19L2 negative globozoospermic patient (Sermondade et al., 2011) and confirmed later in non-genotyped globozoospermic patients by Kashir et al, showing that sperm exhibiting an acrosomal bud presented a punctate pattern of PLCζ staining within the head (Kashir et al., 2012). Finally, there appears to be a clear correlation in Table I between the number of MII oocytes retrieved and the occurrence of 2-PN embryos, the correlation coefficient between MII oocytes and 2-PN embryos being 0.87. This observation suggests that the ability of globozoospermic sperm to activate the eggs is dependent on the number of retrieved oocytes. It may reflect either a difference in oocyte quality or a higher chance that those eggs were injected with sperm with an acrosomal bud. This observation deserves further study.
In conclusion, this study shows that PLCζ is absent or greatly reduced in DPY19L2-deficient sperm. Moreover, for the first time we demonstrate the subcellular location of PLCζ in human sperm, which is consistent with its role as the sperm factor and its absence in Dpy19l2-dependent globozoospermic sperm.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
J.E. and H.C.L. performed ICSI experiments. J.D. and K.P.G. performed EM experiments. S.Y. and J.E. performed confocal experiments on PLCzeta localization and sperm DNA compaction, andWB experiments. R.Z., S.H. and C.M.G. provided clinical data and human sperm samples. G.M., C.C. and T.K. were responsible of molecular biology experiments. P.F.R., R.F. and C.A. coordinated the study. P.F.R., R.F. and C.A. contributed to discussion, design and interpretation of data. C.A. and R.F. wrote the manuscript.
Funding
This study was supported by grants from Gravit Foundation (to C.A.), the Agence National de la Recherche (Grant ICG2I to P.F.R. and C.A.) and NIH (Grant number: R01 HD051872 to R.F.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We are grateful to the patients who gave their informed consent to the use of their samples for research. We thank Dominique Alfandari for technical assistance. We also thank clinicians from the reproductive clinics (Pascale Hoffman, Ulrike Bergues and Dr B. Courbière) and C. Metton and M.J. Fays-Bernardin for technical assistance and Germetheque support.
References
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28:4434–4440. doi: 10.1096/fj.14-256495. [DOI] [PubMed] [Google Scholar]
- Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCzeta): oocyte activation and clinical links to male factor infertility. Adv Biol Regul. 2013;53:292–308. doi: 10.1016/j.jbior.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Audouard C, Christians E. Hsp90beta1 knockout targeted to male germline: a mouse model for globozoospermia. Fertil Steril. 2011;95:1475–1477. doi: 10.1016/j.fertnstert.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Koehler JK, Klein NA, Tucker MJ. Failure of oocyte activation after intracytoplasmic sperm injection using round-headed sperm. Fertil Steril. 1997;68:118–122. doi: 10.1016/s0015-0282(97)81486-0. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Yarali H, Yucel A, Sari T, Gurgan T. Intracytoplasmic sperm injection versus in vitro fertilization for patients with a tubal factor as their sole cause of infertility: a prospective, randomized trial. Fertil Steril. 2000;73:38–42. doi: 10.1016/s0015-0282(99)00449-5. [DOI] [PubMed] [Google Scholar]
- Cooney MA, Malcuit C, Cheon B, Holland MK, Fissore RA, D'Cruz NT. Species-specific differences in the activity and nuclear localization of murine and bovine phospholipase C zeta 1. Biol Reprod. 2010;83:92–101. doi: 10.1095/biolreprod.109.079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutton C, Zouari R, Abada F, Ben Khelifa M, Merdassi G, Triki C, Escalier D, Hesters L, Mitchell V, Levy R, et al. MLPA and sequence analysis of DPY19L2 reveals point mutations causing globozoospermia. Hum Reprod. 2012;27:2549–2558. doi: 10.1093/humrep/des160. [DOI] [PubMed] [Google Scholar]
- Coutton C, Abada F, Karaouzene T, Sanlaville D, Satre V, Lunardi J, Jouk PS, Arnoult C, Thierry-Mieg N, Ray PF. Fine characterisation of a recombination hotspot at the DPY19L2 locus and resolution of the paradoxical excess of duplications over deletions in the general population. PLoS Genet. 2013;9:e1003363. doi: 10.1371/journal.pgen.1003363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam AH, Feenstra I, Westphal JR, Ramos L, van Golde RJ, Kremer JA. Globozoospermia revisited. Hum Reprod Update. 2007a;13:63–75. doi: 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- Dam AH, Koscinski I, Kremer JA, Moutou C, Jaeger AS, Oudakker AR, Tournaye H, Charlet N, Lagier-Tourenne C, van Bokhoven H, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007b;81:813–820. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinati E, Kuentz P, Redin C, Jaber S, Vanden Meerschaut F, Makarian J, Koscinski I, Nasr-Esfahani MH, Demirol A, Gurgan T, et al. Globozoospermia is mainly due to DPY19L2 deletion via non-allelic homologous recombination involving two recombination hotspots. Hum Mol Genet. 2012;21:3695–3702. doi: 10.1093/hmg/dds200. [DOI] [PubMed] [Google Scholar]
- Escalier D. Failure of differentiation of the nuclear-perinuclear skeletal complex in the round-headed human spermatozoa. Int J Dev Biol. 1990;34:287–297. [PubMed] [Google Scholar]
- Fujihara Y, Satouh Y, Inoue N, Isotani A, Ikawa M, Okabe M. SPACA1-deficient male mice are infertile with abnormally shaped sperm heads reminiscent of globozoospermia. Development. 2012;139:3583–3589. doi: 10.1242/dev.081778. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry AC. Mammalian phospholipase Czeta induces oocyte activation from the sperm perinuclear matrix. Dev Biol. 2004;274:370–383. doi: 10.1016/j.ydbio.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008;23:2513–2522. doi: 10.1093/humrep/den280. [DOI] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Parkinson SJ, Carroll J. Conventional PKCs regulate the temporal pattern of Ca2+ oscillations at fertilization in mouse eggs. J Cell Biol. 2004;164:1033–1044. doi: 10.1083/jcb.200311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz R, Zouari R, Pierre V, Ben Khelifa M, Kharouf M, Coutton C, Merdassi G, Abada F, Escoffier J, Nikas Y, et al. A recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am J Hum Genet. 2011;88:351–361. doi: 10.1016/j.ajhg.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009;24:2417–2428. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]
- Heytens E, Schmitt-John T, Moser JM, Jensen NM, Soleimani R, Young C, Coward K, Parrington J, De Sutter P. Reduced fertilization after ICSI and abnormal phospholipase C zeta presence in spermatozoa from the wobbler mouse. Reprod Biomed Online. 2010;21:742–749. doi: 10.1016/j.rbmo.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S. Periodic increase of cytoplasmic free calcium in fertilized hamster eggs measured with calcium-sensitive electrodes. J Physiol. 1986;377:193–205. doi: 10.1113/jphysiol.1986.sp016181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1: an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod. 2008;78:1081–1090. doi: 10.1095/biolreprod.108.067801. [DOI] [PubMed] [Google Scholar]
- Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, van Deursen JM. Lack of acrosome formation in Hrb-deficient mice. Science. 2001;294:1531–1533. doi: 10.1126/science.1063665. [DOI] [PubMed] [Google Scholar]
- Karaca N, Yilmaz R, Kanten GE, Kervancioglu E, Solakoglu S, Kervancioglu ME. First successful pregnancy in a globozoospermic patient having homozygous mutation in SPATA16. Fertil Steril. 2014;102:103–107. doi: 10.1016/j.fertnstert.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Kashir J, Sermondade N, Sifer C, Oo SL, Jones C, Mounce G, Turner K, Child T, McVeigh E, Coward K. Motile sperm organelle morphology evaluation-selected globozoospermic human sperm with an acrosomal bud exhibits novel patterns and higher levels of phospholipase C zeta. Hum Reprod. 2012;27:3150–3160. doi: 10.1093/humrep/des312. [DOI] [PubMed] [Google Scholar]
- Koscinski I, Elinati E, Fossard C, Redin C, Muller J, Velez de la Calle C, Schmitt F, Ben Khelifa M, Ray PF, Kilani Z, et al. DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Genet. 2011;88:344–350. doi: 10.1016/j.ajhg.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA. Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol. 2005;285:376–392. doi: 10.1016/j.ydbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Yoon SY, Alfandari D, Fukami K, Sato K, Fissore RA. Proteolytic processing of phospholipase Czeta and [Ca2+]i oscillations during mammalian fertilization. Dev Biol. 2007;312:407–418. doi: 10.1016/j.ydbio.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Arny M, Grow D, Dumesic D, Fissore RA, Jellerette-Nolan T. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet. 2014;31:749–756. doi: 10.1007/s10815-014-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Liu J, Nagy Z, Joris H, Tournaye H, Devroey P, Van SA. Successful fertilization and establishment of pregnancies after intracytoplasmic sperm injection in patients with globozoospermia. Hum Reprod. 1995;10:626–629. doi: 10.1093/oxfordjournals.humrep.a136000. [DOI] [PubMed] [Google Scholar]
- Liu G, Shi QW, Lu GX. A newly discovered mutation in PICK1 in a human with globozoospermia. Asian J Androl. 2010;12:556–560. doi: 10.1038/aja.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K, Sjogren A, Nilsson L, Hamberger L. Fertilization and pregnancy after intracytoplasmic microinjection of acrosomeless spermatozoa. Fertil Steril. 1994;62:1266–1267. [PubMed] [Google Scholar]
- Malcuit C, Maserati M, Takahashi Y, Page R, Fissore RA. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca2+]i responses and oocyte activation. Reprod Fertil Dev. 2006;18:39–51. doi: 10.1071/rd05131. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, Nounesis G, Lai FA, Swann K. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod. 2014;20:938–947. doi: 10.1093/molehr/gau056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiardi C, Pasini ME, Gioria M, Berruti G. Failure of acrosome formation and globozoospermia in the wobbler mouse, a Vps54 spontaneous recessive mutant. Spermatogenesis. 2011;1:52–62. doi: 10.4161/spmg.1.1.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre V, Martinez G, Coutton C, Delaroche J, Yassine S, Novella C, Pernet-Gallay K, Hennebicq S, Ray PF, Arnoult C. Absence of Dpy19l2: a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development. 2012;139:2955–2965. doi: 10.1242/dev.077982. [DOI] [PubMed] [Google Scholar]
- Sato MS, Yoshitomo M, Mohri T, Miyazaki S. Spatiotemporal analysis of [Ca2+]i rises in mouse eggs after intracytoplasmic sperm injection (ICSI) Cell Calcium. 1999;26:49–58. doi: 10.1054/ceca.1999.0053. [DOI] [PubMed] [Google Scholar]
- Sato K, Wakai T, Seita Y, Takizawa A, Fissore RA, Ito J, Kashiwazaki N. Molecular characteristics of horse phospholipase C zeta (PLCzeta) Anim Sci J. 2013;84:359–368. doi: 10.1111/asj.12044. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Hafhouf E, Dupont C, Bechoua S, Palacios C, Eustache F, Poncelet C, Benzacken B, Levy R, Sifer C. Successful childbirth after intracytoplasmic morphologically selected sperm injection without assisted oocyte activation in a patient with globozoospermia. Hum Reprod. 2011;26:2944–2949. doi: 10.1093/humrep/der258. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Lawrence YM, Kingsland CR, Biljan MM, Cuthbertson KS. Oscillations in intracellular free calcium induced by spermatozoa in human oocytes at fertilization. Hum Reprod. 1993;8:2174–2179. doi: 10.1093/oxfordjournals.humrep.a137999. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Yoon SY, Morshedi MS, Lacey DR, Jellerette T, Fissore RA, Oehninger S. Complete globozoospermia associated with PLCzeta deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online. 2010;20:559–564. doi: 10.1016/j.rbmo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9:511–518. doi: 10.1093/oxfordjournals.humrep.a138537. [DOI] [PubMed] [Google Scholar]
- Xiao N, Kam C, Shen C, Jin W, Wang J, Lee KM, Jiang L, Xia J. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest. 2009;119:802–812. doi: 10.1172/JCI36230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet. 1999;23:118–121. doi: 10.1038/12729. [DOI] [PubMed] [Google Scholar]
- Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, Kuretake S, Yanagida K, Sato A, Toshimori K, Noda T. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci USA. 2002;99:11211–11216. doi: 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Fissore RA. Release of phospholipase C zeta and [Ca2+]i oscillation-inducing activity during mammalian fertilization. Reproduction. 2007;134:695–704. doi: 10.1530/REP-07-0259. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest, 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Perry AC. Piezo-actuated mouse intracytoplasmic sperm injection (ICSI) Nat Protoc. 2007;2:296–304. doi: 10.1038/nprot.2007.7. [DOI] [PubMed] [Google Scholar]
- Young C, Grasa P, Coward K, Davis LC, Parrington J. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril. 2009;91:2230–2242. doi: 10.1016/j.fertnstert.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Zanetti N, Mayorga LS. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod. 2009;81:396–405. doi: 10.1095/biolreprod.109.076166. [DOI] [PubMed] [Google Scholar]
- Zhu F, Gong F, Lin G, Lu G. DPY19L2 gene mutations are a major cause of globozoospermia: identification of three novel point mutations. Mol Hum Reprod. 2013;19:395–404. doi: 10.1093/molehr/gat018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.